Abstract
The performance characteristics of the E-test (AB Biodisk, Solna, Sweden), the ATB Staph, the Rapid ATB Staph, and the Vitek GPS-503 card (bioMérieux, La Balme Les Grottes, France) methods for the detection of oxacillin resistance in a collection of staphylococci with a high proportion of troublesome strains were evaluated. Sixty-four Staphylococcus aureus strains and 76 coagulase-negative staphylococcal strains were tested. All strains were mecA positive and were characterized by the oxacillin agar screen plate test; 75 (53.6%) were found to be heterogeneous by a large-inoculum oxacillin disk diffusion assay, and oxacillin MICs for 89 (63.6%) were ≤32 μg/ml. Three (4.7%) S. aureus strains and 25 (32.9%) coagulase-negative strains were classified as susceptible by the E-test, as defined by the National Committee for Clinical Laboratory Standards (NCCLS) oxacillin breakpoint (MIC ≤ 2 μg/ml). The ATB Staph method failed to detect oxacillin resistance in 7 (11%) S. aureus isolates and 32 (42.1%) coagulase-negative isolates. The MICs for all but six of these discrepant isolates were ≤16 μg/ml. The Rapid ATB Staph method was tested against S. aureus strains only and yielded 15 (23.4%) false-susceptible results for strains for which the MICs were ≤32 μg/ml. The Vitek system was the best-performing system, since it failed to detect oxacillin resistance in only 3 (4.7%) S. aureus strains and 15 (19.7%) coagulase-negative strains, the MICs for all of which were ≤2 μg/ml. These data indicate that (i) the performance of the two ATB Staph systems can be limited when the prevalence of borderline-heteroresistant staphylococci is high and (ii) the unreliability of the E-test and the Vitek methods for detecting resistant coagulase-negative strains might be reduced by the potential revision of the oxacillin breakpoint currently recommended by the NCCLS.
Oxacillin-resistant staphylococci are major nosocomial pathogens with frequent multiple resistance, leading to the overuse of glycopeptides in therapy. One of the priority measures to decrease this strong antibiotic pressure is to optimize the detection of oxacillin resistance in clinical laboratories. The heterogeneous resistance of many strains (4, 9, 24) makes this detection a constant challenge for clinical laboratories. Recent evidence suggests that the heteroresistance of staphylococci is linked to the inactivation of transcription regulators, such as the sar regulon (6) and the sigma-B operon (36). Several studies have raised concerns over the failures of the conventional methods to detect such resistance and have led to various recommendations to enhance the expression of the resistance in vitro (2, 4, 8–11, 17, 20–23, 25, 27, 31, 33, 37). At present, the detection of the mecA gene, which is responsible for methicillin resistance in practically all clinical methicillin-resistant staphylococcal strains (9, 24, 28), is considered the reference test (2, 4, 7, 17, 23, 25–28, 32). In spite of the growing consensus in the literature for this method, it is not yet available in all clinical laboratories, and the alternative reference test remains the oxacillin agar screen plate test (1). Both mecA detection and agar screening have been used as “gold standards” for the evaluation of commercial methods (12–14, 16, 22, 25, 26, 33–35, 38). Automated systems are widely used in clinical laboratories, but they may lack accuracy for the detection of heterogeneously resistant isolates (9, 17, 22, 25). However, in the past few years, several reports have emphasized the performance characteristics of different rapid methods, such as the Rapid ATB Staph (bioMérieux, la Balme-Les Grottes, France) system (26), the Rapid MicroScan panel (Baxter Microscan, West Sacramento, Calif.) (25, 35), and the Vitek system (bioMérieux Vitek, Inc., Hazelwood, Mo.) (13, 25). In particular, Knapp et al. (13) showed the usefulness of the Vitek system for the detection of low-level-expression class isolates of Staphylococcus aureus and Staphylococcus epidermidis. However, these authors raised concerns over the accuracy of the Vitek system for detecting borderline-susceptible isolates that lack mecA (14). Moreover, the Vitek system may miss a significant number of coagulase-negative staphylococci that have the mecA gene and for which the oxacillin MICs are in the susceptible range (1 to 2 μg/ml) (22).
The purpose of this study was to evaluate the E-test system and three automated systems currently used in France, the ATB Staph, the Rapid ATB Staph, and the Vitek systems, and to compare their performance characteristics for the detection of oxacillin-resistant staphylococci. These methods were tested against a difficult population of S. aureus and coagulase-negative staphylococcal strains, previously characterized by the PCR amplification of the mecA gene and the oxacillin agar screen plate test. Half of the challenge strains were selected because they exhibited heteroresistance when tested by a large-inoculum disk diffusion assay.
MATERIALS AND METHODS
Organisms tested.
