Abstract
The liver is a central hub in lipid, carbohydrate and protein metabolism and protects against gut-derived antigens and toxins. The etiology of liver diseases includes altered metabolism, viral infections, autoimmunity, toxins and genetic alterations. Liver-resident cells, including hepatocytes, biliary epithelial cells, endothelial cells, and hepatic stellate cells, are essential for liver function and homeostasis but may also drive the development of inflammation, fibrosis, cirrhosis and liver cancer via interactions with immune cells. This review highlights the often-underappreciated contributions of epithelial, endothelial and mesenchymal liver cells in regulating inflammation and immunity across various liver diseases, emphasizing their importance in disease onset, progression and regression. Immune cells and their mediators also play a role in stimulating liver regeneration and repair following injury. Recent findings on the bidirectional interactions between immune cells and resident liver cells provide deeper insights into the underlying pathophysiology and identify novel therapeutic targets for the treatment of liver disease.
Keywords: Cholangiocytes, liver fibrosis, liver cancer, NAFLD, MASLD, MASH, PSC, PBC
Subject terms: Immunology, Cell biology
Introduction
Foreword
This review highlights the inflammation-related pathogenic mechanisms involved in liver disease initiation and progression from a nonimmune cell perspective. This manuscript reviews recent findings on the immune-modulatory functions of hepatocytes, cholangiocytes or biliary epithelial cells (BECs), hepatic stellate cells (HSCs), and liver endothelial cells (LECs). First, we briefly describe central molecular pathways that drive liver organogenesis but can also be reactivated upon liver injury to stimulate organ repair or, if injury persists, may promote disease progression. Next, we elaborate on the known roles of liver epithelial and stromal cells in modulating disease-driving and inflammation-related cellular responses. Finally, we discuss recent state-of-the-art technologies that will further improve our understanding of how interactions between parenchymal and nonparenchymal cells regulate inflammation and immunity in the liver and hold promise for tackling liver disease from novel angles.
Liver development
The intricate cellular crosstalk networks in the liver take root during embryogenesis, and some of the molecular pathways involved in organogenesis have been found to be reactivated upon liver disease onset, wound repair, and cancer development. Liver development is initiated from the foregut endoderm, which is part of the embryonic digestive tube. Signals such as fibroblast growth factors (FGFs) and bone morphogenetic proteins (BMPs), derived from the cardiac mesoderm and the septum transversum mesenchyme, initiate liver development [1–4]. In addition, the anterior foregut endoderm secretes wingless-related integration site (Wnt) antagonists to inhibit Wnt signaling, allowing for hepatic specification [5]. As the liver grows, cells differentiate into hepatoblasts, which are precursors of both hepatocytes and cholangiocytes or biliary epithelial cells (BECs). By default, hepatoblasts engage in a differentiation path toward hepatocytes. In the portal area, mesenchymal cell-derived signals drive hepatoblasts toward the biliary lineage in mice [6, 7].
During embryonic stages, primitive vascular structures and sinusoids along with early bile ducts guide hepatic cell migration in the liver [8], suggesting a crucial role for endothelial cells during liver development. Indeed, angiocrine signals control liver embryonic growth between E11.5 and E13.5 through the regulation of the Wnt and Notch pathways [9, 10]. During prenatal development, the hemogenic endothelium gives rise not only to endothelial cells but also to hematopoietic stem cells, which seed the fetal liver and contribute to erythro-myeloid hematopoiesis during development and early postnatal stages [11]. The niche that maintains hematopoietic stem cells in the fetal liver is largely supported by stem cell factor (SCF), which is expressed by endothelial cells and hepatic stellate cells [12]. Deletion of SCF in both endothelial cells and hepatic stellate cells leads to nearly complete loss of hematopoietic cells and early lethality in mice [12]. Some mechanisms involved in the close interactions between endothelial cells and hepatic stellate cells during development may also affect these interactions during disease. During postnatal development in mice, there is a distinct subcluster of Kupffer cell-derived Decorin (Dcn)+ macrophages that express liver EC markers such as Cd31, Kdr and Lyve1, as suggested by a recent single-cell RNA sequencing (scRNA-seq) study [13]. These Dcn+ macrophages closely interact with liver sinusoidal endothelial cells (LSECs) through several VEGF-related receptor/ligand pairs and with Tregs through CXCL12-CXCR3/CXCR4 signaling, as shown by CellPhoneDB [13], suggesting a role for endothelial cell/immune cell crosstalk during postnatal liver development.
