Abstract
Relapse and metastasis of malignancy remain the primary causes of treatment failure. Our prior research has revealed that polyploid giant cancer cells (PGCCs), these specialized dormant cells, are capable of triggering cancer recurrence and widespread metastasis. Once awakening, these PGCCs give rise to daughter cells (DCs) through asymmetric division, which has been hypothesized as the primary driver of tumor recurrence and metastasis. Nevertheless, the precise role of DCs in head and neck squamous cell carcinoma (HNSCC) remains elusive. In this study, we have elucidated the characteristics of PGCCs and DCs in HNSCC. Furthermore, we have confirmed that the anoikis-resistance of DCs serves as a crucial mechanism for HNSCC recurrence and metastasis following treatment. Utilizing RNA-seq, we discovered that ITGB6 is upregulated in DCs. Additionally, through in vitro and in vivo experiments, we demonstrated that ITGB6 promotes HNSCC metastasis by activating the FAK/PI3K/AKT pathway, thereby inhibiting anoikis in DCs. Taken together, these findings suggest a potential therapeutic approach targeting DCs in HNSCC.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-18652-0.
Keywords: Tumor, PGCCs, HNSCCs, Tumor recurrence, Metastasis, Anoikis
Subject terms: Head and neck cancer, Metastasis
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a highly malignant tumor originating from the mucosal epithelial cells of the nasal, oral, and pharyngeal regions1. It is the most common type of head and neck cancer and also ranks as the sixth most common cancer worldwide2. Due to its high degree of malignancy, HNSCC poses a significant threat to patients’ health. Despite undergoing standard therapeutic regimens, approximately 50% of patients experience recurrence after treatment, indicating the significant challenges in managing HNSCC3–6. Regrettably, current research pertaining to the metastasis and recurrence of HNSCC remains scant. Consequently, there is an exigent need to elucidate the underlying molecular mechanisms in order to enhance the prognostic outcomes for patients afflicted with HNSCC.
Polyploid giant cancer cells (PGCCs) is a kind of dormant cell that exists in a variety of tumor tissues7. PGCCs can be induced by therapy of tumor like radiation, chemical drugs8,9. Zhang, S. et al. were the first to successfully induce PGCCs using cobalt chloride (CoCl2)7. PGCCs possess distinct morphological features, including a vast cytoplasmic area and either a single enlarged nucleus or multiple nuclei7,10,11. Remarkably, they can generate small daughter cells (DCs) through asymmetric cell divisions resembling budding, splitting, or bursting9. With the increased understanding of PGCCs, growing evidence points to their role in cancer persistence, metastasis, and chemoresistance across a range of malignancies, including ovarian, kidney, pancreatic, urothelial, prostate, melanoma, and breast cancers12–20. DCs derived from PGCCs are believed to be the primary culprit behind tumor recurrence and metastasis. However, metastasis is a highly inefficient process. Upon entering the circulation, tumor cells are induced to undergo programmed cell death, thereby making it difficult for them to form metastatic lesions. Presently, no studies have delineated the molecular mechanisms underlying DCs’ resistance to programmed cell death.
Anoikis is a type of programmed cell death caused by cells detaching from the extracellular matrix21. It is a key mechanism to prevent non-adhesive cells from growing and attaching to inappropriate matrices, thereby avoiding colonization of distant organs22. Due to the existence of this mechanism, circulating tumor cells can only be disseminated and metastasized with extremely low efficiency23,24. However, tumor can acquire the ability of anoikis resistance to survive while they circulate in the bloodstream25. Therefore, elucidating the molecular mechanisms by which tumors acquire resistance to anoikis is crucial for tumor treatment.
In the present study, we attempted to explored role of anoikis in DCs metastasis, and the molecular mechanism. We confirmed the role of ITGB6 playing in anoikis-resistance by In vitro and in vivo experiments. In addition, we determine the downstream molecular mechanisms of ITGB6 using a series of molecular experiments. Together, our findings suggest that ITGB6 enhances DCs resistance to anoikis and promotes HNSCC metastasis.