Sixty-three clinical isolates of S. aureus and 76 clinical isolates of coagulase-negative staphylococci were determined to be oxacillin resistant because of the presence of the mecA gene. The strains were isolated between May 1995 and December 1996 from the following clinical specimens (the numbers of S. aureus and coagulase-negative staphylococcal isolates, respectively, are in parentheses): blood (11 and 20), urogenital tract (24 and 14), cutaneous-mucous specimens (20 and 29), respiratory tract (6 and 4), joint fluid (1 and 1), pericardic fluid (0 and 1), cerebrospinal fluid (0 and 1), digestive tract (1 and 4), and transplant device (0 and 2). The S. aureus and coagulase-negative staphylococcal strains were collected from 27 and 29 different care units, respectively, in the universitary hospital in Rouen. Strain ATCC 43300, a mecA-positive heteroresistant S. aureus strain, was used as the reference strain. Isolates were identified as S. aureus or coagulase-negative staphylococci by colony morphology, Gram stain characteristics, coagulase reactions, and the Pastorex Staph Plus test (Sanofi Diagnostics Pasteur, Marnes la Coquette, France). Strains were stored frozen in glycerol at −70°C and subcultured to ensure purity before testing. All strains were oxacillin resistant, as determined by the PCR amplification of the mecA gene described below. Thirty-two (50%) of the S. aureus isolates and 45 (59.2%) of the coagulase-negative staphylococcal isolates were intentionally included in the study because they exhibited a heterogeneous phenotype when tested by the disk diffusion assay, as described below. All isolates for which the results of different methods were discrepant were tested twice.
Amplification of the mecA gene.
For preparation of a template from staphylococcal cells we used a simplified procedure, which does not require lysostaphin lysis. Two microliters of a 2× McFarland suspension of cells was heated in the presence of 10 μl of Genereleaser (BioVentures, Murfreesboro, Tenn.), a reagent which sequesters cell lysis products, directly in the amplification tube of a GeneAmp PCR system 2400 (Perkin-Elmer Cetus, Norwalk, Conn.). A nine-temperature, one-cycle DNA extraction program was conducted as recommended by the manufacturer. Subsequently, 40 μl of the PCR reagent mixture was added to the PCR tube to initiate amplification. PCR was performed with the following primers, previously designed by Geha et al. (7): mecA 1 (5′-GTA GAA ATG ACT GAA CGT CCG ATA A) and mecA 2 (5′-CCA ATT CCA CAT TGT TTC GGT CTA A). The PCR reagent mixture consisted of 200 μM concentrations of deoxynucleoside triphosphates (dNTPs), 10 mM Tris (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, a 0.25 μM concentration of each primer, and 1.25 U of Taq polymerase (Appligene Oncor, Gaithersburg, Md.). DNA amplification was carried out with the following thermal cycling profile: initial denaturation at 94°C for 5 min, followed by 30 cycles of amplification (denaturation at 94°C for 15 s, annealing at 55°C for 15 s, extension at 72°C for 30 s), ending with a final extension at 72°C for 2 min. A positive result was indicated by the presence of the 310-bp amplified DNA fragment revealed by electrophoresis on a 1.5% agarose gel at 130 V for 45 min. Results were obtained within 4 h. Each PCR assay included strain ATCC 43300 as a positive control and water as a negative control.
Oxacillin agar screen method.
Agar screen tests for susceptibility to oxacillin were performed as directed in National Committee for Clinical Laboratory Standards (NCCLS) guidelines (21). For each isolate, 100 μl of a 0.5× McFarland suspension was streaked on a Mueller-Hinton agar plate supplemented with 4% NaCl and 6 μg of oxacillin per ml. The plates were then incubated for 48 h at 35°C. Any growth on the plate was recorded as indicating oxacillin resistance.
Disk diffusion testing.
The disk diffusion assay was performed with 5-μg oxacillin disks and a 108-CFU/ml inoculum. The disks were placed on Mueller-Hinton agar plates (Becton Dickinson, Cockeysville, Md.) not supplemented with NaCl; the plates were then incubated for 48 h at 30°C. Strains were considered resistant when the diameter of inhibition was <20 mm, in accordance with the French recommendations (3), and when any growth around the disk was observed. Strains were considered heterogeneously resistant when partial growth within the inhibition zone or microcolonies around the oxacillin disk were observed.
Determination of MICs.
The MICs of oxacillin were determined by means of the E-test (AB Biodisk, Solna, Sweden), performed according to the manufacturer’s recommendations. E-test strips were placed on Mueller-Hinton agar plates containing 2% NaCl, which enhance the growth of microcolonies and the expression of the resistance. These plates were inoculated by swabbing the surfaces with a 0.5× McFarland suspension for S. aureus strains or with a 1× McFarland suspension coagulase-negative staphylococcal strains. The plates were then incubated at 35°C for 24 h.
ATB Staph system and Rapid ATB Staph system.
Susceptibility testing was performed according to the manufacturer’s recommendations (bioMérieux). Briefly, a 0.5× McFarland emulsion of isolated colonies in sterile saline was added to 7 ml of the ATB medium (Mueller-Hinton broth supplemented with 5% NaCl). The final inoculum was transferred into an oxacillin (2 μg/ml) well and incubated for 24 h at 35°C. The Rapid ATB Staph system was tested against S. aureus strains only.
Vitek system.
Susceptibility testing with the Vitek GPS-503 card (bioMérieux) was performed according to the manufacturer’s instructions. The cards were inoculated with a 0.5× McFarland suspension of the cells and processed in a Vitek 120 reader-incubator.
RESULTS
All isolates analyzed in this study harbored the mecA gene. The degree of agreement between the results of the reference tests (the PCR amplification of the mecA gene and the oxacillin agar screen plate test) and those of the E-test MIC determination and the automated systems is shown in Table 1. The discrepant results yielded by at least one of the susceptibility testing methods are presented in Table 2 for S. aureus isolates, and in Table 3 for coagulase-negative staphylococcal isolates.
