Dear Editor,
Hutchinson-Gilford Progeria Syndrome (HGPS) is caused by a mutation in the Lamin A/C gene (LMNA), resulting in the synthesis and accumulation of an abnormal protein, progerin, which disrupts the structural integrity and function of the nucleus and nucleolus. Affected individuals exhibit a senescent phenotype and die prematurely due to cardiovascular complications.1 The exceptional case of Sammy Basso, who lived until the age of 28, has brought renewed public attention to this rare disease.
Lonafarnib, a farnesyltransferase inhibitor that prevents the synthesis and accumulation of progerin, is the sole FDA-approved treatment for HGPS. In October 2024, a phase 2a clinical trial of Lonafarnib in combination with Progerinin, an improved progerin-Lamin A binding inhibitor, was started in a small cohort of children affected by HGPS. New cardiovascular treatments that can extend life until a definitive solution from DNA base editing are urgently needed.
We previously demonstrated that supplementing with the longevity variant (LAV) of the BPI fold containing family B, member 4 (BPIFB4), found in healthy supercentenarians, can delay and even reverse the ageing clock in animal models of cardiovascular disease.2 Supercentenarians’ cells exhibit minimal alterations in nuclear envelope components and possess a distinctive resilience to prelamin A accumulation.3 The other way round, progerin inhibits the nuclear translocation of Transcription Factor EB (TFEB), a transcriptional activator of BPIFB4 and a regulator of autophagy and lysosomal biogenesis.4 Based on this background, we elaborated the new hypothesis that BPIFB4 is downregulated in HGPS cells and that the transfer of the BPIFB4 gene protects from LMNA mutation-induced aging.
First, we determined whether LAV-BPIFB4 horizontal transfer could improve early cardiomyopathy in hemizygous transgenic C57BL/6-Tg(LMNA*G608G)HClns/J mice, which mimic the early cardiovascular traits of human HGPS. Second, LAV-BPIFB4 was tested on HGPS patient fibroblasts in vitro. Results are illustrated in Fig. 1 and the online Repository Supplement (https://zenodo.org/records/16792599).
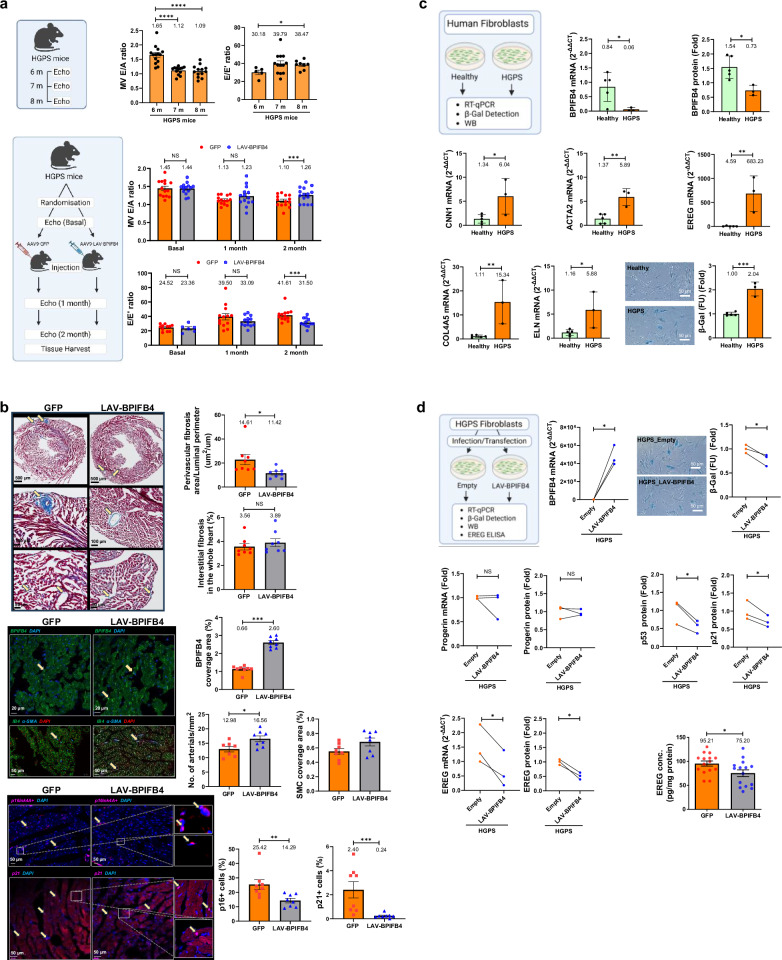
Fig. 1.