Result
The formation of PGCC and DCs in HNSCC
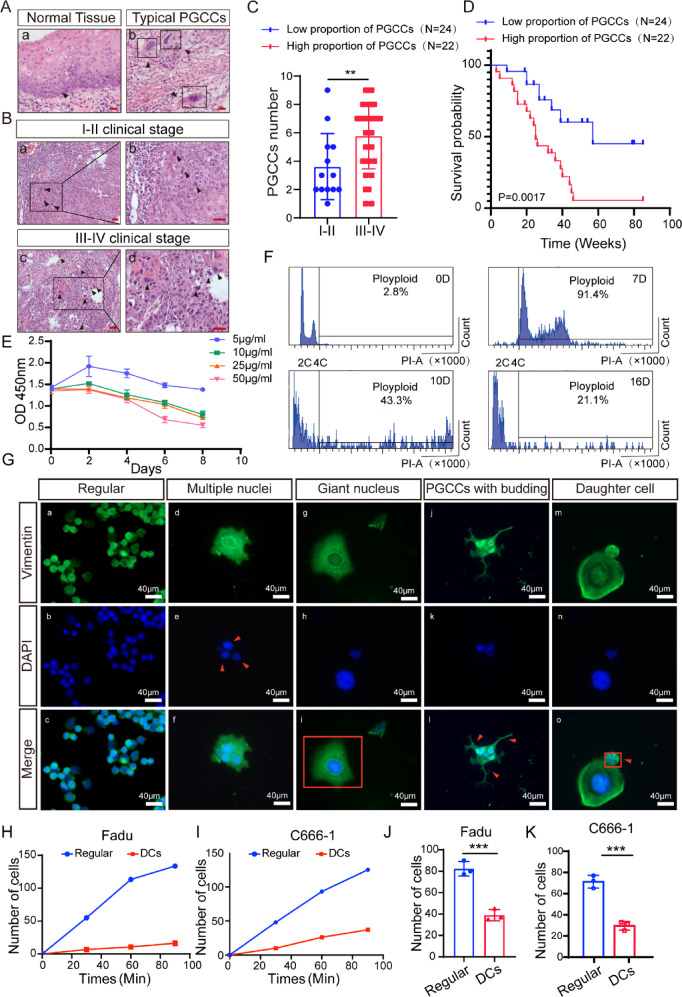
To examine the role of PGCCs and DCs on HNSCC progression, we calculated the number of PGCCs in tissues of patients with HNSCC based on morphological features (Fig. 1A, C). Patients in the late clinical stage showed more PGCCs in the HNSCC tissues (Fig. 1B). Furthermore, patients with more PGCCs had a significantly poorer prognosis (Fig. 1D, p = 0.0017).
Fig. 1.
Morphologic characteristics, and function analysis of PGCCs and DCs. (A) Representative HE images of PGCCs in tumor tissues. (bar:100 µm) (B–C) Representative HE images of PGCCs in tumor tissues with different clinical stages. (**p < 0.001, Student’s t‐test, bar:100 µm) (D) Kaplan–Meier curves showing the correlation between number of PGCCS and overall survival by the log‐rank test. (E) The CCK8 assays were performed to quantify the ability of cell proliferation. O.D., optical density. (F) Cell flow cytometry analysis at different time periods. (G) Morphologic characteristics morphology was visualized by phalloidin. (H–I) Cell adhesion experiment, reflecting the quantity of cell adhesion at different time points. (J-K) Colony formation experiment to quantify cell proliferation ability.
Similar with previous reports, we induced PGCCs formation from normal cancer cells by paclitaxel (PTX). When Fadu cells were exposed to the drug (5–50 μg/ml), cell death increased with paclitaxel concentration, and almost all the cells were killed when the concentration up to 50 μg/ml (Fig. 1E). Thus, we used the PTX at a concentration of 50 μg/ml to ensure follow-up investigations conducted smoothly. We added PTX to the cell culture medium, and cultured the cells for 24 h, then withdrew the drug, and afterwards replaced the medium daily to remove dead cells. The vast majority of the normal cancer cells were killed during 7 days of PTX withdrawal, and only a minority of cells survived to generate PGCCs (Fig. 1F, G). Then, 10 days after PTX treatment some small daughter cells (DCs) began to be formed via asymmetric budding or bursting by PGCCs (Fig. 1F, G). Subsequently, almost entirely of divided and formed DCs 16 days after drug withdrawal (Fig. 1F, G).
Consistent with prior reports, both PGCCs and DCs exhibit distinct morphological characteristics. PGCCs possess a vast cytoplasmic region and either a solitary giant nucleus or multiple nuclei, and the diameter of PGCCs measures about 170 μm (Fig. 1G, Figure S1A). Conversely, he diameter of DCs measures approximately 20 μm (Fig. 1G, Figure S1A). Overall, our study sheds light on the cellular attributes of PGCCs and the developmental process of DCs within HNSCC. Overall, our study sheds light on the cellular attributes of PGCCs and the developmental process of DCs within HNSCC.
To delve deeper into the biological behavior of DCs, we selected C666-1, another nasopharyngeal cell line, and induced the formation of DCs using the established protocol according to the methods established by our team before8. Surprisingly, we observed that the adhesion and proliferation abilities of DCs were inferior to those of typical cancer cells, contradicting previous research findings (Fig. 1H-K, Figure S1B)8. This unexpected result might be attributed to the incomplete restoration of cell function. The weakened adhesive capacity of DCs could potentially lead to their detachment from cancer tissue, potentially seeding distant metastases and relapse following chemotherapy.
DC cells are capable of resisting anoikis and achieving metastasis
To assess the anoikis-resistance capacity of DCs, we plated normal cells, PGCCs, and DCs on poly-HEMA coated plates for 48 h and subsequently conducted a colony formation experiment. Notably, DCs formed significantly more colonies compared to normal cells (Fig. 2A, B). Furthermore, the generation of DCs reduced the levels of cleaved caspase-3 and the pro-apoptotic protein BAX (Fig. 2C, D). Consistent with these findings, we obtained similar results after culturing the cells in suspension for 48 h (Fig. 2E, F). These observations suggest that DCs possess a stronger anti-apoptotic ability than typical cancer cells. Furthermore, the outcomes of the transwell experiments reveal that the migratory capacity of DCs significantly surpasses that of ordinary tumor cells (Fig. 2G, H, Figure S1C). Consequently, we postulate that DCs may possess superior migratory abilities.
Fig. 2.