TABLE 1.
Agreement between the oxacillin E-test, the ATB Staph, the Rapid ATB Staph, and the Vitek systems and the reference methods (PCR amplification of the mecA gene and oxacillin agar screening) for S. aureus and coagulase-negative staphylococcal isolates
| Reference method | Isolate | No. of strains with concordant results (% agreement) for indicated system
|
|||
|---|---|---|---|---|---|
| E-test | ATB Staph | Rapid ATB Staph | Vitek | ||
| PCR of mecA | S. aureus | 61 (95.3) | 57 (89.0) | 49 (76.6) | 61 (95.3) |
| Coagulase-negative staphylococci | 51 (67.1) | 44 (57.9) | —a | 61 (80.3) | |
| Agar screening | S. aureus | 63 (98.4) | 59 (92.2) | 51 (79.7) | 63 (98.4) |
| Coagulase-negative staphylococci | 55 (72.4) | 48 (63.2) | — | 65 (85.5) | |
—, not tested.
TABLE 2.
Characteristics of 16 S. aureus strains misclassified as oxacillin susceptible by at least one of the susceptibility testing methods
| Isolate | Resultc of indicated reference method
|
Result of indicated oxacillin susceptibility testing method
|
|||||
|---|---|---|---|---|---|---|---|
| mecA PCR | Oxacillin agar screen | Disk diffusion | E-test (MIC [μg/ml]) | Rapid ATB Staph | ATB Staph | Vitek | |
| 7079 | R | S | Ra | 0.38 | S | S | S |
| 6710 | R | S | Ra | 1 | S | S | S |
| 5295 | R | R | Rb | 1.5 | S | S | S |
| 5401 | R | R | Rb | 4 | S | S | R |
| 7741 | R | R | Ra | 6 | S | S | R |
| 8879 | R | R | Rb | 6 | S | R | R |
| 1638 | R | R | Rb | 6 | S | R | R |
| 7394 | R | R | Rb | 8 | S | R | R |
| 6019 | R | R | R | 12 | S | S | R |
| 5839 | R | R | Ra | 16 | S | R | R |
| 2471 | R | R | R | 16 | S | R | R |
| REFd | R | R | Rb | 16 | S | R | R |
| 760 | R | R | R | 24 | S | R | R |
| 6229 | R | R | R | 24 | S | R | R |
| 3902 | R | R | R | 32 | S | R | R |
| 5490 | R | R | Rb | >256 | R | S | R |
Strain had a susceptible zone of inhibition (≥20 mm with the 5-μg oxacillin disk) but had colonies within the zone (heteroresistance).
Strain had a resistant zone of inhibition (<20 mm with the 5-μg oxacillin disk) and had colonies within the zone (heteroresistance).
R, resistant; S, susceptible.
REF, low-level-expression strain ATCC 43300.
TABLE 3.
Characteristics of 36 coagulase-negative staphylococcal strains misclassified as oxacillin susceptible by at least one of the susceptibility testing methods
| Isolate | Resultc of indicated reference method
|
Result of indicated oxacillin susceptibility testing method
|
||||
|---|---|---|---|---|---|---|
| mecA PCR | Oxacillin agar screen | Disk diffusion | E-test (MIC [μg/ml]) | ATB Staph | Vitek | |
| 2940 | R | R | Rb | 0.125 | S | S |
| 2910 | R | S | S | 0.25 | S | S |
| 591 | R | R | Ra | 0.25 | S | R |
| 346 | R | R | Ra | 0.38 | S | S |
| 2401 | R | R | Ra | 0.5 | S | S |
| 1089 | R | S | S | 0.75 | S | S |
| 7002 | R | R | Rb | 0.75 | S | S |
| 9992 | R | R | Ra | 1 | S | S |
| 3913 | R | S | Ra | 1 | S | S |
| 1134 | R | R | S | 1 | S | R |
| 4026 | R | R | Ra | 1 | S | R |
| 5220 | R | R | Rb | 1.5 | S | S |
| 5198 | R | R | Ra | 1.5 | S | S |
| 237 | R | R | S | 1.5 | S | S |
| 8378 | R | R | Rb | 1.5 | S | S |
| 1635 | R | R | Rb | 1.5 | S | R |
| 5603 | R | R | Ra | 1.5 | S | R |
| 6120 | R | R | Ra | 1.5 | S | R |
| 0191 | R | R | Rb | 1.5 | R | R |
| 3819 | R | R | Rb | 2 | S | S |
| 777 | R | S | Rb | 2 | S | S |
| 5438 | R | R | Ra | 2 | S | S |
| 4039 | R | R | S | 2 | S | R |
| 3246 | R | R | S | 2 | R | R |
| 5987 | R | R | Rb | 2 | R | R |
| 2971 | R | R | Rb | 3 | S | R |
| 8954 | R | R | S | 4 | R | R |
| 6587 | R | R | Ra | 8 | S | R |
| 8343 | R | R | Ra | 16 | S | R |
| 4380 | R | R | Ra | 16 | S | R |
| 4757 | R | R | R | 16 | S | R |
| 6074 | R | R | Ra | >256 | S | R |
| 7773 | R | R | Ra | >256 | S | R |
| 1096 | R | R | R | >256 | S | R |
| 3149 | R | R | Rb | >256 | S | R |
| 1092 | R | R | R | >256 | S | R |
Strain had a susceptible zone of inhibition (≥20 mm with the 5-μg oxacillin disk) but had colonies within the zone (heteroresistance).
