Abstract
Background
The optimal mechanical ventilation strategy to minimise ventilator-induced lung injury (VILI) remains uncertain. Mechanical power (MP) is a key VILI determinant, but whether and how MP should be normalised to individual patient characteristics is unclear. In this study, we aimed to evaluate whether the discriminatory accuracy of MP for ICU mortality in mechanically ventilated patients improves when normalised to physiologically relevant variables that reflect individual susceptibility to VILI. We also explored whether the relationship between MP, MPratio, and mortality is linear or exhibits a threshold effect.
Methods
In this retrospective observational study, we extracted granular electronic healthcare record data for mechanically ventilated adults in a single centre over a seven-year period. Primary exposures were MP with five normalisations: for dead space (expressed as corrected minute ventilation, ventilatory ratio, or end-tidal to arterial CO2 ratio); aerated lung size (compliance), and normal idealised MP (MPratio). We used logistic regression to assess associations with ICU mortality. We calculated the Area Under the Receiver Operating Characteristic Curve (AUROC) to compare discriminative accuracy of individual models. Additionally, we evaluated the linearity or presence of a threshold for the relationships between MP, MPratio and ICU mortality.
Result
We included 3,578 patients in our analyses. We found MP normalised to compliance (AUROC 0.71, 95% confidence interval (CI) 0.69–0.73, p = 0.007 (DeLong’s test)) and MPratio (AUROC 0.71, 95% CI 0.68–0.73, p = 0.0014) performed better than MP alone (AUROC 0.69, 95% CI 0.66–0.71) for predicting ICU mortality. Other methods of MP normalisation were no more discriminative than MP without normalisation. The relationship between MP and MPratio with ICU mortality showed a statistically significant but small departure from linearity.
Conclusions
Mechanical power normalised to compliance and MPratio had better discrimination for ICU mortality than MP, although the difference was modest and absolute predictive power remained limited.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-025-01562-9.
Keywords: Mechanical power, Normalised mechanical power, Mechanical ventilation, Ventilatory ratio, Ventilator-induced lung injury, Ergotrauma, Acute respiratory distress syndrome
Background
Invasive mechanical ventilation is an important intervention in the management of patients with acute respiratory distress syndrome (ARDS), but carries the risk of ventilator-induced lung injury (VILI) [1]. The mechanisms underlying VILI include barotrauma (excessive pressure), volutrauma (excessive volume), atelectrauma (repeated opening and closing of lung units), and biotrauma (inflammatory response to ventilation) [2]. Furthermore, there is increasing recognition that the energy applied to the respiratory system during mechanical ventilation (Mechanical power -MP) is a significant determinant of VILI - a concept termed ergotrauma [3]. Mechanical power quantifies the energy transferred to the respiratory system per unit of time and includes key ventilatory parameters such as positive end expiratory pressure (PEEP), tidal volume, driving pressure, inspiratory flow and respiratory rate [4]. Higher MP has been associated with increased mortality in ARDS patients [5] and experimental models [6, 7] demonstrate a direct link between MP levels and histological and pathological markers of VILI [8].
One key unresolved question is whether MP should be normalised to individual patient characteristics. While MP reflects total energy delivered, its effect on the respiratory system likely varies depending on anthropometric characteristics, and amount of functional, aerated lung tissue. This is conceptually similar to the argument made for tidal volume, where its normalisation to compliance—the driving pressure—provides superior discriminative accuracy for mortality compared to indexing by predicted body weight alone [9]. Several approaches to MP normalisation have been proposed. These include adjustment for predicted body weight (PBW) [10], lung gas volumes measured by computed tomography (CT) scan [11], and compliance [12]. Some studies suggest that MP normalised to compliance or well-aerated lung tissue is more strongly associated with mortality in ARDS [11]. Another study predicting outcome of a short weaning trial found MP normalized to respiratory system compliance was enhanced by adjustment for PaCO2 [13]. A novel metric, the mechanical power ratio (MPratio), represents the ratio of delivered MP to the theoretical MP required for normal minute ventilation in a healthy individual of the same age, sex, weight and height [6]. MPratio has shown early promise in predicting treatment escalation to invasive ventilation in COVID-19 patients receiving non-invasive continuous positive airway pressure (CPAP) [14]. In a small cohort of ARDS patients, MPratio outperformed MP, PaO2/FiO2 ratio, driving pressure, and alveolar dead space for mortality prediction [15]. In addition, studies of animals with healthy lungs indicate that a MPratio above 4.5 has a threshold effect above which VILI occurs [6].
