Abstract
In this work, we describe the assembly of a synthetic gene coding for several antigenic determinants found in different Leishmania infantum antigens. Selected epitopes were derived from the ribosomal proteins LiP2a, LiP2b, and LiP0 and from the histone H2A. The resulting gene was overexpressed in Escherichia coli either as a fusion protein (with the vector pMAL-c2) or alone (with the vector pQE). In both cases, high-level bacterial production of the recombinant protein was achieved and the products were found to be stable. Enzyme-linked immunosorbent assay (ELISA) and Western blotting experiments confirmed that the corresponding epitopes are present in the engineered protein. Finally, a serological evaluation of this multiple-epitope protein by Falcon assay screening test-ELISA revealed a sensitivity of 79 to 93% and a specificity of 96 to 100% in diagnosis of canine visceral leishmaniasis, indicating that this protein represents a valuable tool for serodiagnosis.
Leishmaniases are a spectrum of diseases having a worldwide distribution that are caused by different species of the genus Leishmania. Leishmania infantum, distributed in many areas of the Mediterranean basin, causes visceral leishmaniasis (VL) in both humans and dogs. In fact, Leishmania-infected dogs are the main animal reservoir of the parasite, particularly during the long incubation period before clinical symptoms are observed (20). Epidemiologic data indicate that there is a direct correlation between the prevalence of canine leishmaniasis and the transmission of the parasite to humans (13, 19). Hence, early detection of infected animals may be critical in controlling the spread of the disease. Given the frequent lack of signs in dogs and the difficulty of direct detection of the organism, rapid and accurate diagnosis has become an essential part of VL control. The presence of high titers of circulating antibodies against parasite proteins in the sera of Leishmania-infected dogs that are detected even during the asymptomatic phase of the disease in both natural (2) and experimental (1, 6, 7, 18) infections has been reported elsewhere.
In the last few years, an increasing number of Leishmania antigens have been characterized. Some of them can be considered Leishmania-specific proteins, such as the surface protease gp63 (25), the surface glycoprotein gp46 (16), and the lipophosphoglycan-associated protein KMP11 (35). An additional group of antigens is integrated by evolutionarily conserved proteins: kinesin (5), heat shock proteins (3, 8, 17, 24, 26), and actin and tubulin (22). As a strategy to develop a specific serodiagnostic test for canine leishmaniasis, we carried out a search of parasite antigens by immunoscreening of an L. infantum expression library with sera from dogs with active disease. Remarkably, most of the characterized antigens were found to belong to evolutionarily conserved protein families. However, the B-cell epitope mapping of these antigens revealed that in all cases the antigenic determinants are located on regions specific for the parasite proteins. Thus, the L. infantum acidic ribosomal proteins, LiP2a and LiP2b, which are recognized by more than 80% of canine VL sera contain disease-specific antigenic determinants (29). In fact, we have demonstrated that engineered LiP2a and LiP2b recombinant proteins can be used as specific tools to distinguish between VL and Chagas’ disease (32). We showed that the L. infantum P0 ribosomal protein is also recognized by a high percentage of the sera from dogs with VL (31). The main antigenic determinant of the LiP0 protein during canine VL is located at the C-terminal end of the protein, a region with low evolutionary conservation. Antibodies reacting against the L. infantum histone H2A were observed in 78% of canine VL sera. Interestingly, despite the high conservation of the histone H2A sequences among eukaryotic organisms, the humoral response against this protein is specifically elicited by the Leishmania histone H2A antigenic determinants. The antigenic determinants of the histone H2A that are recognized by canine VL sera were located at both ends of the protein (30).
In the present work, on the basis of previous knowledge of the B-cell epitopes of the L. infantum antigens LiP2a, LiP2b, LiP0, and H2A, we carried out the assembly of a novel synthetic gene containing the DNA regions coding for the antigenic determinants of these proteins. The gene was expressed in Escherichia coli and the chimeric product was analyzed for its antigenic properties, confirming that this protein could be an excellent serodiagnostic tool for canine VL.
MATERIALS AND METHODS
Sera.
Canine VL sera were obtained from two different regions of Spain. A total of 26 canine VL serum samples were collected in the Extremadura region of Spain. Infected animals were clinically and analytically evaluated at the Department of Parasitology, Veterinary School, Extremadura University, Cáceres, Spain. All sera were positive when tested by indirect immunofluorescence, and the presence of amastigote forms of the parasites was confirmed by direct observation in popliteal and prescapular lymphoid nodes. A second group of 33 canine VL serum samples was from the Mataró Veterinary Hospital (Barcelona, Spain). These sera were diagnosed as positive after an enzyme-linked immunosorbent assay (ELISA) against parasite total extracts and/or by indirect immunofluorescence. Also, sera from dogs affected by diseases other than VL were obtained from the Mataró Veterinary Hospital (44 serum samples) and from the Veterinary School of Extremadura University (5 serum samples). Within this group, sera from dogs with the following infection-causing organisms were used: Mesocestoides spp. (one serum sample), Diphylidium caninum (one serum sample), Uncinaria stenocephala (one serum sample), Toxocara canis (one serum sample), Dipetalonema dranunculoides (one serum sample), Demodex canis (one serum sample), Babesia canis (two serum samples), Ehrlichia canis (three serum samples), and Rickettsia rickettsiae (one serum sample). The rest of the sera were obtained in veterinary surgery from dogs that showed different clinical symptoms that were not associated with demonstrated infectious processes. Finally, control sera were obtained from 15 healthy animals carefully maintained at the Department of Parasitology (Veterinary School, Extremadura University).