Strain had resistant zones of inhibition (<20 mm with the 5-μg oxacillin disk) and had colonies within the zone (heteroresistance).
R, resistant; S, susceptible.
Two mecA-positive S. aureus isolates, for which the oxacillin MICs were 0.38 and 1 μg/ml, did not grow on the oxacillin agar screen plate but expressed heteroresistance when tested by the large-inoculum disk diffusion assay (Table 2). Of the 76 mecA-positive coagulase-negative staphylococcal isolates, 4 did not grow on the oxacillin agar screen plate (Table 3). Among these four discrepant isolates, two, for which the MICs were 0.25 and 0.75 μg/ml, were not detected by the other methods and the others, for which the MICs were 1 and 2 μg/ml, were detected by the disk diffusion assay after 48 h of incubation (Table 3).
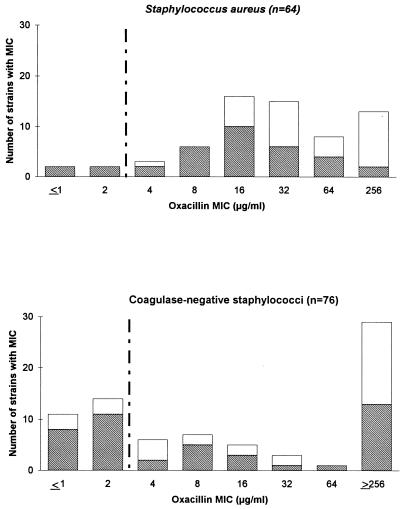
The distribution of the oxacillin MICs, as determined by the E-test, is presented in Fig. 1. The MIC for reference strain ATCC 43300 was 16 μg/ml. The MICs of oxacillin for 43 (67%) of the S. aureus strains and 46 (60.5%) of the coagulase-negative staphylococcal strains were ≤32 μg/ml. Whereas the MICs of oxacillin for most of the heterogeneous S. aureus strains were around 16 μg/ml, the MICs for the heterogeneous coagulase-negative staphylococcal strains exhibited a bimodal distribution (Fig. 1). According to the NCCLS breakpoint (≤2 μg/ml), the E-test identified 3 S. aureus strains (Table 2) and 25 coagulase-negative staphylococcal strains (Table 3) as susceptible strains. Therefore, the percentages of agreement of the E-test with the PCR amplification of the mecA gene and the oxacillin agar screen test were 95.3 and 98.4%, respectively, for the S. aureus strains and 67.1 and 72.4%, respectively, for the coagulase-negative strains (Table 1).
FIG. 1.
Distribution bar graphs of E-test-determined oxacillin MICs for mecA-positive S. aureus and coagulase-negative staphylococcal strains analyzed in the study. Hatched bars indicate heterogeneously resistant strains. Vertical broken lines indicate the breakpoint for distinguishing resistant from susceptible strains recommended by the NCCLS.
The ATB Staph system generated results within 18 h for all strains. Compared to the PCR amplification of the mecA gene, the ATB Staph system failed to detect resistance in 7 (11%) S. aureus isolates (Table 2) and 32 (42.1%) coagulase-negative staphylococcal isolates (Table 3). Among the seven falsely susceptible S. aureus isolates, five expressed heteroresistance when tested by disk diffusion and the MICs for six were ≤16 μg/ml (Table 2). Among the 32 coagulase-negative staphylococcal isolates, with undetected resistance, 24 expressed heteroresistance; 22 were susceptible to oxacillin (oxacillin MICs ≤ 2 μg/ml), 5 were borderline (MICs ≤ 16 μg/ml), and 5 (15.6%) were highly resistant (MICs > 256 μg/ml) (Table 3).
The Rapid ATB Staph system was tested against S. aureus strains exclusively, as recommended by the manufacturer. Results were provided within 5 h for all isolates. The Rapid ATB expression system yielded 15 (23.4%) false-susceptible results (Table 2). Thus, the percentages of agreement of the Rapid ATB Staph method with the PCR amplification of the mecA gene and with the agar screen plate test were 76.6 and 79.7%, respectively (Table 1). The MICs of oxacillin for all of the discrepant strains identified in this comparison of results were ≤32 μg/ml (Table 2).
No strain failed to grow in the Vitek GPS-503 card. Results were obtained within 8 h for 62 (96.9%) S. aureus isolates. For two S. aureus strains the Vitek system yielded results within 9 and 13 h. For the coagulase-negative staphylococci, final reports were achieved within 6 to 8 h for 63 (82.9%) isolates and required 12 h for 6 isolates. The mean time required to generate a final report was slightly longer for S. aureus isolates (8 h) than for coagulase-negative staphylococcal isolates (7.6 h). The oxacillin susceptibility results yielded by the Vitek system correlated with the presence of the mecA gene for 61 (95.3%) S. aureus isolates and 61 (80.3%) coagulase-negative staphylococcal isolates (Table 1). The percentages of agreement between the Vitek system and the oxacillin screen test were 98.4 and 85.5% for S. aureus and coagulase-negative staphylococcal isolates, respectively (Table 1). The MICs for all isolates that were undetectable by the Vitek system were ≤2 μg/ml, and all isolates expressed heteroresistance, except for strains 2910 and 1089, which did not express any resistance. None of these isolates was detectable by the ATB Staph or the Rapid ATB Staph system (Tables 1 and 2).