DC cells can resist anoikis and metastasize. (A–B) Colony formation assay after induction of anoikis (* p < 0.05, *** p < 0.001, one‐way ANOVA). (C–F) Western blot quantification of apoptosis-related markers in regular cells and DCs for 24 h (C-D) and 48 h (E–F). (G–H) Quantification of transwell experiment reveals cell migration ability (**p < 0.01, Student’s t‐test). (I–J) Whole-body BLI was used to track tumor metastasis (**p < 0.01, ***p < 0.001, Student’s t‐test). (K–L) Quantitative statistics of in vivo imaging. (** p < 0.01, *** p < 0.001, Student’s t‐test).
We then injected the same number of normal cells and DCs into nude mice via tail vein injection, and monitored distant metastasis by measuring the resulting bioluminescence. We found that the formation of DCs significantly enhance the metastatic ability of cancer (Fig. 2I-L). Therefore, we concluded that DCs has a weak adhesive ability and strong anoikis-resistance capacity, so they can divide from tumor tissue, survive in the circulation system, and cause distant metastasis.
The overexpression of ITGB6 in DCs contributes to the poor prognosis of HNSCC
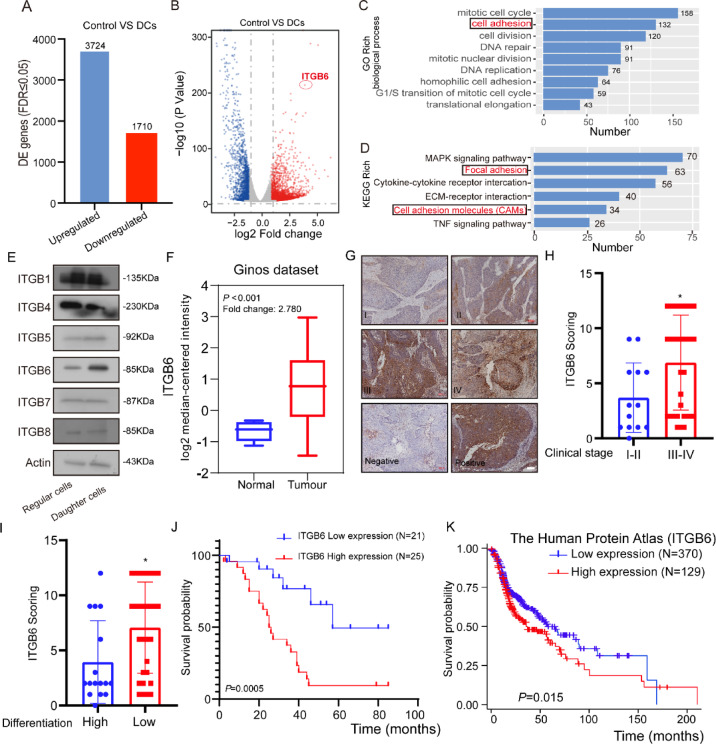
The cancer progression and distant metastasis‐promoting role of DCs has been known extensively, but the mechanism has not been elucidated yet. Therefore, we performed whole‐transcriptome sequencing of normal Fadu cells and DCs induced from Fadu cells. A total of 5437 genes with differential transcript expression were detected (Figure S2A, Fig. 3A). By comparing normal cancer cells, we observed 1710 down‐ and 3724 up‐regulated transcripts (Fig. 3A, B). Then the GO enrichment analyses and KEGG pathway enrichment analyses were performed on the 5437 transcripts that were significantly regulated, which revealed several significant KEGG pathways26–28. Of note, the cell adhesion was highly enriched in GO analyses and focal adhesion was highly enriched in KEGG analyses, which prompted us to focus on members of integrin family (Fig. 3C, D).
Fig. 3.
Clinical characteristics of ITGB6 in HNSCC. (A) The bar chart shown the absolute numbers of upregulated and downregulated genes identified by RNA-seq experiments. (B) The volcano plot reflects that ITGB6 is upregulated in DCs. (C) GO enrichment analysis for genes regulated in DCs. (D) KEGG pathways enrichment analysis for genes regulated in DCs. (E) Quantification of integrin expression in DCs and normal tumor cells by western blotting. (F) Quantification of integrin expression in DCs and normal tumor cells (Data form Ginod daraset, Student’s t‐test) (G) Representative IHC analysis images of ITGB6 staining in tumor tissues of different clinical grades from patients with HNSCC, bar: 100 µm. (H) Statistical comparison of ITGB6 scores across different clinical grades revealed significant differences (* p < 0.05, Student’s t‐test) (I) A statistical comparison of ITGB6 scores among different differentiation levels also showed significant variations. (* p < 0.05, Student’s t‐test) (J–K) Kaplan–Meier curves depicting the correlation between ITGB6 expression and overall survival were analyzed using the log-rank test. Specifically, (J) represents the analysis with data from IHC analysis, while (K) utilizes data from the HPA database.
Integrins, a significant class of transmembrane proteins, serve as critical conduits for transducing extracellular signals into intracellular responses29. They occupy a central position in orchestrating numerous cellular processes that are fundamental to cancer biology, including cell survival, proliferation, apoptosis, migration, and immune cell function30–33. Among the diverse family of integrins, ITGB6 emerges as a particularly noteworthy member due to its distinctively differential regulation patterns (Fig. 3B, E). The significance of ITGB6 in the context of cancer malignancy lies in its role in promoting cell migration and invasion34. These processes are essential for the dissemination of cancer cells to distant sites, a hallmark of malignant metastasis. ITGB6’s involvement in these critical steps suggests that it may serve as a key regulator of the metastatic cascade.