Cloning strategy.
The strategy followed for the cloning of the DNA sequences coding for each one of the selected antigenic determinants was the same in all cases (see below for details). In a first step, the sequence of interest was PCR amplified with specific oligonucleotides containing restriction enzyme sites at both ends. In a second step, the PCR product was digested by the appropriate restriction enzyme, cloned into the corresponding restriction site of plasmid pUC18, and sequenced. Afterwards, the insert was recovered and subcloned in the corresponding restriction site of a modified plasmid, pMAL-c2 (New England Biolabs, Inc., Cambridge, Mass.). The modification of the plasmid was done by insertion of a stop translation codon downstream from the HindIII site of the pMAL-c2 polylinker. The resulting plasmid was named pMAL-c2*.
Cloning of DNA sequences coding for the antigenic determinants of histone H2A.
The cDNA clone cL71, coding for the L. infantum histone H2A (28), was used as the template in the PCRs. For the amplification of the DNA coding for the N-terminal region of histone H2A, namely, rLiH2A-Nt-Q, the following oligonucleotides were used: sense, 5′-CCTTTAGCTACTCCTCGCAGCGCCAAG-3′ (positions 84 to 104 of the cL71 sequence); antisense, 5′-CCTGGGGGCGCCAGAGGCACCGATGCG-3′ (reverse of and complementary to positions 204 to 224 of the cL71 sequence). In boldface are those sequences, included for cloning purposes in the oligonucleotides, that are not in the cL71 sequence. This amplified DNA was directly cloned into the XmnI restriction site of the plasmid pMAL-c2* and sequenced with the no. 1237 malE primer (New England Biolabs). In order to express the antigenic C-terminal region of histone H2A, namely, rLiH2A-Ct-Q, the coding DNA was amplified with the following oligonucleotides: sense, 5′-GAATTCTCCGTGAAGGCGGCCGCGCAG-3′ (positions 276 to 296 of the cL71 sequence); antisense, 5′-GAATTCGGGCGCGCTCGGTGTCGCCTTGCC-3′ (reverse of and complementary to positions 456 to 476 of the cL71 plasmid). A proline-encoding triplet (indicated in boldface) was included in the antisense oligonucleotide. Underlined is the EcoRI restriction site that was included in both oligonucleotides for cloning purposes.
Cloning of the rLiP2a-Q and rLiP2b-Q coding sequences.
The cloning strategy for and construction of the rLiP2a-Q- and rLiP2b-Q-expressing plasmids have been described elsewhere (32).
Cloning of the rLiP0-Q-encoding sequence.
Cloning of the DNA sequence coding for the C-terminal region of L. infantum P0 protein, namely, rLiP0-Q, was performed by PCR amplification with the cDNA L27 as template (27) and the following oligonucleotides: sense, 5′-CTGCAGCCCGCCGCTGCCGCGCCGGCCGCC-3′ (positions 1 to 24 of the L27 cDNA), and the 17-mer pUC18 sequencing primer (no. 1211; New England Biolabs). Amplified DNA was PstI-HindIII digested and cloned in the plasmid pMAL-c2, and the resulting clone was named pPQI. Note that the PstI restriction site was included in the sense oligonucleotide (underlined sequence) and that the HindIII restriction site is present in the cDNA sequence of clone L27 (27).
Cloning of the chimeric gene.
The above-described DNA sequences coding for the five antigenic determinants were assembled into a chimeric gene. The starting clone was pPQI, and the coding regions for the antigenic regions LiP2a-Q (the resulting clone was named pPQII), LiP2b-Q (clone pPQIII), LiH2A-Ct-Q (clone pPQIV), and LiH2A-Nt-Q (clone pPQV) were sequentially added. Finally, the insert obtained after SacI-HindIII digestion of the final clone, pPQV, was subcloned in the expression plasmid pQE-31 (Qiagen, Inc., Chatsworth, Calif.), and the resulting clone was named pPQ.
Protein purification.
Purification of the recombinant proteins expressed by the pMAL-c2-derived clones was performed by affinity chromatography on amylose columns according to the supplier’s method (New England Biolabs). Purification of the recombinant protein expressed by clone pQV was performed on Ni-nitrilotriacetic acid resin columns under denaturing conditions according to the method provided by the supplier (Qiagen).