DISCUSSION
The purpose of this study was to compare the efficiencies of different commercial and widely used methodologies for the detection of oxacillin heteroresistance. The PCR amplification of the mecA gene and the oxacillin screen plate test were used as the “gold standards.” Oxacillin MICs were determined by the E-test. In previous evaluations of automated susceptibility testing methods (13, 37, 38), the problem was mostly one of accurate detection of oxacillin resistance and not of false resistance to oxacillin. Therefore, we focused the present work on a collection of mecA-positive staphylococci. Fifty percent of the isolates were selected for the study because they exhibited a heterogeneous phenotype when tested by a large-inoculum disk diffusion assay. Therefore, the whole population of strains tested in this study has no epidemiological significance and does not reflect the relative frequencies of staphylococci with heterogeneous phenotypes in our hospital. In order to screen for heteroresistant isolates, we performed the disk diffusion method according to the French recommendations, i.e., with 5-μg oxacillin disks and salt-free Mueller-Hinton agar plates incubated at 30°C, except that we increased the inoculum (108 instead of 107 CFU/ml) and the incubation time (48 instead of 24 h). Although we did not perform the differential inoculum disk diffusion method (4), we observed that most of our heteroresistant isolates were undetectable when the assay was performed with a 106 inoculum (data not shown). This and the fact that the oxacillin MICs for 43 (67%) S. aureus isolates and 46 (60.5%) coagulase-negative staphylococcal isolates were low (≤32 μg/ml) suggest a predominance of heterogeneously resistant strains belonging to phenotypic expression class 1 or 2 (4, 28).
The usefulness of the detection of the mecA gene for the detection of oxacillin resistance has extensively been shown (2, 7, 8, 10, 17, 25–32). Several PCR-based methods have successfully been used (7, 27, 29, 30, 32). In the present work, the preparation of the template from staphylococcal cells was simplified by heating the cells in the presence of a reagent which sequesters cell lysis products (Genereleaser; BioVentures Inc.). This procedure is easy and as efficient as lysostaphin lysis. Moreover, the fact that all heating reactions can be performed on the thermal cycler within a single tube might contribute to the automation of the PCR amplification.
There is a growing consensus in the literature that the oxacillin agar screen plate test (1, 21) is the most reliable phenotypic test for the detection of the oxacillin resistance (7–9, 11, 17, 23, 25, 26, 31, 37). In the present evaluation, there was concordance between the results of the PCR amplification of mecA and those of the agar screening test, except for two S. aureus isolates and four coagulase-negative staphylococcal isolates. Surprisingly, these two S. aureus strains and two of the four coagulase-negative staphylococcal strains were detectable by the large-inoculum oxacillin disk diffusion assay (Tables 2 and 3). Such discrepancies might be resolved by reevaluating the NaCl incorporation of the oxacillin agar plates, as suggested by other investigators (8, 11). The absence of any growth of coagulase-negative staphylococcal strains 2910 and 1089 may be related to the absence of expression of the mecA gene. Whether the mecA gene was functional and whether the production of PBP 2′ was inducible in these strains were not investigated. Such mecA-positive strains susceptible to oxacillin, for which the MICs ranged between 0.25 and 2 μg/ml, have been found in other studies (7, 11, 22, 27). The reduced beta-lactam resistance relies on the down-regulation of mecA transcription (19) and is influenced by auxiliary genes such as mecR, mecI (15), and the fem genes (5). However, these cryptic methicillin-resistant strains, also called preMRSA (10, 15), are potentially highly resistant, since they can generate highly resistant subclones in vitro (10, 27). Therefore, their detection appears to determine the choice of antibiotic therapy and relies only on the detection of the mecA gene.
In this study, the oxacillin MICs were determined by the E-test, which has been reported as a reliable alternative to the conventional agar or broth dilution methods (11, 12, 32, 34). We found that the E-test was acceptable for detecting oxacillin-resistant S. aureus isolates, as shown by the agreement of 98.4% with the oxacillin agar screen plate results (Table 1). In contrast, when testing the coagulase-negative staphylococcal isolates, we found only 67.1 and 72.4% agreement with the PCR amplification of mecA and the agar screen test, respectively. However, 25 coagulase-negative staphylococcal strains were misinterpreted as susceptible by the E-test because the oxacillin MICs for them ranged from 0.125 to 2 μg/ml. Such mecA-positive coagulase-negative staphylococcal strains for which the oxacillin MICs cluster around 1 or 2 μg/ml have also been observed by using conventional MIC determination methods (12, 17, 18). In agreement with these previous studies, our results suggest that NCCLS MIC interpretative criteria may underestimate oxacillin resistance among coagulase-negative staphylococcal strains. The use of an oxacillin breakpoint of ≥0.5 μg/ml for resistance, previously proposed by McDonald et al. (18), would lead us to revise the MIC interpretations of 16 coagulase-negative staphylococcal isolates in our study and would increase the agreement of the E-test with the PCR of the mecA gene to 94.7%.