Although several studies have examined mechanical power (MP) and its normalisation in small and heterogeneous populations, none has compared these parameters in a large cohort of ventilated patients spanning the full spectrum of severity—from no ARDS to severe ARDS.
In this study, we aimed to evaluate whether the discriminatory accuracy of MP, for ICU mortality in mechanically ventilated patients, improves when normalised to physiologically relevant variables that reflect individual susceptibility to VILI. We also explored whether the relationship between MP, MPratio, and mortality is linear or exhibits a threshold effect.
Methods
Study design and setting
We conducted a retrospective cohort study using routinely collected patient data at a tertiary centre with a quaternary severe respiratory failure service in London, United Kingdom (UK).
Study participants
We included all patients aged 18 years or older who were admitted to the ICU between 1 January 2015 and 31 December 2021 (7 years), received invasive mechanical ventilation in a mandatory mode without spontaneous effort, and had evaluable mechanical power data within 24 h of initiating mandatory ventilation. We excluded patients who were ventilated using airway pressure release ventilation (APRV), required extracorporeal membrane oxygenation (ECMO), required renal replacement therapy for pre-existing chronic kidney disease, had an ICU stay of less than 72 h, or exhibited spontaneous breathing efforts, defined as a difference of more than one breath per minute between the set and measured respiratory rates.
Data sources
We extracted demographic characteristics, ventilator parameters and the corresponding arterial blood gas results, physiological variables, and ICU mortality from the hospital electronic records (ICIP; Philips, Netherlands) of all patients meeting our inclusion criteria and none of our exclusion criteria.
Mechanical power calculation and normalisation
We calculated MP using the simplified formula for pressure control ventilation as described by Becher et al. [16]. Measured minute volume (VE Meas) was calculated as the product of respiratory rate (RR, min− 1) and tidal volume (VT, Litres). MP was calculated whenever available data were documented in the medical record. We then calculated the maximum values in the first 24-hours of mechanical ventilation. All five defined normalisation variants were calculated using maximum MP. For normalisations requiring etCO2 or PaCO2, the closest value within 2 h of the maximal recorded MP was used. If these data were unavailable, the data was considered missing and was not imputed.
We used the following formulae for MP and the five normalisation (MPcorr, MPVR, MPCO2, MPCdyn and MPratio ) variants
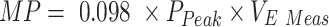 |
1 |
MPcorr was defined by the substitution of corrected minute volume (VE Corr) for VE Meas in Eq. 1. VE Corr was calculated as VE Meas multiplied by the ratio of PaCO2 to an ideal PaCO2 of 5.3 kPa (40mmHg) [17].
 |
2 |
MPVR was defined as the product of MP and ventilatory ratio (VR), with VR calculated as the ratio of the product of VE Meas and PaCO2, to the product of an ideal minute volume of 0.1 L/kg/min, an ideal PaCO2 of 5 kPa (37.5mmHg), and PBW [18].
 |
3 |
Where: 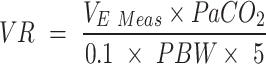 and PBW was calculated as per the ARDSnet definitions [19] of:
and PBW was calculated as per the ARDSnet definitions [19] of:

MPCO2 was defined as MP divided by the etCO2/PaCO2 ratio.
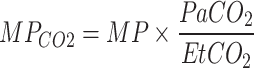 |
4 |
MPCdyn was defined as the ratio of MP and dynamic compliance of the respiratory system (Crs).
 |
5 |
MPratio was defined as the ratio of MP and a patient-specific ideal MP.
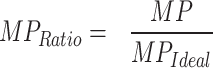 |
6 |
Where: 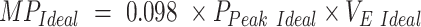
MPideal was derived as per the method detailed by Pozzi et al. [15]
Statistical analysis
We fitted six univariable logistic regression models to the dependent variable of ICU mortality with MP and each of the five defined variants as predictor variables. We calculated odds ratios (OR) with 95% confidence intervals (CI) for ICU mortality considering each MP variant individually. We constructed receiver operating characteristic (ROC) curves to calculate AUROC for each model. The difference between AUROC values for each MP variant was tested against MP using DeLong’s test [20].