Protein electrophoresis and immunoblot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% polyacrylamide gels was performed under standard conditions (15) in a Mini-Protean system (Bio-Rad Laboratories, Richmond, Calif.). For immunoblot analysis, the electrophoresed proteins were transferred to nitrocellulose membranes (Amersham, Aylesbury, United Kingdom). The transferred proteins were blocked with 5% nonfat dried milk powder in phosphate-buffered saline (PBS)–0.5% Tween 20. The filters were sequentially probed with primary and secondary antisera in blocking solution. A peroxidase immunoconjugate (Nordic Immunology, Tilburg, The Netherlands) was used as secondary antibody, and the specific binding was revealed with the ECL Western blotting detection system (Amersham).
FAST-ELISA measurements.
The Falcon assay screening test-ELISA (FAST-ELISA; Becton Dickinson Labware, Lincoln Park, N.J.) was used instead of the classic ELISA. The coating of the lids was performed overnight at room temperature with 100 μl of the antigen diluted in PBS. The antigen concentration was 2 μg/ml for all the recombinant proteins. Afterwards, the lids were washed three times by immersion in 200 μl of PBS–0.5% Tween 20. After the washing process, the antigen-coated lids were incubated for 1 h with the blocking solution (5% nonfat dried milk powder in PBS–0.5% Tween 20). The sera to be assayed were diluted 1:300 in blocking solution. The lids were immersed in the microtiter plates containing the diluted sera and incubated for 2 h at room temperature with shaking. After exposure to antibody, the lids were washed as described above. As secondary antibody, horseradish peroxidase-labelled antibodies (dilution, 1:2,000) were used. After incubation for 1 h at room temperature and washing, the lids were developed by ortho-phenylenediamine (0.4 mg/ml) as substrate. The absorbance was read at 450 nm.
RESULTS
Cloning of selected epitopes of the L. infantum antigens.
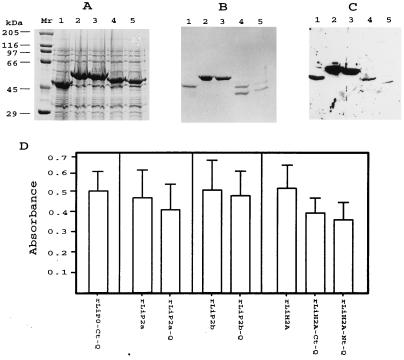
Previous studies allowed us to define the location of the B-cell epitopes from the L. infantum antigenic proteins LiH2A, LiP0, LiP2a, and LiP2b, which are specifically recognized by canine VL sera (29–31). The first goal of this work was the cloning of the relevant antigenic determinants of those proteins, avoiding other regions that could be recognized by sera from diseases other than VL. With specific oligonucleotides and by the PCR-amplification technique, different clones expressing the recombinant proteins rLiP0-Ct-Q, rLiP2a-Q, rLiP2b-Q, rLiH2A-Ct-Q, and rLiH2A-Nt-Q were constructed (see Materials and Methods for cloning details). The recombinant protein rLiP0-Ct-Q corresponds to the C-terminal 30 residues of the ribosomal protein LiP0. The recombinant proteins rLiP2a-Q and rLiP2b-Q, as described elsewhere (32), were derived from the ribosomal proteins LiP2a and LiP2b, respectively. Two subregions of the histone H2A, corresponding to the N-terminal 46 residues (rLiH2A-Nt-Q) and the C-terminal 67 residues (rLiH2A-Ct-Q), were separately expressed. All the recombinant proteins, fused to the maltose-binding protein (MBP), were overexpressed in E. coli (Fig. 1A) and purified by affinity chromatography on amylose columns (Fig. 1B). After the purification process, cleavage products were observed in the lanes containing the recombinant proteins rLiH2A-Nt-Q and rLiH2A-Ct-Q, indicating that those proteins are unstable (Fig. 1B, lanes 4 and 5).
FIG. 1.
Expression, purification, and antigenicity of the engineered Leishmania proteins. (A) SDS-PAGE analysis of the E. coli lysates harboring MBP-LiP0-Ct-Q (lane 1), MBP-LiP2a-Q (lane 2), MBP-LiP2b-Q (lane 3), MBP-LiH2A-Ct-Q (lane 4), and MBP-LiH2A-Nt-Q (lane 5). Lane Mr shows molecular mass markers. (B) SDS-PAGE analysis of the corresponding recombinant proteins after purification through the amylose column. (C) Western blot analysis of the reactivity of three pooled canine VL serum samples (final dilution, 1:100) against the purified proteins. The Western blot was obtained by transfer of a gel similar to that shown in panel B. (D) FAST-ELISA evaluation of the reactivities (means and SDs) of 26 VL serum samples against the purified antigens. All sera were assayed at a 1:300 dilution.