a Progeria mice exhibit early cardiac diastolic dysfunction, which is attenuated by AAV9-LAV-BPIFB4 gene transfer. (Top) Heart function was assessed using echocardiography in 6–8-month-old progeria mice. Diastolic function was determined with the E/A (mitral valve peak E and peak A waves ratio) and E/E’ (mitral valve peak E wave and mitral annular peak E’ wave ratio). Then, we studied the same parameters in progeria mice that received AAV9-GFP or AAV9-LAV-BPIFB4 (Bottom). GFP: AAV9-GFP-treated mice; LAV-BPIFB4: AAV9-LAV-BPIFB4-treated mice. Two months post the AAV9-GFP or AAV9-LAV-BPIFB4 injections, the E/A and E/E’ indexes were determined (n = 13–14 for E/A at each time point; n = 6–11 for E/E’ at baseline and 12–14 for 1- and 2-month post-injection). b Treatment with AAV9-LAV-BPIFB4 reduces cardiac perivascular fibrosis and improves cardiac microvasculature in progeria mice. (Top) Fibrosis was determined by collagen staining in the whole left ventricle of AAV9-GFP- and AAV9-LAV-BPIFB4-treated mice two months after virus injection (n = 8 for AAV9-GFP- and AAV9-LAV-BPIFB4-treated progeria mice). Bar graphs summarize the quantitative analysis of the perivascular fibrosis index of subepicardial arteries, expressed as the perivascular fibrosis area standardized against the luminal perimeter in male and female combined groups. The treatment did not affect interstitial fibrosis. (Middle) The BPIFB4 expression was expressed as the percentage of BPIFB4 coverage area in total tissue area in each image. The number of microvessels (IB4, green) and arterials (a-SMA, blue), as well as the coverage area of microvessels and a-SMA+ cells, were normalized against the total tissue area in each image (nuclei stained with DAPI, red). In addition, (Bottom) treatment with AAV9-LAV-BPIFB4 reduced senescence in the heart. The percentage of senescent cells in the whole left ventricle in AAV9-GFP- and AAV9-LAV-BPIFB4-treated mice was determined, with the data reported as the percentage of p16+ cells (pink) or p21+ cells (pink) relative to the total cell number (nuclei stained with DAPI, blue). GFP: AAV9-GFP-treated mice; LAV-BPIFB4: AAV9-LAV-BPIFB4-treated mice. c HGPS patient fibroblasts exhibit downregulation of endogenous BPIFB4 expression, activation of typical myofibroblast genes and increased senescence. (n = 5 for healthy controls and 3 for HGPS patients). (Top) Endogenous BPIFB4 mRNA and protein levels were assayed by RT-qPCR and Western blot with corresponding densitometric quantification. (Middle and Bottom, left) Genes associated with myofibroblast activation were assayed by RT-qPCR. In addition, (Bottom, right) senescent fibroblasts were identified by histochemical staining and quantitative β–Galactosidase assay. Healthy: fibroblasts from healthy controls; HGPS: fibroblasts from HGPS patients. d Ectopic expression of LAV-BPIFB4 normalizes HGPS patient fibroblasts (n = 3). (Top, left) BPIFB4 expression was increased by vector-mediated transfection of HGPS fibroblasts. (Top, right) Staining and measurement of β-Galactosidase levels were carried out in HGPS fibroblasts transfected with LAV-BPIFB4. (Middle) HGPS fibroblasts infected with LAV-BPIFB4 lentivirus were analyzed for progerin mRNA and protein by semi-quantitative PCR and Western blot, respectively. Senescence in LAV-BPIFB4-infected HGPS fibroblasts was evaluated by Western blot analysis of p53 and p21, with relative quantification. (Bottom, left and center) EREG transcript and protein released into the supernatant from LAV-BPIFB4-infected HGPS fibroblasts were measured by RT-qPCR and ELISA assay, respectively. Empty: HGPS fibroblasts transfected/infected with empty vectors; LAV-BPIFB4: HGPS fibroblasts transfected/infected with vectors containing LAV-BPIFB4. Furthermore, (Bottom, right) EREG protein expression was assessed in cardiac tissues of AAV9-GFP- and AAV9-LAV-BPIFB4-treated mice two months post-virus injections; AAV9-LAV-BPIFB4 reduced EREG expression in the progeria mouse heart. β–Gal: β–Galactosidase, EREG: epiregulin. Data are represented as mean ± SEM. The descriptive scientific graphics were created using BioRender and the corresponding licences are detailed in the online Repository Supplement