To explore the linearity of relationships between MP and MPratio with ICU mortality, we calculated and plotted ORs for 11 quantiles with reference to the median quantile. Additionally, we constructed logistic regression models including quadratic terms for the relevant MP variant.
Data are presented as median and interquartile ranges (IQR) for continuous data and counts and percentages for categorical data. Complete case analysis was performed with no imputation or substitution of missing data. An alpha level of 0.05 was chosen as the threshold for statistical significance. All statistical analyses were performed using R version 4.4 [21].
Ethical Considerations
This study was approved by the Institution (No: 14632) with the need for informed consent waived due to the use of anonymised and routinely collected data.
Results
Baseline characteristics
We included 3,578 patients in our analysis (See Fig. 1 for CONSORT diagram). Of these, 2,306 (64%) were male; median age was 60 (IQR 47, 71) years; median APACHE II score of 15 (IQR 12, 20) (Table 1). Utilising the worst PaO2/FiO2 ratio recorded in the first 24 h of invasive mechanical ventilation, we identified 763 (21.3%) patients with a PF ratio ≥ 40 kPa (≥ 300mmHg) (no ARDS); 1035 (28.9%) 26.6 kPa to 39.9 kPa (200 to 299mmHg) (mild ARDS), 1295 (36.2%) 13.3 kPa to 26.5 kPa (100mmHg to 199mmHg) (moderate ARDS), and 485 (13.6%) < 13.3kPa (< 100mmHg) (severe ARDS).
Fig. 1.
Patient inclusion CONSORT flow diagram
Table 1.
Baseline patient characteristics
| Patient Characteristic | N = 3,578 |
|---|---|
| Age, years | 60 (47–71) |
| Male | 2,306 (64) |
| Height, cm | 170 (165, 175) |
| Weight, kg | 75 (65, 86) |
| Predicted body weight, kg | 66 (57, 71) |
| APACHE II score | 15 (12, 20) |
| Surgical admission | 1,217 (34) |
| Elective admission | 935 (26) |
| Infection related admission | 762 (21) |
| Admission Diagnosis by Organ System: | |
| Cardiovascular | 1052 (29) |
| Respiratory | 1023 (29) |
| Gastrointestinal | 538 (15) |
| Neurological (including eyes) | 328 (9.2) |
| Poisoning | 184 (5.1) |
| Genito-urinary | 171 (4.8) |
| Endocrine, Metabolic, Thermoregulation and Poisoning | 161 (4.5) |
| Musculoskeletal | 44 (1.2) |
| Haematological/Immunological | 31 (0.9) |
| Dermatological | 22 (0.6) |
| Psychiatric | 13 (0.4) |
| Trauma | 11 (0.3) |
| Comorbidities: | |
| Cardiovascular disease | 971 (27) |
| Pulmonary disease | 772 (22) |
| Renal disease | 448 (13) |
| Liver disease | 181 (5.1) |
| Diabetes | 718 (20) |
| Cancer | 243 (6.8) |
| Volume control mode | 232 (6) |
| Pressure control mode | 3,346 (94) |
| Tidal volume, ml | 537 (458, 625) |
| Tidal volume PBW, ml/kg | 8.4 (7.1, 9.9) |
| Minute volume, L/min | 8.9 (7.2, 10.9) |
| PEEP, cmH2O | 6 (5, 8) |
| Peak inspiratory pressure, cmH2O | 22 (18, 26) |
| Driving pressure, cmH2O | 14 (11.5, 17.0) |
| Dynamic compliance, ml/cmH2O | 38 (30, 49) |
| Minute volume corrected, L/min | 8.5 (6.7, 11.2) |
| Ventilatory ratio | 1.33 (1.06, 1.75) |
| End-tidal: arterial pCO2 ratio | 0.88 (0.76, 1.02) |
Median (IQR) for continuous variables, n (%) for categorical variables. For missing data counts see Supplementary materials.
The median maximum MP in the first 24 h of ventilation was 19 J/min (IQR 14, 26). Median values for MP normalisations are presented in Table 2.
Table 2.