In order to analyze whether the new recombinant proteins are recognized by canine VL sera, a Western blot containing these proteins was exposed to a pool of three canine VL serum samples (Fig. 1C). Interestingly, all recombinant proteins were recognized, and therefore, it could be concluded that the antigenic determinants were maintained in these proteins. Also, the antigenic properties of the engineered proteins were compared with those of parental antigens by FAST-ELISA with a collection of 26 canine VL serum samples (Fig. 1D). The fact that the sera showed similar reactivity values against either the selected regions or the corresponding complete proteins was taken as a demonstration that no alterations in the antigenic epitopes were introduced during cloning procedures.
Construction of a chimeric gene coding for a polypeptide containing all the selected antigenic determinants.
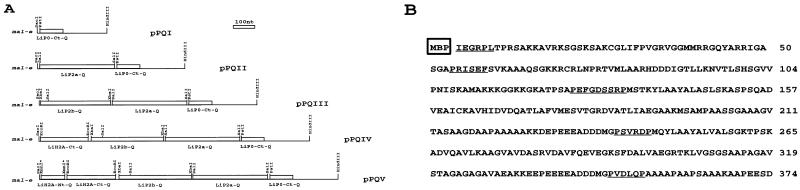
The second goal of the present work was the assembly of the five selected antigenic determinants in a contiguous polypeptide. Figure 2A illustrates the cloning strategy and shows the intermediates generated during the process. The clone expressing rLiP0-Ct-Q (namely, pPQI) was used as the starting clone. By use of the appropriate restriction sites, the DNA inserts coding for LiP2a-Q, LiP2b-Q, LiH2A-Ct-Q, and LiH2B-Nt-Q were added sequentially. After each addition step, the correct orientation of the inserts was deduced from the size of the expression products. Finally, the complete nucleotide sequence of the clone pPQV was determined, and the deduced amino acid sequence is shown in Fig. 2B. The encoded polypeptide has a deduced molecular mass of 38 kDa with an isoelectric point of 7.37. Spacer sequences, containing proline residues (underlined in Fig. 2B), were included to effectively separate the antigenic domains.
FIG. 2.
(A) Diagrammatic representation of the successive clones obtained during the cloning of the chimeric gene. The pMAL-c2 polylinker restriction enzyme cut sites employed in the cloning strategy are indicated. The asterisks at the XmnI sites indicate that these restriction sites were lost after cloning. nt, nucleotides. (B) Deduced amino acid sequence of the chimeric protein. Regions encoded by the linker sequences are underlined. The position of the maltose-binding fusion protein (MBP) is also indicated.
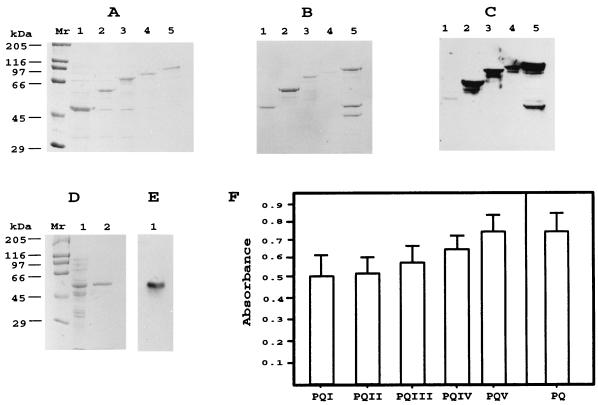
The expression and recovery yields for each of the intermediate products are shown in Fig. 3A and B. As expected, after each addition step, the size of the expression products increased until there was a molecular mass of 80 kDa for the recombinant protein PQV, which includes the 42-kDa MBP moiety. Although the recombinant protein PQV was more stable than proteins rLiH2A-Ct and rLiH2A-Nt (Fig. 1B), a certain cleavage of the protein PQV was evidenced after purification. Also, we cloned the chimeric gene in the plasmid pQE-31, a vector that permits the expression and purification of proteins with only a six-His tag placed in the N terminus. The resulting clone and recombinant protein were named pPQ and PQ, respectively. The expression level of the protein in bacterial cultures transformed by plasmid pPQ and the purification yield are shown in Fig. 3D. Furthermore, the purified protein PQ, obtained by affinity chromatography under denaturing conditions, was found to be more stable than the recombinant protein PQV (Fig. 3B).
FIG. 3.
Expression, purification, and antigenicity of all the intermediates and the final chimeric protein. (A) SDS-PAGE analysis of the E. coli lysates harboring PQI (lane 1), PQII (lane 2), PQIII (lane 3), PQIV (lane 4), and PQV (lane 5). Lane Mr shows molecular mass markers. (B) SDS-PAGE analysis of the corresponding recombinant proteins after purification through the amylose column. (C) Western blot analysis of the reactivity of three pooled canine VL serum samples (final dilution, 1:100) against the purified antigens. The filter was obtained by electrotransfer of a gel similar to that in panel B. (D) SDS-PAGE analysis of the pQE chimeric expression product. Lane 1, lysates of E. coli harboring the PQ protein; lane 2, the PQ protein after purification through a Ni-nitrilotriacetic acid column. Lane Mr shows molecular mass markers. (E) Western blot analysis of the reactivity of a pool of three canine VL serum samples against the purified PQ protein. (F) FAST-ELISA evaluation of the reactivities (means and SDs) of 26 VL serum samples against each one of the intermediate proteins PQI to PQIV and final products PQV and PQ. All sera were assayed at a 1:300 dilution.