In order to elucidate the significance of ITGB6 in the progression of HNSCC, we analyzed its expression in head and neck squamous cell carcinoma (HNSCCs) (n = 41) and adjacent sections of HNSCCs tissues (n = 13) from the Ginos Head-Neck dataset in Oncomine. Our analysis revealed a striking upregulation of ITGB6 expression in HNSCCs compared to their adjacent normal mucosa tissues (Fig. 3F).
To further validate this observation, we performed immunohistochemical (IHC) analysis on 46 HNSCC tissue specimens (Fig. 3G). Intriguingly, our findings indicated that HNSCCs at later clinical stages tend to express higher levels of ITGB6 (Fig. 3H).
The high expression of ITGB6 was notably associated with poor tumor differentiation (Fig. 3I). Moreover, survival analysis revealed that patients exhibiting high ITGB6 expression had significantly poorer prognosis compared to those with lower expression, as shown in Fig. 3J (p = 0.0005). This finding was further corroborated by the analysis of data from the Human Protein Atlas (HPA) database, which also indicated a significant association between high ITGB6 expression and poorer prognosis (Fig. 3K, p = 0.015). In addition, xenograft tumor experiments revealed that the expression of itgb6 in subcutaneous tumors formed by DCs was significantly higher than that in normal tumor cells (Figure S3A, B).
ITGB6 promotes metastasis of HNSCC by inhibiting anoikis
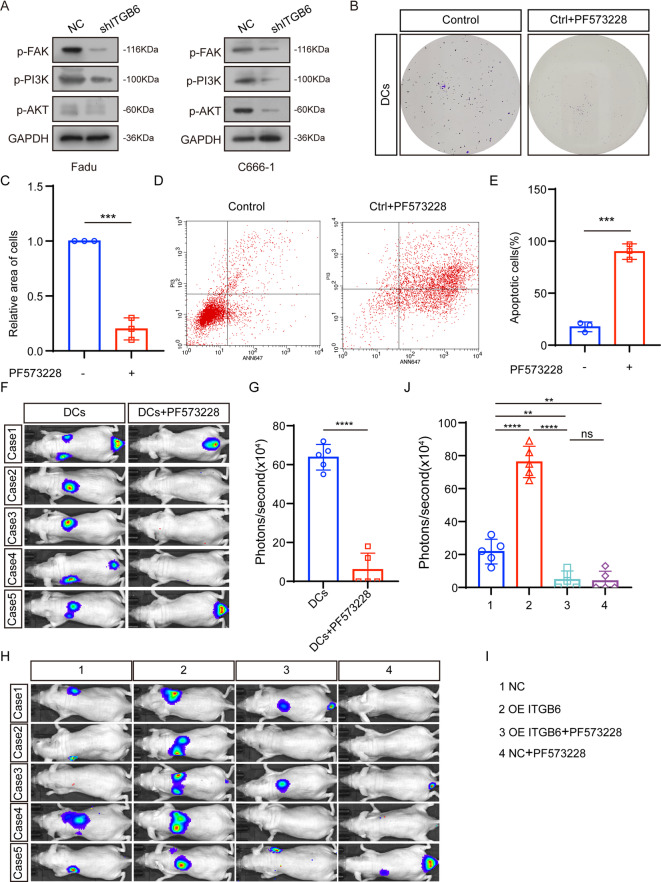
Based on the aforementioned results, we formulate a hypothesis that ITGB6 has the potential to facilitate metastasis of HNSCC. Then, we transfected Fadu cells and C666-1 cells with shITGB6 lentivirus or the vector virus, and then proceeded to subsequent experiments (Fig. 4A). In addition, after the knockdown of itgb6, the proliferation capacity of FaDu cells and C666-1 cells was significantly reduced, and apoptosis-related markers also increased accordingly. This indicates that itgb6 enhances the ability of FaDu cells and C666-1 cells to resist anoikis (Fig. 4B-E). Therefore, we investigated whether ITGB6 promote metastasis in vivo. We injected these two groups of cells into the tail vein of nude mice. Upon comparison with the NC group, the shITGB6 group displayed a significantly reduced incidence of distant metastases (Fig. 4F-I).
Fig. 4.
ITGB6 promotes metastasis of HNSCC. (A) ITGB6 level of Fadu-NC, -Fadu-shITGB6, C666-1-NC and C666-1-shITGB6uwere measured by Western blotting. (B–C) Colony formation assay after induction of anoikis. (D) Western blot quantification of apoptosis-related markers. (E) Quantification of apoptosis-related markers by immunoblotting after induction of anoikis. (F, H) Whole-body BLI was used to track tumor metastasis. (G, I) Quantitative statistics of in vivo imaging. (*p < 0.05, *** p < 0.001, **** p < 0.0001, Student’s t‐test).
To attempt to identify the function of ITGB6 in HNSCC, we tried to knockdown ITGB6 in HNSCC cells and induce it to be PGCCs and DCs. However, what is interesting, PTX killed cells quickly even in an extremely low concentration, and DCs dead quickly after knockdown ITGB6 (data not shown). Therefore, we speculate that ITBGB6 is critical for sustaining life and cellular homeostasis of PGCCs and DCs.