Comparison of mechanical power variants
| MP Variant | Median (IQR) | OR (95% CI) | AUROC (95% CI) | p-value |
|---|---|---|---|---|
| MP | 19 J/min (14, 26) | 1.03 (1.02, 1.05) | 0.69 (0.66,0.71) | N/A |
| MPcorr | 19 J/min (13, 28) | 1.03 (1.03, 1.04) | 0.68 (0.65, 0.70) | 0.6 |
| MPVR | 25 J/min (15, 44) | 1.01 (1.01, 1.01) | 0.67 (0.65, 0.70) | 0.5 |
| MPCO2 | 22 J/min (15, 33) | 1.02 (1.01, 1.02) | 0.70 (0.68, 0.73) | 0.4 |
| MPCdyn | 0.53 J.cmH2O /min.ml (0.34, 0.86) | 1.21 (1.11, 1.31) | 0.71 (0.69, 0.73) | 0.007 |
| MPratio | 5.17 (3.80, 7.05) | 1.23 (1.19, 1.27) | 0.71 (0.68, 0.73) | 0.0014 |
MP variants, median and Meandiand interquartile ranges for each MP variant, odds ratios for mortality from the univariable logistic regression model and discriminatory accuracy of models as assessed by calculation of the AUROC. P-values for each MP variant using DeLong’s test was performed with reference to MP. For missing data counts see Supplementary Material.
Outcomes
ICU mortality was 485/3578 (14%). As presented in Table 2, the AUROC values for MP variants ranged from 0.67 to 0.71. MPCdyn and MPratio demonstrated the most accurate prediction of ICU mortality. As assessed using the DeLong’s test, AUROCs for MPcorr, MPVR, and MPCO2 were not different to MP.
Quantile analysis
The odds ratio of ICU mortality for 11 quantiles of MP compared with the median reference quantile are plotted in Fig. 2. The inclusion of quadratic terms to logistic regression models of MP and MPratio indicated statistically significant departures from linearity for both variables. However, the quadratic coefficient was small in absolute value with minimal influence over the range of MP observed. See Supplementary Material for further details.
Fig. 2.
a/b Odds Ratios for 11 Quantiles of Mechanical Power and Mechanical Power Ratio. Odds ratios for mortality plotted with 95% CIs for 11 quantiles of MP (a) and MPratio (b). Odds ratios are relative to the median quantile (green line)
Discussion
In this large cohort of mechanically ventilated ICU patients, we found that MPratio and MP normalised to compliance had a modest but statistically significant improvement in the discriminatory accuracy for ICU mortality compared to conventional MP. While the absolute increase in AUROC was limited, both measures outperformed MP normalised to dead space surrogates, which offered no additional discriminative value compared to conventional MP. Our findings are consistent with previous studies in smaller cohorts of ARDS patients that found MP normalised to compliance had a greater discriminatory accuracy than conventional MP [11, 12]. Similarly, MPratio outperformed MP normalised for calculated dead space fraction in predicting mortality in ARDS patients [15].
We selected MP normalisation variants to adjust MP based on individual patient susceptibility to VILI. These included ventilated lung size (i.e., compliance), dead space, and ventilatory efficiency. MPratio is an alternative approach, normalising MP to an ideal MP to achieve normal PaCO2 in healthy individuals, using idealised values of respiratory mechanics and patient-specific anthropometrics.
MPcorr utilises VE corrected instead of VE Measured as a surrogate of dead space that has been independently associated with mortality in ARDS patients [22]. MPVR is conventional MP multiplied by the ventilatory ratio. First described in 2009, VR is a unitless ratio of VE Measured and PaCO2 to predicted VE (0.1 L/kg PBW) and an ideal PaCO2 of 5 kPa (37.5mmHg) [18]. An increase in VR may be due to increased CO2 production or to reduced ventilatory efficiency (i.e., increased dead space ventilation). MPCO2 is conventional MP divided by the ratio of end-tidal to arterial CO2. This ratio is used to assess gas exchange, and particularly the alveolar dead space, although venous admixture (shunt) will also decrease the value. Lung perfusion will influence this ratio although cardiac output data was not included in our data set. A low end-tidal to arterial CO2 ratio is strongly associated with dead space fraction, as assessed by volumetric capnography, and mortality in ARDS patients [23]. MPCdyn is conventional MP divided by the compliance which reflects the functional lung size which may be greatly reduced in ARDS patients – it has been shown to correlate with the dimensions of the baby lung [24].