Like HGPS children, 26-week-old progeria mice have growth retardation as compared with age-matched normal C57Bl/6 mice (Jackson Laboratories dataset, online Repository Supplementary Fig. 1a, b). Three-dimensional echocardiography showed that untreated progeria mice spontaneously acquire left ventricular (LV) diastolic dysfunction at 7 or 8 months of age, as demonstrated by a decreased E/A ratio and higher E/E’ ratio (Fig. 1a). In contrast, systolic function parameters remained unchanged (online Repository Supplementary Fig. 2a–e). Prakash et al. found diastolic dysfunction as the first echocardiographic abnormality in children with HGPS. They hypothesized that myocardial fibrosis may be the structural cause of altered LV relaxation.1
In a randomized study, male and female progeria mice received a single intraperitoneal injection of LAV-BPIFB4 or GFP via an adeno-associated subtype-9 viral vector (AAV9, 1 × 1012 GC/ml in 100 μl PBS). AAV9-LAV-BPIFB4 enhanced cardiac BPIFB4 protein expression 2.5-fold and boosted female mice’s body weight (online Repository Supplementary Fig. 1c, d). AAV9-LAV-BPIFB4 significantly reduced age-related changes in LV diastolic function compared to AAV9-GFP-treated controls (Fig. 1a), but did not affect systolic function or LV mass (online Repository Supplementary Fig. 3a–e).
In progeria mice, histological analyses demonstrated that AAV9-LAV-BPIFB4 decreased perivascular fibrosis, but not interstitial fibrosis. Moreover, as shown using fluorescent immunohistochemistry, gene therapy increased the expression of BPIFB4, as well as the number of coronary arterioles along with vascular smooth muscle cell coverage, while reducing cardiac p16- or p21-positive senescent cells (Fig. 1b). The anti-fibrotic, pro-survival, and vascular actions of AAV9-LAV-BPIFB4 may account for improving diastolic dysfunction.
Aortas were harvested and examined after HGPS mice were culled after 2-month follow up and there was no evidence of atherosclerotic lesions in the aortas of 8-month-old progeria animals. This, along with the liver’s modest collagen deposition, suggests that the model is an early form of HGPS. On the other end, senescent cells, identified by p16 or p21 markers, were abundant in the livers of progeria mice. AAV9-LAV-BPIFB4 decreased p21-positive senescent cells but not p16-positive ones (online Repository Supplementary Fig. 4).
We studied fibroblast cell lines from three HGPS patients and five unaffected parents to investigate the effects of LAV-BPIFB4 on fibrosis and senescence. All cell lines expressed Lamin A/C, but only HGPS fibroblasts expressed progerin mRNA and protein (online Repository Supplementary Fig. 5). HGPS fibroblasts exhibited lower BPIFB4 mRNA and protein levels along with marked increases in calponin-1 (CNN1), smooth muscle α-actin (ACTA2), and epiregulin (EREG) as well as pro-fibrotic and senescent markers, such as Col4A5 and Elastin, and β-galactosidase (Fig. 1c). Lentivirus infection or plasmid transfection of LAV-BPIFB4 reduced senescence, p53 and p21 levels, and EREG mRNA and protein secretion into the medium without affecting progerin accumulation (Fig. 1d). The cardiac tissue of progeria animals treated with AAV9-LAV-BPIFB4 exhibited lower EREG levels, consistent with the in vitro results (Fig. 1d). These data indicate that LAV-BPIFB4 reduces cellular senescence via the p53–p21 and p16 axes and modulates early fibrotic signaling via the EREG ligand of the Epidermal Growth Factor Receptor (EGFR) pathway. The treatment did not directly affect terminal myofibroblast differentiation or extracellular matrix deposition. Altogether, results indicate that LAV-BPIFB4 decreases progerin toxicity rather than suppressing this abnormal protein.