ITGB6 enhances anoikis-resistance and metastasis of HNSCC via the FAK/ PI3K/AKT pathway
FAK/PI3K/AKT pathwayplay a primary role in human growth and development, progression of benign and malignant diseases. Marie-Josée Demers et al. reported that the FAK/PI3K/AKT pathway can promote anoikis resistance of cancer. The role of integrins in activating FAK is well known. Our previous research confirmed that ITGB6 can promote nasopharyngeal metastasis via FAK pathway. We speculate that ITGB6 may inhibit anoikis in DCs by activating FAK/ PI3K/AKT pathway. Thus, we examined the phosphorylation level of FAK, PI3K and AKT. ITGB6 knockdown significantly inhibited the activity of FAK, PI3K and AKT (Fig. 5A). PF573228, the kinase inhibitor of FAK, can inhibit the anoikis-resistance of DCs (Fig. 5B-C). Further, PF573228 treatment blocked the phenotype induced by ITGB6 overexpression (Fig. 5D-E). Consistent with those results in vitro, PF573228 treatment can inhibit the distant metastasis of DCs, and can significantly inhibited the HNSCC metastasis promoted by ITGB6 (Fig. 5D-J).
Fig. 5.
ITGB6 enhances anoikis-resistance and metastasis of HNSCC via the FAK/ PI3K/AKT pathway (A) P-FAK, P-PI3K, P-AKT level of Fadu and C666-1 were measured by Western blotting. (B–C) Colony formation assay after induction of anoikis. (D–E) Flow cytometry experiment to detect cell proliferation ability. (F, H) Whole-body BLI was used to track tumor metastasis. (G, J) Quantitative statistics of in vivo imaging. (ns p > 0.05, ** p < 0.01, **** p < 0.0001).
Discussion
The enduring struggle between humans and malignant tumors has been ongoing for a long time. With continuous research on malignant tumors, scientists have made remarkable progress in understanding their genesis and progression35. As research progresses, we have witnessed significant advancements in the treatment of hematological malignancies. However, the treatment of solid tumors, particularly HNSCCs such as hypopharyngeal and nasopharyngeal cancers, remains a significant challenge2,4,16,36–39. During initial treatment phases, the growth of tumor cells is often effectively suppressed or even eradicated. Nevertheless, a small subset of cunning tumor cells manages to evade therapeutic intervention by entering a dormant state, resisting the effects of chemotherapy drugs and radiation therapy. Upon the occurrence of certain triggers, these latent tumor cells can reawaken, acquire drug resistance and enhance metastatic potential. Therefore, patients with recurrent malignant tumors often resistant to multiple therapeutic approaches. Hence, it is imperative to delve deeper into the underlying mechanisms behind the reactivation of dormant cells and their acquisition of drug resistance and enhanced metastatic abilities. Such research holds the key to developing more effective treatment strategies for HNSCCs and other solid tumors. The reactivation of dormant cancer cells, known as PGCCs, continues to pose a significant challenge due to their quiescent state with low metabolism and activity, making them resilient to complete elimination by drugs and radiotherapy8,40,41. Once resuscitated, PGCCs rapidly generate numerous descendant cells (DCs) in a short period8. Therefore, unraveling these mechanisms is crucial for developing more effective cancer treatments. Future research should focus on elucidating the molecular and cellular pathways involved in PGCCs and DCs, while exploring potential therapeutic targets to disrupt their role in cancer progression. This knowledge could pave the way for novel therapeutic strategies targeting these dormant cells and their progeny, ultimately improving outcomes for cancer patients. To further investigate the role of DCs in tumor recurrence and metastasis, we induced and cultured PGCCs and DCs from tumor cell lines using paclitaxel. Notably, compared to normal cancer cells, DCs derived from PGCCs exhibit two distinct characteristics: decreased adhesion and increased viability. These unique features enhance the ability of DCs to detach from tumor cells and survive in the blood circulation. Using RNA-seq, we discovered that ITGB6 is significantly upregulated in DCs. Furthermore, using Western blot, colony formation, and in vivo experiments, we confirmed that ITGB6 enhances the survival, proliferation, and metastasis capabilities of tumor cells. Subsequently, we identified that ITGB6 enhances the resistance of DCs to anoikis and metastasis. Interestingly, our study revealed that the survival of DCs appears to depend on ITGB6 expression, suggesting that ITGB6 may play a critical role in the formation of these daughter cells. While the specific molecular mechanisms underlying this dependency remain to be elucidated, it raises the possibility that ITGB6 could modulate the biological properties of daughter cells through sustaining their survival, thereby influencing processes related to their functional roles. Overall, our findings reveal that ITGB6 promotes tumor metastasis by activating the FAK/PI3K/AKT pathway to inhibit anoikis in DCs. Blocking ITGB6 expression or inhibiting the activation of the FAK pathway completely abrogates this effect.
Anoikis, a specialized type of apoptosis, serves as a potential obstacle to cancer cell metastasis. It occurs when individual cells detach from their original microenvironment, triggering apoptosis42–45. However, some malignant tumor cells are able to evade anoikis-induced cell death by activating various pathways, such as the FAK/PI3K/AKT or FAK/MAPK/AKT etc.45. Tumor cells acquiring the ability to resist anoikis will be prone to metastasis, leading to a poor prognosis46. Understanding these mechanisms is crucial for developing more effective cancer treatments that can target these resistant cells and improve patient outcomes.