Normalising MP for PBW or Crs (MPratio, MPCdyn) outperformed methods estimating dead space ventilation (MPcorr, MPVR, MPCO2). This may be because MP is an extensive variable, whose absolute value depends on system size [25]. Normalising MP to lung volume (Crs) or PBW (as used in MPideal) leads to an intensive variable, offering a more reliable indicator. EtCO₂ and PaCO₂ can fluctuate significantly, and using daily peak MP variant values in a heterogeneous cohort may affect their representativeness of underlying physiology.
Threshold effects and MP relationship with mortality
Defining a threshold MP value for increased lung injury remains a key objective in ventilation research. Serpa Neto et al. found that in an ARDS cohort, MP > 17 J/min was associated with a consistent increase in mortality [5]. Manrique et al. found that the risk of ICU mortality increased by 0.1% for each hour with MP > 18 J/min [26]. In a porcine model, D’Albo et al. identified a MPratio threshold of 4.5 for increased risk of VILI [6]. In our cohort we found that there was a near-linear relationship of and ICU mortality without a significant threshold effect despite the wide range of severity in our population spanning from no ARDS to severe ARDS. This finding may suggest that, in a heterogeneous cohort of patients where the primary cause of organ failure is not necessarily the lung, using MP as a predictor of mortality may not be the variable with the highest sensitivity and specificity. Alternatively, the lack of a clear threshold value in our study may indicate the need for more robust and refined MP normalisation to better capture individual risk profiles.
Previous research suggests that MP normalisation variants may differ by patient subgroup. Zhang et al. reported that MP normalized to PBW was only significantly associated with mortality in moderate-to-severe ARDS [10]. We did not find a similar interaction between MP and PaO2/FiO2 ratio (see supplementary material) which may, in part, reflect the heterogeneity of our study population. Although PaO2/FiO2 ratio is commonly used to enrich ARDS trial design and select patients for interventions which reduce VILI risk (prone positioning, ECMO), there is increasing recognition that PaO2/FiO2 ratio alone does not necessarily correlate with the susceptibility to VILI. Therefore it may not be the ideal parameter to base respiratory support strategies on [27].
Despite incremental improvement in the discriminative ability of MP for ICU mortality, the absolute predictive performance of these models remains limited and should not be considered a replacement for established risk stratification scores. Nevertheless, our analysis of a range of MP normalisation variants offers insight into their potential physiological relevance, even if not immediately applicable for prognostic use. As MP is a variable determined not only by the pathophysiologic state but by clinical decisions in relation to ventilation strategy, any signal for an association with mortality is worth exploring. The small differences seen in this study between MP and two normalisation variants may suggest that calculating MP alone already captures the relevant signal without need for further adjustment. Alternatively, it may reflect the heterogenous nature of our cohort and the limitations of ICU mortality as an outcome measure.
Limitations
Strengths of this study include the large, diverse cohort of invasively ventilated ICU patients.
Although the heterogeneity of our cohort supports the generalisability of our findings to a wider mechanically ventilated population, it may have masked associations within specific subgroups. Conducted at a single multi-ICU centre with a focus on respiratory failure, the study’s external validity may be limited. We advocate incorporating MP normalisation variants into prospective, phenotype-stratified studies and validating our findings in larger or open datasets to clarify the metrics’ utility and limitations.
The findings may not apply to patients ventilated using volume control, as 94% received pressure-controlled ventilation. We also did not differentiate between resistive and static elastic components of MP and VILI, but used total MP [28]. Maximal MP in the first 24 h was used to capture the most injurious early exposure and allow direct comparison of MP and its normalisations at a common time point. Future studies should examine cumulative exposure, MP trajectories, and time above threshold.
ICU mortality, though important, is an indirect marker of lung injury, influenced by extra-pulmonary failure, comorbidities, and treatment complications. Given the established link between ventilation strategy, VILI, and mortality, prospective work should assess MP normalisation against direct VILI measures such as biomarkers or imaging.