The reactivity of canine VL sera against the chimeric protein and against each one of the intermediates was assayed by Western blotting. As expected, the antigenicity of all intermediates, and that of the final product PQV, was maintained throughout the cloning procedure (Fig. 3C). Also, the recombinant protein expressed by plasmid pPQ was recognized by the VL sera (Fig. 3E). In order to more accurately analyze the antigenic properties of the chimeric protein and the intermediate expression products, the reactivity of 26 canine VL serum samples against those recombinant proteins was assayed by FAST-ELISA (Fig. 3F). As expected, both absorbance values and the sensitivity for the different intermediates increased after each addition step. Thus, the protein PQI was recognized by 19 of the 26 VL serum samples; the protein PQII was recognized by 21 serum samples; the protein PQIII was recognized by 22 serum samples; and proteins PQIV, PQV, and PQ were recognized by 23 serum samples. Also, the percentage of recognition (88%) shown by these 26 VL serum samples was similar when assayed against either the chimeric protein (PQV or PQ) or a mixture of recombinant proteins rLiP0-Ct-Q, rLiP2a, rLiP2b, and rLiH2A (data not shown). Altogether, the present data indicate that the antigenic properties of each one of the five selected regions are present in the final expression product PQ and that, therefore, the recombinant protein PQ, instead of a mixture of the individually expressed antigens, can be used for diagnostic purposes.
Sensitivity and specificity of the chimeric protein PQ in serodiagnosis of canine VL.
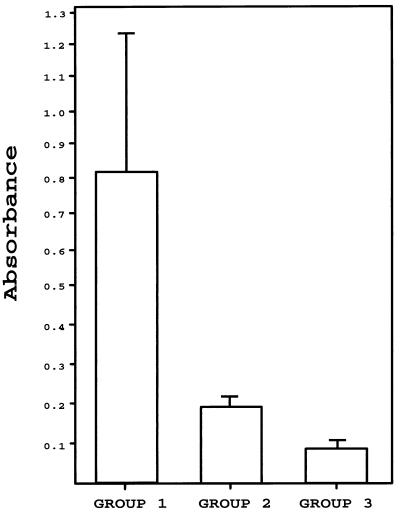
In order to determine whether the chimeric protein could be considered a valuable tool for serodiagnosis of canine VL, we analyzed the reactivity of 123 canine serum samples against this protein. According to the clinical features of donor animals, sera were separated into three groups. The first group was composed of sera from 59 dogs with demonstrated L. infantum infection. The second group was composed of sera from 49 dogs showing different clinical symptoms but no Leishmania infection. In this heterogeneous group, 12 serum samples were from dogs infected with parasites other than Leishmania (see Materials and Methods for more details) and the rest of the sera were from dogs showing clinical symptoms that could be confused with those found during leishmaniasis. The third group was composed of 15 control serum samples from healthy dogs. In Fig. 4 are shown the mean reactivity values for each group of sera. The VL sera (group 1) reacted with a mean absorbance value of 0.8 (standard deviation [SD], 0.4). Within this group, 12 serum samples reacted with values lower than 0.35, 10 serum samples reacted with values between 0.35 and 0.5, 23 serum samples reacted with values between 0.5 and 1, and 14 serum samples reacted with values higher than 1. The mean absorbance value of sera from group 2 was 0.2 (SD, 0.05), and that of control sera (group 3) was 0.1 (SD, 0.003).
FIG. 4.
FAST-ELISA evaluation of the diagnostic value of the recombinant PQ protein. Shown are mean absorbance values (plus SDs) of sera from dogs infected with L. infantum (group 1; n = 59), sera from dogs infected with other pathogens or suffering from diseases other than leishmaniasis (group 2; n = 49), and sera from healthy dogs (group 3; n = 15). Sera were assayed at a 1:300 dilution.
Based on these data (Table 1), it was calculated that the chimeric protein PQ in FAST-ELISAs has a sensitivity of 79% for the diagnosis of VL, the cutoff value being defined as the mean reactivity of sera from group 2 plus 3 SDs (i.e., 0.35). The sensitivity of the assay rises to 93% when the cutoff is defined by using the reactivity values of control sera (group 3). The data indicate, moreover, that the protein PQ had 96% specificity for diagnosis of VL when the cutoff value was defined by the sera from group 2. Only two serum samples from group 2 showed reactivity values slightly higher than 0.35 (in both cases, lower than 0.4). The specificity of the test reached 100% when the reactivity values of sera from healthy animals (group 3) were considered.