The ability for cancer cells to evade the immune system is critical at every stage of cancer progression. Thus, the capability of immune tolerance or immune escape is essential for DCs to maintain efficient metastasis. However, we did not find common immune-related protein expression abnormalities, such as PD-L1. An interesting finding is that the expression of PD-L2 was markedly up-regulated in DCs, which regulate immune system bidirectionally. Due to the technical limitations, we cannot construct a HNSCC model in mice with fully functional immune systems. DCs may have a unique mechanism of immune tolerance or immune escape. And our future studies will continue to explore this direction.
Currently, the efficacy of treatments in patients with HNSCC remain deplorable. Our` data offer a significant advancement in understanding the mechanism of DCs in induced metastasis. Therefore, our finding provides a novel prognostic indicator for patients with HNSCC and pave the way for innovative therapeutic approaches.
Materials and methods
Human HNSCC specimens
Based on the approval granted by the ethics committee of the Affiliated Hospital of Nantong University (approval number 2023-L131), 46 tumor tissues were surgically obtained from consenting patients in accordance with established operative guidelines. Prior to biopsy, all patients provided informed consent and refrained from any cancer treatments. Informed consent was obtained from all individual participants included in the study. For participants who are minors or lack the capacity to provide consent, informed consent was obtained from their legal guardians. All procedures involving human participants were conducted in accordance with the ethical standards of the Declaration of Helsinki. Hematoxylin and eosin staining (HE) was conducted to assess the quantity of PGCCs, a specific cellular component of interest. For the survival analysis of ITGB6 in HNSCC using data from the HPA database (https://www.proteinatlas.org/).
Cell culture
The FaDu cells were cultured in DMEM High Glucose medium containing 10% FBS, and the C666-1 cells were cultured in RPMI 1640 containing 10% FBS. Cells were grown at 37 °C with 5% CO2. FaDu cells and C666-1 cells were purchased from Shanghai Guandao Bioengineering Co., Ltd., and underwent DNA verification.
Transfection with lentiviral vectors
Cells were seeded in 6-well plates at a density of 5 × 10^4 cells/well. 24 h prior to transfection, ensuring ~ 30–40% confluence at the time of virus addition. Lentiviruses were added at a multiplicity of infection (MOI) of 10 with 8 μg/mL Polybrene to enhance transduction efficiency. After 24 h, the medium was replaced with fresh complete medium. At 48 h post-transfection, puromycin selection was initiated at a pre-determined optimal concentration 2 μg/mL, determined by a kill curve assay in untransfected cells. Selection was maintained for 48 h to eliminate untransfected cells. The efficiency of ITGB6 knockdown was verified by western blot. Plasmids, lentiviral vectors, and their corresponding negative controls were constructed and produced by GeneChem (Shanghai, China). The sequence of short hairpin RNA (shRNA) is as follows:
shITGB6: gcCTCCAAACATTCCCATGAT.
Flow cytometry experiment
Determination of DNA ploidy and cell cycle phase distribution of HNSCC-PGCC and HNSCC-DC by flow cytometry analysis. Cell pellets were obtained by digesting cells and washed with 1 × PBS. The cells were then fixed in 70% pre-cooled ethanol at 4 °C overnight; then centrifuged and the supernatant was discarded, and the cells were washed three times with 1 × PBS to remove ethanol. The cells were resuspended in 1 ml of propidium iodide (PI) staining solution containing RNase A and stained at room temperature for 15 min; finally, the cell DNA content was analyzed using a flow cytometer, and the results were analyzed using Flowjo software v10.8.1 (https://www.flowjo.com/).
Cell adhesion assay
Cells were dissociated using trypsin without EDTA. The dissociated cells were then resuspended in a suitable medium to create a cell suspension with a concentration of 5 × 104 cells per milliliter. Next, 200 μl of the prepared cell suspension were gently pipetted into each well of a 96-well plate. The 96-well plates containing the cell suspensions were then incubated under standard cell culture conditions. After incubation for 30 min, 60 min, and 90 min, respectively, the cells in each well were washed to remove any residual culture medium or unbound cells. Cells were fixed to preserve their morphology and structure during subsequent staining procedures. Subsequently, the fixed cells were stained with appropriate dyes to enhance their visibility under a microscope. Three random fields of view were selected for photographing and counting the cells at each time point (30 min, 60 min, and 90 min).
BALB/c nude mice models
To assess the ability of tumor cells to metastasize to the lungs in vivo, cells were transfected with lentivirus. Next, 2 × 106 luciferase‐labeled cells in 200 µl DMEM High Glucose medium were injected into the tail veins of 5‐to 6‐week‐old male nude mice. After the completion of the experimental period, which lasted for 6 weeks, bioluminescence imaging (BLI) was conducted. The BALB/c mice used in this study were sourced from the Laboratory Animal Center of Nantong University. We confirm that the care and use of animals in this study were in full compliance with the institutional guidelines and ethical standards established to ensure the welfare and well-being of laboratory animals. This study was approved by the Ethics Committee of the Animal Experiment Center of Nantong University, following ARRIVE guidelines.
Anoikis quantitative assay
The cells were digested with trypsin, counted, and seeded in ultra-low adsorption culture plates at 105 cells per well and cultured for 48 h. After induction of anoikis, cells floating in the supernatant were collected. The collected cells were continued for colony formation experiments.