The automated methodologies for susceptibility testing are used in a large number of clinical laboratories. A multicentric study focusing on the detection of low-level-expression class reference strain ATCC 43300 (16) showed that the automated methods were generally more reliable than the disk diffusion method. However, in that study, many types of equipment and preprepared MIC panels were represented and the number of laboratories that used any one method was too small to allow comparisons between the different systems. In the present work, we compared the performance characteristics of the ATB Staph, the Rapid ATB Staph, and the Vitek GPS-503 card systems. The ATB Staph system failed to detect oxacillin resistance in 7 (11%) S. aureus isolates and 32 (42.1%) coagulase-negative staphylococcal isolates. The MICs for the falsely susceptible strains were ≤16 μg/ml, except for one S. aureus strain (Table 2) and five coagulase-negative staphylococcal strains (Table 3). Considering the collection of strains tested in this study, the performance of the ATB Staph system can be considered acceptable for testing S. aureus strains, in agreement with the great sensitivity reported by other investigators (38). In contrast, the ATB Staph system generated a high rate of false-susceptible results among the coagulase-negative staphylococcal strains, since its results correlated with the presence of the mecA gene for 44 (57.9%) of the coagulase-negative staphylococcal strains only (Table 1). This lack of accuracy of commercial systems for the detection of oxacillin-resistant coagulase-negative staphylococci has also been reported for the BBL Crystal MRSA (33) and the rapid fluorogenic MicroScan systems (35).
The Rapid ATB Staph system, evaluated for S. aureus strains only, misinterpreted as susceptible 15 (23.4%) strains, for which the MICs were all ≤32 μg/ml (Table 2). Therefore, the Rapid ATB Staph system was less reliable than the ATB Staph system, as illustrated by its lower percentage of agreement with the PCR amplification of the mecA gene (76.6 versus 89.0%) (Table 1). In spite of previous data reporting 97 to 99% accuracy for the Rapid ATB Staph system (26), we conclude that the accuracy may not be acceptable when the prevalence of heterogeneously resistant isolates is high.
Among the automated systems tested herein, the Vitek system was the most reliable at detecting oxacillin heteroresistance. None of the 3 (4.7%) S. aureus strains and 15 (19.7%) coagulase-negative staphylococcal strains misdetected by the Vitek system was found to be resistant by the ATB Staph systems. Moreover, the MICs for all these strains were ≤2 μg/ml, whereas the ATB systems miscategorized many strains for which the MICs were >2 μg/ml (Tables 2 and 3). Considering the percentage of agreement of the Vitek system with the PCR amplification of the mecA gene (95.3%), we found that it is a reliable method for the detection of oxacillin-resistant S. aureus strains. It is difficult to draw a similar conclusion for the coagulase-negative staphylococcal isolates, since the agreement of the Vitek system with the PCR amplification of the mecA gene is only 80.3% (Table 1). This failure of the Vitek system to detect oxacillin resistance in some mecA-positive coagulase-negative staphylococcal strains has been reported by other investigators (22, 25). However, in our study, the lack of accuracy of the Vitek system for the detection of oxacillin resistant coagulase-negative staphylococci is related to the high number of coagulase-negative staphylococcal strains for which the MICs are ≤2 μg/ml. False-susceptible results for strains for which the MICs are ≤2 μg/ml have been observed with the Microscan system as well (25). If the NCCLS MIC interpretative criteria were to be revised for coagulase-negative staphylococcal strains, as was previously suggested (18), the subsequent revision of the expert system softwares would probably increase the accuracy of such automated methodologies. For example, if the Vitek system interpretation could be modified on the basis of an oxacillin breakpoint of ≥0.5 μg/ml for resistance, as suggested above, 12 of the 15 coagulase-negative staphylococcal isolates initially undetected by the Vitek system could be classified as resistant. The agreement of the Vitek system with the PCR of the mecA gene would then be 96%.
In this study we did not evaluate the abilities of the commercial systems to differentiate between borderline oxacillin-susceptible and -resistant staphylococci. Recently, Knapp et al. reported the ability of the Vitek system to differentiate borderline-susceptible S. aureus isolates from heterogeneous class 1 and 2 resistant strains and found a correct classification by the Vitek card for 86% of the strains (14). Concerning the detection of borderline oxacillin-resistant staphylococci, our data emphasize the superiority of the Vitek system over the ATB Staph and the Rapid ATB Staph systems. However, considering that the Vitek system failed to detect mecA-positive staphylococci for which the oxacillin MICs were ≤2 μg/ml, a confirmation test remains essential for the treatment of serious infections. This confirmation can be provided by the oxacillin agar screen plate test, which is accessible to all clinical laboratories. However, this test requires 48 h to confirm oxacillin susceptibility. Alternatively, the rapid BBL Crystal MRSA test provides results within 4 h, but it may misclassify some borderline and/or heterogeneously resistant strains (38), and it is less reliable for coagulase-negative staphylococcal than for S. aureus isolates (33). Finally, the most rapid and reliable procedure providing the definitive discrimination for such isolates remains the PCR amplification of the mecA gene.
In conclusion, among the commercial systems compared in the present study, we found that the E-test and the Vitek system were the most accurate at detecting oxacillin heteroresistance in staphylococci. The potential revision of the 2-μg/ml oxacillin NCCLS breakpoint was previously proposed for coagulase-negative staphylococcal strains (18) and might reduce the relative lack of efficiency of these methods for such strains.