TABLE 1.
Diagnostic performance of the recombinant protein PQ
| Group | No. of serum samples
|
Sensitivityb | Specificityc | |
|---|---|---|---|---|
| Tested | Positivea | |||
| 1 (VL sera) | 59 | 47 | 79 | |
| 2 (diseases other than VL) | 49 | 2 | 96 | |
| 3 (healthy controls) | 15 | 0 | 100 | |
Cutoff value was defined as the mean reactivity value of sera from group 2 plus 3 SDs (0.35).
Sensitivity was calculated as the percentage of VL samples that showed reactivity values higher than the cutoff value.
The specificity, defined as the percentage of sera with reactivity values below the cutoff value, was calculated independently for group 2 and group 3.
DISCUSSION
Given the strong humoral response that accompanies leishmanial infections, it is not surprising that tests based on serological techniques are the most frequent method actually used for VL diagnosis (reviewed in reference 14). Furthermore, an additional advantage of serodiagnosis is that results can be obtained rapidly. This feature is particularly important for canine VL, since early detection of infected animals may be critical in controlling the spread of the disease among dogs and potentially between dogs and humans (10, 21, 34). However, due to the fact that actual tests for VL serodiagnosis use crude preparations of leishmanial antigens, the most serious drawback of this method is the specificity. In order to bypass this problem, several laboratories are seeking Leishmania antigen-encoding genes for the development of more specific serodiagnostic tests by the use of recombinant proteins (4, 9, 11, 12, 25).
As a result of our search for L. infantum antigens that are recognized by the sera from dogs with VL, several antigenic proteins were identified: the ribosomal proteins LiP2a and LiP2b (29), the histone H2A (30), and the protein P0 (31). Although those antigens belong to evolutionarily conserved protein families, it was observed that the humoral response elicited in dogs with VL is specifically directed against the parasite proteins. However, given the conserved nature of these proteins, as was demonstrated for the proteins LiP2a and LiP2b (32), they could be recognized by sera from animals suffering from diseases other than VL. Hence, the aim of the present work was the design, assembly, and expression of a novel synthetic gene that contains the antigenic epitopes of those four L. infantum proteins, avoiding the well-conserved regions. Finally, five antigenic determinants were expressed together as a recombinant chimeric protein, namely, PQ, that showed several interesting features. First, high levels of E. coli expression of this novel gene have been achieved and the gene product is stable. Second, the recombinant protein can be purified with ease. Third, the recombinant protein exhibits the antigenic epitopes found in the L. infantum antigens.
On the other hand, we have tested the chimeric protein by FAST-ELISA for serodiagnosis of canine VL. From the study, we determined that such a diagnostic test has a sensitivity of 80% and, what is more important, a specificity of 96%. These parameters were calculated by having as controls the sera from animals either infected by diverse pathogens or presenting clinical symptoms similar to those accompanying leishmaniasis. The assay reaches 93% sensitivity and 100% specificity when the borderline is established with sera from healthy dogs. Thus, we can conclude that the chimeric protein PQ is a highly specific molecule for serodiagnosis of canine VL. Most likely, if convenient, we believe that the addition to this recombinant protein of the antigenic determinants from other L. infantum antigens described recently (3, 23, 33) will be able to increase the sensitivity of the assay based on the protein PQ, without affecting its specificity.
ACKNOWLEDGMENTS
This work was supported by grants I+D 0020/94 from Comunidad Autónoma de Madrid, PTR94-0091 from Plan Nacional de Investigación Científica y Desarrollo, and BIO96-0405 from Programa Nacional de Biotecnología and a CDTI grant to Laboratorios LETI. An institutional grant from the Fundación Ramón Areces is also acknowledged.