Our study is the first to demonstrate that a supercentenarian gene mutation protects the HGPS heart against fibrosis and promotes cell survival, thereby preserving diastolic function. Mechanistic investigations on HGPS fibroblasts showed lower endogenous BPIFB4 expression, probably due to progerin-induced transcriptional suppression, and an enhanced aged and fibrotic phenotype, which was rescued by exogenous LAV-BPIFB4.
LAV-BPIFB4 transduction may affect nucleolar activities, including ribosome biogenesis and ribonucleoprotein assembly, downstream of the LMNA mutation, resulting in therapeutic actions.5 The creation of an interactive complex with a nucleolar RNA-binding protein that affects ribosome assembly, biogenesis, DNA transcription, and telomere preservation and repair represent some of LAV-BPIFB4’s known molecular functions.2 LAV-BPIFB4 may also protect cardiomyocytes through paracrine signaling systems, as shown by its favorable chronotropic and inotropic effects on human iPSC-derived cardiomyocytes.2
Other gene variations contribute to healthy aging than LAV-BPIFB4. The genetics of supercentenarians may therefore disclose new global treatments for premature or accelerated cardiac senescence.
Supplementary information
Acknowledgements
The authors would like to express their gratitude to the Progeria Research Foundation (PRF) Cell & Tissue Bank for providing the primary human dermal fibroblast cell lines. We also appreciate the support and assistance of the Wolfson Bioimaging Facility at the University of Bristol, UK. Additionally, we would like to acknowledge our colleagues Dr. Valeria V. Alvino, Dr. Khaled A. K. Mohammed, and Dr. Svetlana Mastitskaya from the University of Bristol for their valuable contributions to the in vivo studies, histology, and data analysis involved in this project. This research was funded by the Medical Research Council (MRC) and the Italian Ministry of Health, Ricerca Corrente, through IRCCS MultiMedica.
Author contributions
Y.Q. conducted most of the in vivo experiments and analyses and drafted the manuscript. M.C. performed the majority of the in vitro experiments and analyses and revised the manuscript. A.M. contributed to in vitro experiments and analyses. A.A.P. contributed to planning and data interpretation. P.M. designed and oversaw the project and also revised the manuscript. All authors reviewed and approved the final version. Y.Q. and M.C. contributed equally as first authors; A.A.P. and P.M. contributed equally as senior authors.
Data availability
The authors declare that all data supporting the findings of this study are available within the article, its Supplementary Materials file and the online Repository Supplement (https://zenodo.org/records/16792599).
Competing interests
A.A.P. have shares of LGV1 Inc. and has filed a patent. All the other authors declare that there is no conflict of interest.
Ethics declaration
All animal procedures conformed to the guidelines from Directive 2010/63/EU of the European Parliament on protecting animals used for scientific purposes and were approved by the University of Bristol and the British Home Office (PPL: PP1377882). Human primary dermal fibroblast cell lines were obtained from the Progeria Research Foundation (PRF) Cell and Tissue Bank. The HGPS cell lines included: HGADFN167, HGADFN169, and HGADFN367. The control cell lines were: HGMDFN718, HGFDFN168, HGMDFN368, HGFDFN369, and HGMDFN717.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yan Qiu, Monica Cattaneo.
These authors jointly supervised this work: Annibale A Puca, Paolo Madeddu.
Contributor Information
Yan Qiu, Email: y.qiu@bristol.ac.uk.
Annibale A. Puca, Email: annibale.puca@multimedica.it
Supplementary information
The online version contains supplementary material available at 10.1038/s41392-025-02416-3.
References
- 1.Prakash, A. et al. Cardiac abnormalities in patients with hutchinson-gilford progeria syndrome. JAMA Cardiol.3, 326–334 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calligaris, M. et al. Protective role of the longevity-associated BPIFB4 gene on cardiac microvascular cells and cardiac aging. Vasc. Pharmacol.158, 107470 (2025). [DOI] [PubMed]
- 3.Lattanzi, G. et al. Lamins are rapamycin targets that impact human longevity: a study in centenarians. J. Cell Sci.127, 147–157 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Gambardella, G. et al. GADD34 is a modulator of autophagy during starvation. Sci. Adv.6 (2020). [DOI] [PMC free article] [PubMed]
- 5.Phan, T., Khalid, F. & Iben, S. Nucleolar and ribosomal dysfunction-a common pathomechanism in childhood progerias? Cells8. (2019). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the article, its Supplementary Materials file and the online Repository Supplement (https://zenodo.org/records/16792599).