ACKNOWLEDGMENT
We gratefully thank Jean-Louis Pons for help in preparing the manuscript.
REFERENCES
- 1.Archer G L, Pennell E. Detection of methicillin resistance in staphylococci by using a DNA probe. Antimicrob Agents Chemother. 1990;34:1720–1724. doi: 10.1128/aac.34.9.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bignardi G E, Woodford N, Chapman A, Johnson A P, Speller D C E. Detection of the mecA gene and phenotypic detection of resistance in Staphylococcus aureus isolates with borderline or low-level methicillin resistance. J Antimicrob Chemother. 1996;37:53–63. doi: 10.1093/jac/37.1.53. [DOI] [PubMed] [Google Scholar]
- 3.Comité de l’Antibiogramme de la Société Française de Microbiologie. Valeurs critiques pour l’antibiogramme. Bull Soc Fr Microbiol. 1996;11:315–320. [Google Scholar]
- 4.de Lencastre H, Sa Figueiredo A M, Urban C, Rahl J, Tomasz A. Multiple mechanisms of methicillin resistance and improved methods for detection in clinical isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 1991;35:632–639. doi: 10.1128/aac.35.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Lencastre H, Tomasz A. Reassessment of the number of auxiliary genes essential for expression of high-level methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1994;38:2590–2598. doi: 10.1128/aac.38.11.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duran S P, Kayser F H, Berger-Bächi B. Impact of sar and agr on methicillin-resistance in Staphylococcus aureus. FEMS Microbiol Lett. 1996;141:255–260. doi: 10.1111/j.1574-6968.1996.tb08394.x. [DOI] [PubMed] [Google Scholar]
- 7.Geha D J, Uhl J R, Gustaferro C A, Persing D H. Multiplex PCR for identification of methicillin-resistant staphylococci in the clinical laboratory. J Clin Microbiol. 1994;32:1768–1772. doi: 10.1128/jcm.32.7.1768-1772.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerberding J L, Miick C, Liu H H, Chambers H F. Comparison of conventional susceptibility tests with direct detection of penicillin-binding protein 2a in borderline oxacillin-resistant strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1991;35:2574–2579. doi: 10.1128/aac.35.12.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hackbarth C J, Chambers H F. Methicillin-resistant staphylococci: detection methods and treatment of infections. Antimicrob Agents Chemother. 1989;33:995–999. doi: 10.1128/aac.33.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiramatsu K, Kihara H, Yokota T. Analysis of borderline-resistant strains of methicillin-resistant Staphylococcus aureus using polymerase chain reaction. Microbiol Immunol. 1992;36:445–453. doi: 10.1111/j.1348-0421.1992.tb02043.x. [DOI] [PubMed] [Google Scholar]
- 11.Huang M B, Gay T E, Baker C N, Banerjee S N, Tenover F C. Two percent sodium chloride is required for susceptibility testing of staphylococci with oxacillin when using agar-based dilution methods. J Clin Microbiol. 1993;31:2683–2688. doi: 10.1128/jcm.31.10.2683-2688.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ieven M, Jansens H, Ursi D, Verhoeven J, Goossens H. Rapid detection of methicillin resistance in coagulase-negative staphylococci by commercially available fluorescence test. J Clin Microbiol. 1995;33:2183–2185. doi: 10.1128/jcm.33.8.2183-2185.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knapp C C, Ludwig M D, Washington J A. Evaluation of differential inoculum disk diffusion method and Vitek GPS-SA card for detection of oxacillin-resistant staphylococci. J Clin Microbiol. 1994;32:433–436. doi: 10.1128/jcm.32.2.433-436.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knapp C C, Ludwig M D, Washington J A, Chambers H F. Evaluation of Vitek GPS-SA card for testing of oxacillin against borderline-susceptible staphylococci that lack mec. J Clin Microbiol. 1996;34:1603–1605. doi: 10.1128/jcm.34.7.1603-1605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuwohara-Arai K, Kondo N, Hori S, Tateda-Susuki E, Hiramatsu K. Suppression of methicillin resistance in a mecA-containing pre-methicillin-resistant Staphylococcus aureus strain is caused by the mecI-mediated repression of PBP2′ production. Antimicrob Agents Chemother. 1996;40:2680–2685. doi: 10.1128/aac.40.12.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackenzie A M R, Richardson H, Missett P, Wood D E, Groves D J. Accuracy of reporting of methicillin-resistant Staphylococcus aureus in a provincial quality control program: a 9-year study. J Clin Microbiol. 1993;31:1275–1279. doi: 10.1128/jcm.31.5.1275-1279.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackenzie A M R, Richardson H, Lannigan R, Wood D. Evidence that the National Committee for Clinical Laboratory Standards disk test is less sensitive than the screen plate for the detection of low-expression-class methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1995;33:1909–1911. doi: 10.1128/jcm.33.7.1909-1911.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald C L, Maher W E, Fass R J. Revised interpretation of oxacillin MICs for Staphylococcus epidermidis based on mecA detection. Antimicrob Agents Chemother. 1995;39:982–984. doi: 10.1128/aac.39.4.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mempel M, Feucht H, Ziebuhr W, Endres M, Laufs R, Grüter L. Lack of mecA transcription in slime-negative phase variants of methicillin-resistant Staphylococcus epidermidis. Antimicrob Agents Chemother. 1994;38:1251–1255. doi: 10.1128/aac.38.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A5. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 3rd ed. Approved standard M7-A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 22.Ramotar K, Jessamine P, Bobrowska-Gacek M, Woods W, Coultish I, Toye B. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Detection of methicillin-resistance in coagulase negative staphylococci (CNS), abstr. C-177; p. 32. [Google Scholar]