REFERENCES
- 1.Abranches P, Santos-Gomes G, Rachamim N, Campino L, Schnur L F, Jaffe C L. An experimental model for canine visceral leishmaniasis. Parasite Immunol. 1991;13:537–550. doi: 10.1111/j.1365-3024.1991.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 2.Abranches P, Silva-Pereira M C D, Conceiçao-Silva F M, Santos-Gomes G M, Janz J G. Canine leishmaniasis: pathological and ecological factors influencing transmission of infection. J Parasitol. 1991;77:557–561. [PubMed] [Google Scholar]
- 3.Angel S O, Requena J M, Soto M, Criado D, Alonso C. During canine leishmaniasis a protein belonging to the 83-kDa heat-shock protein family elicits a strong humoral response. Acta Trop. 1996;62:45–56. doi: 10.1016/s0001-706x(96)00020-4. [DOI] [PubMed] [Google Scholar]
- 4.Badaró R, Benson D, Eulálio M C, Freire M, Cunha S, Netto E M, Pedral-Sampaio D, Madureira C, Burns J M, Houghton R L, David J R, Reed S G. rK39: a cloned antigen of Leishmania chagasi that predicts active visceral leishmaniasis. J Infect Dis. 1996;173:758–761. doi: 10.1093/infdis/173.3.758. [DOI] [PubMed] [Google Scholar]
- 5.Burns J M, Jr, Shreffler W G, Benson D R, Ghalib H W, Badaro R, Reed S G. Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc Natl Acad Sci USA. 1993;90:775–779. doi: 10.1073/pnas.90.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabral M, O’Grady J, Alexander J. Demonstration of Leishmania specific cell mediated and humoral immunity in asymptomatic dogs. Parasite Immunol. 1992;14:531–539. doi: 10.1111/j.1365-3024.1992.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 7.Carrera L, Fermín M L, Tesouro M, García P, Rollán E, González J L, Méndez S, Cuquerella M, Alunda J M. Antibody response in dogs experimentally infected with Leishmania infantum: infection course antigen markers. Exp Parasitol. 1996;82:139–146. doi: 10.1006/expr.1996.0018. [DOI] [PubMed] [Google Scholar]
- 8.de Andrade C R, Kirchhoff L V, Donelson J E, Otsu K. Recombinant Leishmania Hsp90 and Hsp70 are recognized by sera from visceral leishmaniasis patients but not Chagas’ disease patients. J Clin Microbiol. 1992;30:330–335. doi: 10.1128/jcm.30.2.330-335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dillon D C, Day C H, Whittle J A, Magill A J, Reed S G. Characterization of a Leishmania tropica antigen that detects immune responses in Desert Storm viscerotropic leishmaniasis patients. Proc Natl Acad Sci USA. 1995;92:7981–7985. doi: 10.1073/pnas.92.17.7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dye C. The logic of visceral leishmaniasis control. Am J Trop Med Hyg. 1996;55:125–130. doi: 10.4269/ajtmh.1996.55.125. [DOI] [PubMed] [Google Scholar]
- 11.Fargeas C, Hommel M, Maingon R, Dourado C, Monsigny M, Mayer R. Synthetic peptide-based enzyme-linked immunosorbent assay for serodiagnosis of visceral leishmaniasis. J Clin Microbiol. 1996;34:241–248. doi: 10.1128/jcm.34.2.241-248.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gicheru M M, Olobo J O. Evaluation of recombinant gp63, the major Leishmania surface glycoprotein, as a diagnostic molecule for leishmaniasis in vervet monkeys. Acta Trop. 1994;58:345–348. doi: 10.1016/0001-706x(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 13.Gramiccia M, Gradoni L, di Martino L, Romano R, Ercolini D. Two syntopic zymodemes of Leishmania infantum cause human and canine visceral leishmaniasis in the Naples area, Italy. Acta Trop. 1992;50:357–359. doi: 10.1016/0001-706x(92)90072-6. [DOI] [PubMed] [Google Scholar]
- 14.Kar K. Serodiagnosis of leishmaniasis. Crit Rev Microbiol. 1995;21:123–152. doi: 10.3109/10408419509113537. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Lohman K L, Langer P J, McMahon-Pratt D. Molecular cloning and characterization of the immunologically protective surface glycoprotein GP46/M-2 of Leishmania amazonensis. Proc Natl Acad Sci USA. 1990;87:8393–8397. doi: 10.1073/pnas.87.21.8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacFarlane J, Blaxter M L, Bishop R P, Kelly J M. Identification and characterisation of a Leishmania donovani antigen belonging to the 70-kDa heat-shock protein family. Eur J Biochem. 1990;190:377–384. doi: 10.1111/j.1432-1033.1990.tb15586.x. [DOI] [PubMed] [Google Scholar]
- 18.Martínez-Moreno A, Moreno T, Martínez-Moreno F J, Acosta L, Hernández S. Humoral and cell-mediated immunity in natural and experimental canine leishmaniasis. Vet Immunol Immunopathol. 1995;48:209–220. doi: 10.1016/0165-2427(95)05434-8. [DOI] [PubMed] [Google Scholar]
- 19.Marty P, Le Fichoux Y, Giordana D, Brugnetti A. Leishmanin reaction in the human population of a highly endemic focus of canine leishmaniasis in Alpes-Maritimes, France. Trans R Soc Trop Med Hyg. 1992;86:249–250. doi: 10.1016/0035-9203(92)90295-n. [DOI] [PubMed] [Google Scholar]