Conclusions
In this large cohort of invasively ventilated patients, normalising mechanical power to compliance (MPCdyn) and using MPratio yielded modest improvements in ICU mortality prediction compared with conventional MP. Other normalisation approaches conferred no additional discriminatory value. Although the small AUROC gains indicate only incremental improvements, none of the normalisation variants demonstrated strong predictive ability and should not be used for mortality risk stratification. Both MP and MPratio exhibited near-linear associations with mortality, without evidence of a threshold effect.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- APACHE II
Acute Physiology and Chronic Health Evaluation II
- APRV
Airway Pressure Release Ventilation
- ARDS
Acute Respiratory Distress Syndrome
- AUROC
Area Under the Receiver Operating Characteristics Curve
- CI
Confidence Interval
- Crs
Compliance of the respiratory system
- CT
Computed Tomography
- ECMO
Extracorporeal Membrane oxygenation
- etCO2
End-tidal Carbon Dioxide
- ICIP
Intellivue Clinical Information Portfolio
- ICNARC
Intensive Care National Audit and Research Centre
- ICU
Intensive Care Unit
- IQR
Interquartile Range
- MP
Mechanical Power
- MPcorr
Mechanical Power substituting VEcorr for VEMeas
- MPVR
Mechanical Power multiplied by the ventilatory ratio
- MPCO2
Mechanical Power divided by the etCO2/PaCO2 ratio
- MPCdyn
Mechanical Power divided by the dynamic compliance
- MPratio
Mechanical Power divided by MPideal
- PaCO2
Partial pressure of arterial carbon dioxide
- PaO2
Partial pressure of arterial oxygen
- PaO2/FiO2
Partial pressure of arterial oxygen to inspired fraction of oxygen ratio
- PBW
Predicted Body Weight
- PCV
Pressure Control Ventilation
- PEEP
Positive End-Expiratory Pressure
- Ppeak
Peak airway Pressure
- P-V
ressure-Volume
- ROC
Receiver Operating Characteristics
- RRT
Renal Replacement Therapy
- UK
United Kingdom
- VCV
Volume Control Ventilation
- VE
Minute Ventilation
- VEcorr
Corrected minute ventilation
- VEMeas
Actual measured minute ventilation
- VILI
Ventilator-Induced Lung Injury
- VR
Ventilatory Ratio
Author contributions
RK, BS, PDC, and LC conceived and designed the study. RK co-ordinated the project. LC supervised the project. BS extracted the data and conducted statistical analysis. RK wrote the initial draft of the manuscript. RK, BS, ET, PDC, RDS, SS, MC, FP, FC, LR, LG, and LC critically reviewed and contributed to editing of the manuscript. All authors have read and approved the final manuscript.
Funding
This research did not receive any external funding.
Data availability
Data cannot be shared publicly due to institutional ethics, privacy, and confidentiality regulations.
Declarations
Ethics approval and consent to participate
This study had institutional approval (number: 14632) with consent waived due to the retrospective observational use of routinely collected data. All data were anonymized before analysis.
Consent for publication
Due to the retrospective observational use of routinely collected data with informed consent waived there is no requirement to seek consent for publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369(22):2126–36. [DOI] [PubMed] [Google Scholar]
- 2.Gattinoni L, Collino F, Camporota L. Ventilator induced lung injury: a case for a larger umbrella? Intensive Care Med. 2024;50(2):275–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tonetti T, Vasques F, Rapetti F, Maiolo G, Collino F, Romitti F, et al. Driving pressure and mechanical power: new targets for VILI prevention. Ann Transl Med. 2017;5(14):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gattinoni L, Tonetti T, Cressoni M, Cadringher P, Herrmann P, Moerer O, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med. 2016;42(10):1567–75. [DOI] [PubMed] [Google Scholar]
- 5.Serpa Neto A, Deliberato RO, Johnson AEW, Bos LD, Amorim P, Pereira SM, et al. Mechanical power of ventilation is associated with mortality in critically ill patients: an analysis of patients in two observational cohorts. Intensive Care Med. 2018;44(11):1914–22. [DOI] [PubMed] [Google Scholar]
- 6.D’Albo R, Pozzi T, Nicolardi RV, Galizia M, Catozzi G, Ghidoni V, et al. Mechanical power ratio threshold for ventilator-induced lung injury. Intensive Care Med Exp. 2024;12:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scharffenberg M, Wittenstein J, Ran X, Zhang Y, Braune A, Theilen R, et al. Mechanical power correlates with lung inflammation assessed by positron-emission tomography in experimental acute lung injury in pigs. Front Physiol. 2021;12:717266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vassalli F, Pasticci I, Romitti F, Duscio E, Aßmann DJ, Grünhagen H, et al. Does iso-mechanical power lead to iso-lung damage? An experimental study in a Porcine model. Anesthesiology. 2020;132(5):1126–37. [DOI] [PubMed] [Google Scholar]