The cellular landscape of the healthy adult liver
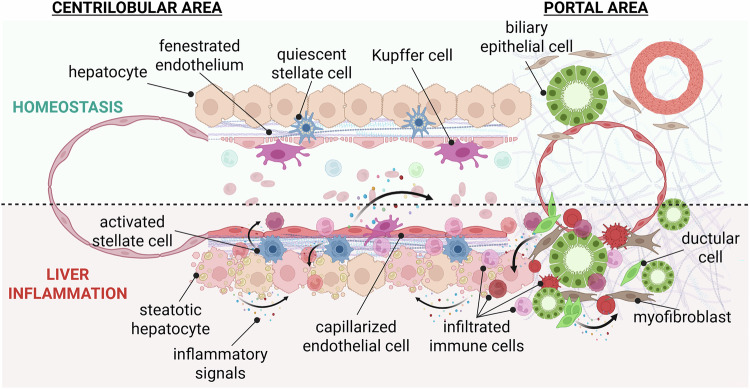
Hepatocytes represent approximately 80% of the liver mass and 60% of all liver cells and are organized as a monolayer of cells along the portal‒centrilobular vein axis. Hepatocytes are classically regarded as the key epithelial cell type in the liver and are responsible for most liver functions, including glucose, lipid, protein, and bile acid metabolism as well as detoxification [14]. Biliary epithelial cells, often also termed cholangiocytes, account for ~3–5% of all liver cells. They line the bile ducts and are mostly studied for their roles in transporting and regulating the composition of bile, which is produced by hepatocytes, stored in the gallbladder, and released into the intestinal lumen [15]. Liver sinusoidal endothelial cells (LSECs) form a fenestrated endothelium that lines the liver blood sinusoids and allows for the circulation of solutes and particles between the vascular compartment and the hepatocyte layers. HSCs are a quiescent mesenchymal cell population in the healthy liver and are best known for their function in most of the body’s vitamin A storage in the form of retinyl esters in their characteristic lipid droplets [16, 17]. Increasing evidence points toward important roles of LSECs and HSCs in the regulation of hepatocyte zonation and metabolic functions via the WNT pathway [18, 19]. The healthy liver also contains Kupffer cells as a large resident immune cell population [20]. Since two-thirds of the hepatic supply is via the portal vein, the liver is constantly exposed to gut-derived nutrients, bacteria, and bacterial components. Kupffer cells act as a firewall against bacterial infection [21] and clear blood from lipopolysaccharides (LPS) [22]. In addition to Kupffer cells, hepatocytes, LSECs, and HSCs also possess important immune-sensing capacities via their expression of Toll-like receptors [23, 24], and their response potential to environmental cues contributes to the hepatic response to pathogenic stimuli. The diseased liver is characterized by profound changes in epithelial, mesenchymal, and stromal compartments and is accompanied by a strong accumulation of immune cells (Fig. 1) [25, 26].
Fig. 1.
Schematic view of liver histology in homeostasis and upon liver inflammation. The liver microenvironment undergoes drastic alterations upon liver inflammation, which are driven by intricate cellular crosstalk pathways, including the release of damage- and stress-induced inflammatory cytokines or molecular patterns by liver epithelial, stromal, and mesenchymal cells. This figure displays a schematic representation of the cellular organization along the porto-central axis during homeostasis and upon steatotic liver disease, as an example of drastic alterations in the liver cell landscape upon tissue inflammation
Interactions of epithelial liver cells with immune cells in liver disease
Hepatocytes
Hepatocytes are mature epithelial cells responsible for most synthetic functions of the liver; these cells have a wide range of systemic effects and affect organismal health. Accordingly, hepatocyte injury and stress can contribute to both local and systemic inflammation through the release of damage-associated molecular patterns (DAMPs), cytokines, and acute phase proteins (APPs). The involvement of these proteins in a wide range of metabolic processes as well as the receptor systems and enzymes needed to import, export, and metabolize substrates and toxins renders hepatocytes a common “victim” in liver injuries caused by a wide range of etiologies, including metabolic, toxic, biliary, and viral liver diseases. Owing to their central position in the organ’s functions and architecture, hepatocytes are fundamentally interconnected with surrounding cells. Following liver injury, hepatocytes, together with surrounding cells, exert a profound influence on inflammatory and immune responses and change and often overcome the normally immunotolerant hepatic environment. Accordingly, hepatocyte–immune interactions actively participate in virtually all steps of liver diseases, from initial hepatitis to progression toward fibrosis, cirrhosis, and cancer. Conversely, immune‒hepatocyte interactions aid in liver regeneration and the restoration of liver architecture after liver injury [27].