- 20.Molina R, Amela C, Nieto J, San-Andres M, González F, Castillo J A, Lucientes J, Alvar J. Infectivity of dogs naturally infected with Leishmania infantum to colonized Phlebotomus perniciosus. Trans R Soc Trop Med Hyg. 1994;88:491–493. doi: 10.1016/0035-9203(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 21.Ozbel Y, Turgay N, Ozensoy S, Ozbilgin A, Alkan M Z, Ozcel M A, Jaffe C L, Schnur L, Oskam L, Abranches P. Epidemiology, diagnosis and control of leishmaniasis in the Mediterranean region. Ann Trop Med Parasitol. 1995;89:89–93. doi: 10.1080/00034983.1995.11813018. [DOI] [PubMed] [Google Scholar]
- 22.Pateraki E, Portocala R, Labrousse H, Guedson J-L. Antiactin and antitubulin antibodies in canine visceral leishmaniasis. Infect Immun. 1983;42:496–500. doi: 10.1128/iai.42.2.496-500.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quijada L, Requena J M, Soto M, Gómez L C, Guzman F, Patarroyo M E, Alonso C. Mapping of the linear antigenic determinants of the Leishmania infantum hsp70 recognized by leishmaniasis sera. Immunol Lett. 1996;52:73–79. doi: 10.1016/0165-2478(96)02585-0. [DOI] [PubMed] [Google Scholar]
- 24.Quijada L, Requena J M, Soto M, Alonso C. During canine viscero-cutaneous leishmaniasis the anti-Hsp70 antibodies are specifically elicited by the parasite protein. Parasitology. 1996;112:277–284. doi: 10.1017/s0031182000065793. [DOI] [PubMed] [Google Scholar]
- 25.Shreffler W G, Burns J M, Jr, Badaró R, Ghalib H W, Button L L, McMaster W R, Reed S G. Antibody responses of visceral leishmaniasis patients to gp63, a major surface glycoprotein of Leishmania species. J Infect Dis. 1993;167:426–430. doi: 10.1093/infdis/167.2.426. [DOI] [PubMed] [Google Scholar]
- 26.Skeiky Y A W, Benson D R, Guderian J A, Whittle J A, Bacelar O, Carvalho E M, Reed S G. Immune responses of leishmaniasis patients to heat shock proteins of Leishmania species and humans. Infect Immun. 1995;63:4105–4114. doi: 10.1128/iai.63.10.4105-4114.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soto M, Requena J M, Alonso C. Isolation, characterization and analysis of the expression of the Leishmania ribosomal P0 protein genes. Mol Biochem Parasitol. 1993;61:265–274. doi: 10.1016/0166-6851(93)90072-6. [DOI] [PubMed] [Google Scholar]
- 28.Soto M, Requena J M, Gomez L C, Navarrete I, Alonso C. Molecular characterization of a Leishmania donovani infantum antigen identified as histone H2A. Eur J Biochem. 1992;205:211–216. doi: 10.1111/j.1432-1033.1992.tb16770.x. [DOI] [PubMed] [Google Scholar]
- 29.Soto M, Requena J M, Quijada L, Angel S O, Gomez L C, Guzman F, Patarroyo M E, Alonso C. During active viscerocutaneous leishmaniasis the anti-P2 humoral response is specifically triggered by the parasite P proteins. Clin Exp Immunol. 1995;100:246–252. doi: 10.1111/j.1365-2249.1995.tb03661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soto M, Requena J M, Quijada L, García M, Guzman F, Patarroyo M E, Alonso C. Mapping of the linear antigenic determinants from the Leishmania infantum histone H2A recognized by sera from dogs with leishmaniasis. Immunol Lett. 1995;48:209–214. doi: 10.1016/0165-2478(95)02473-5. [DOI] [PubMed] [Google Scholar]
- 31.Soto M, Requena J M, Quijada L, Guzman F, Patarroyo M E, Alonso C. Identification of the Leishmania infantum P0 ribosomal protein epitope in canine visceral leishmaniasis. Immunol Lett. 1995;48:23–28. doi: 10.1016/0165-2478(95)02436-0. [DOI] [PubMed] [Google Scholar]
- 32.Soto M, Requena J M, Quijada L, Alonso C. Specific serodiagnosis of human leishmaniasis with recombinant Leishmania P2 acidic ribosomal proteins. Clin Diagn Lab Immunol. 1996;3:387–391. doi: 10.1128/cdli.3.4.387-391.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soto M, Requena J M, Quijada L, Gomez L C, Guzman F, Patarroyo M E, Alonso C. Characterization of the antigenic determinants of the Leishmania infantum histone H3 recognized by antibodies elicited during canine visceral leishmaniasis. Clin Exp Immunol. 1996;106:454–461. doi: 10.1046/j.1365-2249.1996.d01-865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tesh R B. Control of zoonotic visceral leishmaniasis: is it time to change strategies? Am J Trop Med Hyg. 1995;52:287–292. doi: 10.4269/ajtmh.1995.52.287. [DOI] [PubMed] [Google Scholar]
- 35.Tolson D L, Jardim A, Schnur L F, Stebeck C, Tuckey C, Beecroft R P, Teh H-S, Olafson R W, Pearson T W. The kinetoplastid membrane protein 11 of Leishmania donovani and African trypanosomes is a potent stimulator of T-lymphocyte proliferation. Infect Immun. 1994;62:4893–4899. doi: 10.1128/iai.62.11.4893-4899.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]