- 23.Richard P, Meyran M, Carpentier E, Thabaut A, Drugeon H B. Comparison of phenotypic methods and DNA hybridization for detection of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1994;32:613–617. doi: 10.1128/jcm.32.3.613-617.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryffel C, Kayser F H, Berger-Bächi B. Correlation between regulation of mecA transcription and expression of methicillin resistance in staphylococci. Antimicrob Agents Chemother. 1992;36:25–31. doi: 10.1128/aac.36.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skulnick M, Simor A E, Gregson D, Patel M, Small G W, Kreiswirth B, Hathoway D, Low D E. Evaluation of commercial and standard methodology for determination of oxacillin susceptibility in Staphylococcus aureus. J Clin Microbiol. 1992;30:1985–1988. doi: 10.1128/jcm.30.8.1985-1988.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Struelens M J, Nonhoff C, Van der Auwera P, Mertens R, Serruys E Groupement pour le Dépistage; l’Etude et la Prévention des Infections Hospitalières-Groep Ter Opsporing; Studie en Preventie van Infecties in de Ziekenhuizen. Evaluation of Rapid ATB Staph for 5-hour antimicrobial susceptibility testing of Staphylococcus aureus. J Clin Microbiol. 1995;33:2395–2399. doi: 10.1128/jcm.33.9.2395-2399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tokue Y, Shoji S, Satoh K, Watanabe A, Motomiya M. Comparison of a polymerase chain reaction assay and a conventional microbiologic method for detection of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:6–9. doi: 10.1128/aac.36.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomasz A, Nachman S, Leaf H. Stable classes of phenotypic expression in methicillin-resistant clinical isolates of staphylococci. Antimicrob Agents Chemother. 1991;35:124–129. doi: 10.1128/aac.35.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ubakata K, Nakagami S, Nitta A, Yamane A, Kawakami S, Sugiura M, Konno M. Rapid detection of the mecA gene in methicillin-resistant staphylococci by enzymatic detection of polymerase chain reaction products. J Clin Microbiol. 1992;30:1728–1733. doi: 10.1128/jcm.30.7.1728-1733.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ünal S, Hoskins J, Flokowitsch J E, Wu C Y E, Preston D A, Skatrud P L. Detection of methicillin-resistant staphylococci by using the polymerase chain reaction. J Clin Microbiol. 1992;30:1685–1691. doi: 10.1128/jcm.30.7.1685-1691.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ünal S, Werner K, DeGirolami P, Barsanti F, Eliopoulos G. Comparison of tests for detection of methicillin-resistant Staphylococcus aureus in a clinical microbiology laboratory. Antimicrob Agents Chemother. 1994;38:345–347. doi: 10.1128/aac.38.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanuffel P, Gigi J, Ezzedine H, Vandercam B, Delmee M, Wauters G, Gala J-L. Specific detection of methicillin-resistant Staphylococcus species by multiplex PCR. J Clin Microbiol. 1995;33:2864–2867. doi: 10.1128/jcm.33.11.2864-2867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallet F, Roussel-Delvallez M, Courcol R J. Choice of a routine method for detecting methicillin-resistance in staphylococci. J Antimicrob Chemother. 1996;37:901–909. doi: 10.1093/jac/37.5.901. [DOI] [PubMed] [Google Scholar]
- 34.Weller T M A, Crook D W, Crow M R, Ibrahim W, Pennington T H, Selkon J B. Methicillin susceptibility testing of staphylococci by Etest and comparison with agar dilution and mecA detection. J Antimicrob Chemother. 1997;39:251–253. doi: 10.1093/jac/39.2.251. [DOI] [PubMed] [Google Scholar]
- 35.Woods G L, LaTemple D, Cruz C. Evaluation of MicroScan rapid gram-positive panels for detection of oxacillin-resistant staphylococci. J Clin Microbiol. 1994;32:1058–1059. doi: 10.1128/jcm.32.4.1058-1059.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu S, de Lencastre H, Tomasz A. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J Bacteriol. 1996;178:6036–6042. doi: 10.1128/jb.178.20.6036-6042.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.York M K, Gibbs L, Chehab F, Brooks G F. Comparison of PCR detection of mecA with standard susceptibility testing methods to determine methicillin resistance in coagulase-negative staphylococci. J Clin Microbiol. 1996;34:249–253. doi: 10.1128/jcm.34.2.249-253.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zambardi G, Fleurette J, Schito G C, Auckenthaler R, Bergogne-Berezin E, Hone R, King A, Lenz W, Lohner C, Makristhatis A, Marco F, Müller-Serieys C, Nonhoff C, Phillips I, Rohner P, Rotter M, Schaal K P, Struelens M, Viebahn A. European multicentre evaluation of a commercial system for identification of methicillin-resistant Staphylococcus aureus. Eur J Clin Microbiol. 1996;15:747–749. doi: 10.1007/BF01691964. [DOI] [PubMed] [Google Scholar]