RNA sequencing
GENEWIZ conducted RNA sequencing (RNA-seq) and bioinformatics analysis. Initially, total RNA was extracted from samples with utmost precision, followed by rigorous quality assessment and quantification to guarantee the integrity and purity of the RNA. Subsequently, next-generation sequencing libraries were constructed strictly according to the manufacturer’s instructions. RNA-seq was then performed on an Illumina HiSeq platform, adhering strictly to the manufacturer’s instructions. The resulting clean data were processed using Hisat2 (v2.0.1, https://daehwankimlab.github.io/hisat2/) and HTSeq software (v0.6.1, https://htseq.readthedocs.io/en/release_0.11.1/) for alignment and gene quantification, respectively. Differential expression analysis was carried out using the DESeq2 Bioconductor package, enabling us to identify differentially expressed genes.
Immunoblotting
Immunoblotting was conducted on whole-cell lysates to assess protein levels. The protein content was accurately measured using a BCA kit from Thermo Fisher Scientific. The protein samples were then resolved by polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were incubated with specific antibodies overnight at 4 °C (indicated in the Table 1), after blocking with the blocking buffer for an hour. The original pictures are stored in Figure S4.
Cell fluorescence staining
Cells were plated in 15-mm glass-bottomed dishes at a concentration of 5 × 104 cells per dish. After 24 h, the cells were rinsed three times with PBS and then treated with 0.2% Triton X-100 to enhance permeability at room temperature for 10 min. Subsequently, the cells were washed three times with PBS and incubated overnight in the dark with phalloidin at 4 °C. After further washing three times with PBS in the dark, an antifluorescence quencher containing Hoechst stain was applied, and the cells were imaged. This protocol allowed us to visualize the cytoskeletal structure and nuclear staining of the cells.
Quantification and statistical analysis
The statistical analysis in this study was conducted using GraphPad Prism 6 software 6.01 (https://www.graphpad.com/). For each dataset, the specific details of the statistical methods and tests employed are outlined in the corresponding figure legends. A p value of less than 0.05 was considered statistically significant, indicating that the observed difference or association is unlikely to have occurred due to chance alone and is therefore considered meaningful in the context of the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
B.Y. T.X. and Z.Z. proposed the research topic and conceived the overall research design. Led the collection, organization, and analysis of literature resources. S.P. wrote the main sections of the paper, including the introduction, methodology, and results. Revised the entire paper multiple times to ensure logical coherence and academic rigor. Participated in discussions to refine the research design and provided valuable insights. H.L. and H.X. Participated in discussions to refine the research design and provided valuable insights. Responsible for collecting and processing experimental data, ensuring accuracy and reliability. Assisted in writing the results section, providing detailed interpretation and analysis of the data. L.Y. and K.Z. provided expert guidance and support in the specialized field throughout the research process.Reviewed the academic quality of the paper and suggested modifications. Participated in the final proofreading of the paper to ensure its quality. H.Y. contributed to the literature review and provided additional references. Assisted in data analysis and interpretation. Helped in drafting specific sections of the paper. Y.H. participated in the design of experimental procedures. Carried out experiments and collected data. Contributed to the methods section, detailing the experimental protocols. R.W. provided statistical expertise and conducted data analysis. Helped in interpreting the statistical results.
Data availability
All the raw data of this study have been deposited in the NCBI Sequence Read Archive (SRA) database under the BioProject accession number PRJNA1247116.
Declarations
Competing interests
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tian Xia, Email: xt15358772378@163.com.
Zhenxin Zhang, Email: zhangzhenxinent@ntu.edu.cn.
Bo You, Email: youbo19891014@163.com.
References
- 1.Johnson, D. E. et al. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers.6(1), 92 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2019. CA Cancer. J. Clin.69(1), 7–34 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Ma, J. et al. Establishment and characterization of a novel hypopharyngeal squamous cell carcinoma cell line CZH1 with genetic abnormalities. Hum. Cell.37(2), 546–559 (2024). [DOI] [PubMed] [Google Scholar]
- 4.Sewnaik, A. et al. Treatment of hypopharyngeal carcinoma: Analysis of nationwide study in the Netherlands over a 10-year period. Clin. Otolaryngol.30(1), 52–57 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Pulte, D. & Brenner, H. Changes in survival in head and neck cancers in the late 20th and early 21st century: A period analysis. Oncologist.15(9), 994–1001 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferris, R. L. et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl. J. Med.375(19), 1856–1867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang, S. et al. Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene33(1), 116–128 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.You, B. et al. AMPK-mTOR-mediated activation of autophagy promotes formation of dormant polyploid giant cancer cells. Cancer Res.82(5), 846–858 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang, D. et al. Daughter cells and erythroid cells budding from PGCCs and their clinicopathological significances in colorectal cancer. J. Cancer.8(3), 469–478 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niu, N. et al. Linking genomic reorganization to tumor initiation via the giant cell cycle. Oncogenesis.5(12), e281 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fei, F. et al. Formation of polyploid giant cancer cells involves in the prognostic value of neoadjuvant chemoradiation in locally advanced rectal cancer. J. Oncol.2019, 2316436 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, J. et al. Polyploid giant cancer cells (PGCCs): The evil roots of cancer. Curr. Cancer Drug Targets.19(5), 