- 9.Network ARDS, Brower R, Matthay M, Morris A, Schoenfeld D, Taylor Thompson B, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–8. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, Zheng B, Liu N, Ge H, Hong Y. Mechanical power normalized to predicted body weight as a predictor of mortality in patients with acute respiratory distress syndrome. Intensive Care Med. 2019;45(6):856–64. [DOI] [PubMed] [Google Scholar]
- 11.Coppola S, Caccioppola A, Froio S, Formenti P, De Giorgis V, Galanti V, et al. Effect of mechanical power on intensive care mortality in ARDS patients. Crit Care Lond Engl. 2020;24(1):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pistillo N, Castelluccio P, Suzuki I, Castiblanco L. Mechanical power correlates with stress, strain, and atelectrauma Anly when normalized to aerated lung size in patients with acute respiratory distress syndrome. Crit Care Explor. 2023;5(10):e0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghiani A, Paderewska J, Walcher S, Tsitouras K, Neurohr C, Kneidinger N. Mechanical power normalized to lung-thorax compliance indicates weaning readiness in prolonged ventilated patients. Sci Rep. 2022;12(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gattarello S, Coppola S, Chiodaroli E, Pozzi T, Camporota L, Saager L, et al. Mechanical power ratio and respiratory treatment escalation in COVID-19 pneumonia: a secondary analysis of a prospectively enrolled cohort. Anesthesiology. 2023;138(3):289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pozzi T, Fratti I, Tomarchio E, Bruno G, Catozzi G, Monte A, et al. Early time-course of respiratory mechanics, mechanical power and gas exchange in ARDS patients. J Crit Care. 2024;79:154444. [DOI] [PubMed] [Google Scholar]
- 16.Becher T, van der Staay M, Schädler D, Frerichs I, Weiler N. Calculation of mechanical power for pressure-controlled ventilation. Intensive Care Med. 2019;45(9):1321–3. [DOI] [PubMed] [Google Scholar]
- 17.Wexler HR, Lok P. A simple formula for adjusting arterial carbon dioxide tension. Can Anaesth Soc J. 1981;28(4):370–2. [DOI] [PubMed] [Google Scholar]
- 18.Sinha P, Fauvel NJ, Singh S, Soni N. Ventilatory ratio: a simple bedside measure of ventilation. Br J Anaesth. 2009;102(5):692–7. [DOI] [PubMed] [Google Scholar]
- 19.NHLBI ARDS Network |. Tools [Internet]. [cited 2025 Jan 31]. Available from: http://www.ardsnet.org/tools.shtml
- 20.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 21.R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing. 2024. Available from: https://www.R-project.org
- 22.Fusina F, Albani F, Bertelli M, Cavallo E, Crisci S, Caserta R, et al. Corrected minute ventilation is associated with mortality in ARDS caused by COVID-19. Respir Care. 2021;66(4):619–25. [DOI] [PubMed] [Google Scholar]
- 23.Kallet RH, Lipnick MS. End-tidal-to-arterial PCO2 ratio as signifier for physiologic dead-space ratio and oxygenation dysfunction in acute respiratory distress syndrome. Respir Care. 2021;66(2):263–8. [DOI] [PubMed] [Google Scholar]
- 24.Gattinoni L, Pesenti A. The concept of baby lung. Intensive Care Med. 2005;31(6):776–84. [DOI] [PubMed] [Google Scholar]
- 25.Giosa L, Collins PD, Shetty S, Lubian M, Del Signore R, Chioccola M, et al. Bedside assessment of the respiratory system during invasive mechanical ventilation. J Clin Med. 2024;13(23):7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manrique S, Ruiz-Botella M, Murillo N, Canelles S, Victoria ID, Samper MA, et al. Impact of mechanical power on ICU mortality in ventilated critically ill patients: a retrospective study with continuous real-life data. Eur J Med Res. 2024;29:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catozzi G, Pozzi T, Nocera D, et al. Rethinking ARDS classification: oxygenation impairment fails to predict VILI risk. Intensive Care Med. 2025;51(1):62–71. [DOI] [PMC free article] [PubMed]
- 28.von Düring S, Parhar KKS, Adhikari NKJ, Urner M, Kim SJ, Munshi L, et al. Understanding ventilator-induced lung injury: the role of mechanical power. J Crit Care. 2025;85:154902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data cannot be shared publicly due to institutional ethics, privacy, and confidentiality regulations.