360–367 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Kudo-Saito, C. et al. IL33 is a key driver of treatment resistance of cancer. Cancer Res.80(10), 1981–1990 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Tagal, V. & Roth, M. G. Loss of aurora kinase signaling allows lung cancer cells to adopt endoreplication and form polyploid giant cancer cells that resist antimitotic drugs. Cancer Res.81(2), 400–413 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nehme, Z. et al. Polyploid giant cancer cells, stemness and epithelial-mesenchymal plasticity elicited by human cytomegalovirus. Oncogene40(17), 3030–3046 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Liu, G. et al. Clinical characteristics and preliminary morphological observation of 47 cases of primary anorectal malignant melanomas. Melanoma Res.28(6), 592–599 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Sirois, I. et al. A unique morphological phenotype in chemoresistant triple-negative breast cancer reveals metabolic reprogramming and PLIN4 expression as a molecular vulnerability. Mol. Cancer Res.17(12), 2492–2507 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Zhang, L. et al. Number of polyploid giant cancer cells and expression of EZH2 are associated with VM formation and tumor grade in human ovarian tumor. Biomed Res. Int.2014, 903542 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qu, Y., Zhang, L., Rong, Z., He, T. & Zhang, S. Number of glioma polyploid giant cancer cells (PGCCs) associated with vasculogenic mimicry formation and tumor grade in human glioma. J. Exp. Clin. Cancer Res.32(1), 75 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirzayans, R. et al. Multinucleated giant cancer cells produced in response to ionizing radiation retain viability and replicate their genome. Int. J. Mol. Sci.18(2), 360s (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strilic, B. & Offermanns, S. Intravascular survival and extravasation of tumor cells. Cancer Cell32(3), 282–293 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Raeisi, M. et al. Anoikis in cancer: The role of lipid signaling. Cell Biol Int.46(11), 1717–1728 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Sawyer, B. T. et al. Targeting fatty acid oxidation to promote anoikis and inhibit ovarian cancer progression. Mol. Cancer Res.18(7), 1088–1098 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fanfone, D. et al. Confined migration promotes cancer metastasis through resistance to anoikis and increased invasiveness. Elife11, e73150 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fares, J., Fares, M. Y., Khachfe, H. H., Salhab, H. A. & Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target Ther.5(1), 28 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res.28(1), 27–30 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci.28(11), 1947–1951 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res.51(D1), D587–D592 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niu, J. & Li, Z. The roles of integrin alphavbeta6 in cancer. Cancer Lett.403, 128–137 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Weiss, F., Lauffenburger, D. & Friedl, P. Towards targeting of shared mechanisms of cancer metastasis and therapy resistance. Nat Rev Cancer.22(3), 157–173 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.del Pozo, M. A. et al. Integrins regulate Rac targeting by internalization of membrane domains. Science303(5659), 839–842 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Tod, J. et al. Pro-migratory and TGF-beta-activating functions of alphavbeta6 integrin in pancreatic cancer are differentially regulated via an Eps8-dependent GTPase switch. J Pathol.243(1), 37–50 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giancotti, F. G. Complexity and specificity of integrin signalling. Nat Cell Biol.2(1), E13–E14 (2000). [DOI] [PubMed] [Google Scholar]
- 34.You, B. et al. Extracellular vesicles rich in HAX1 promote angiogenesis by modulating ITGB6 translation. J. Extracell Vesic.11(5), e12221 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71(3), 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Chen, Y. P. et al. Nasopharyngeal carcinoma. Lancet394(10192), 64–80 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Takes, R. P. et al. Current trends in initial management of hypopharyngeal cancer: The declining use of open surgery. Head Neck.34(2), 270–281 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Zhou, X. et al. PKM2 promotes lymphatic metastasis of hypopharyngeal carcinoma via regulating epithelial-mesenchymal transition: An experimental research. Diagn Pathol.19(1), 48 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vigneswaran, N. & Williams, M. D. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac. Surg. Clin. North Am.26(2), 123–141 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiao, Y. et al. Dormant cancer cells and polyploid giant cancer cells: The roots of cancer recurrence and metastasis. Clin. Transl. Med.14(2), e1567 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirzayans, R., Andrais, B. & Murray, D. Roles of polyploid/multinucleated giant cancer cells in metastasis and disease relapse following anticancer treatment. Cancers (Basel).10(4), 118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grossmann, J., Walther, K., Artinger, M., Kiessling, S. & Scholmerich, J. Apoptotic signaling during initiation of detachment-induced apoptosis (“anoikis”) of primary human intestinal epithelial cells. Cell Growth Differ.12(3), 147–155 (2001). [PubMed] [Google Scholar]
- 43.Paoli, P., Giannoni, E. & Chiarugi, P. Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta.1833(12), 3481–3498 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Taddei, M. L., Giannoni, E., Fiaschi, T. & Chiarugi, P. Anoikis: An emerging hallmark in health and diseases. J Pathol.226(2), 380–393 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Chiarugi, P. & Giannoni, E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem. Pharmacol.76(11), 1352–1364 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Guadamillas, M. C., Cerezo, A. & Del Pozo, M. A. Overcoming anoikis–pathways to anchorage-independent growth in cancer. J. Cell Sci.124(Pt 19), 3189–3197 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the raw data of this study have been deposited in the NCBI Sequence Read Archive (SRA) database under the BioProject accession number PRJNA1247116.