Hepatocytes drive systemic and local immune responses via acute-phase proteins
The liver plays a central role in the regulation of immune defenses during systemic infections, with roles in bacterial sensing and clearance, APP and cytokine production, and metabolic adaptation to inflammation [28]. Following infection or sterile injury, the liver releases up to 30 APPs into the systemic circulation [28, 29]. The APP response involves the crosstalk of multiple liver cell types with a central role in hepatocytes. Monocytes and macrophages, which sense and trap the majority of bacteria and bacterial pathogen-associated molecular patterns (PAMPs), trigger the production of APPs in hepatocytes via inflammatory cytokines such as interleukin (IL)-1 and IL-6 and the downstream mediators NF-κB and STAT3 [28–31]. Consistent with their function as the primary synthetic cell type in the liver, hepatocytes are the predominant producers of major APPs, including C-reactive protein (CRP), serum amyloid A (SAA), serum amyloid P, complement factors, ferritin, and lipopolysaccharide binding protein [28, 32]. Most of these hepatocyte-produced APPs are key regulators of systemic inflammation and immunity [33] but can also act locally, thereby affecting the development of liver disease. Via APPs, hepatocytes directly contribute to extracellular pathogen clearance via complement-mediated antigen opsonization [34–36]. Importantly, complement cascade activation leads to potent immune responses, and when inappropriate, this can have severe consequences for the organism [37]. Notably, recent studies have investigated the implications of the complement system in liver diseases. C3 complement protein is increased in human metabolic dysfunction–associated steatohepatitis (MASH) livers, and the activation of the alternative complement pathway is associated with disease hallmarks [38]. In addition, C5a levels are increased in patients with liver ischemia after transplantation, but its potential role in ischemia‒reperfusion injury and liver transplant rejection remains under debate [39–42]. Moreover, C5 inhibition is protective in mouse models of acute liver failure, presumably by reducing proinflammatory immune cell recruitment and thrombosis [43]. Similarly, C3 inhibition or a complement receptor CD59 inhibitor improved ischemia‒reperfusion injury, and CD59 inhibition also drastically improved liver regeneration and survival after 90% partial hepatectomy in mice, along with increased TNF-α and IL-6 levels [44]. SAA proteins constitute another group of APPs with key roles in host defense and inflammation [34]. Its two human isoforms, SAA1 and SAA2, are predominantly produced by hepatocytes and contribute to systemic immune responses in sepsis but also in a range of other inflammatory conditions, such as arthritis [34, 45]. In liver injury induced by acetaminophen, SAA1 and SAA2 increase hepatocyte and LSEC damage, thus potentiating acetaminophen-induced microvascular dysfunction [46]. SAA1 may also increase concanavalin-A-induced liver inflammation and injury via a Toll-like receptor (TLR)2-dependent pathway by potentiating Th17 lymphocyte and monocyte activation [47]. The roles of APPs remain diverse and can also drive anti-inflammatory immune responses. As such, the secretion of SAA1 and SAA2 by hepatocytes inhibits dendritic cell differentiation and thus limits T-cell activation and infiltration in extrahepatic cancers [48]. Accordingly, pancreatic adenocarcinoma patients with lower levels of SAA have higher circulating T-cell counts and longer survival after surgery [48]. Similarly, the IL-6/STAT3 pathway leading to SAA production drives the establishment of a prometastatic niche in the liver [49]. Moreover, together with CXCL1, SAA contributes to the recruitment of anti-inflammatory CD11b+Gr1+ myeloid-derived suppressor cells (MDSCs) in a mouse model of polymicrobial sepsis, leading to innate immune response control and increased survival via the gp130–STAT3 signaling pathway [50]. CRP and amyloid P are APPs of the pentraxin family that are predominantly secreted by hepatocytes and increase 1000-fold following infection or injury [35, 36]. CRP primarily binds to phosphocholine (present on the surface of bacteria and necrotic and apoptotic cells) and C1, driving complement activation, immune responses, and the removal of dead cells [35, 36]. The fact that deletions of CRP have not been reported in patients suggests an essential role in humans [36]. It has been suggested that the ability of CRP and other pentraxins, such as serum amyloid P, to aid in the removal of dead cells may prevent autoimmunity [35, 51]. C-reactive protein (CRP) attenuates complement-driven liver injury in CCl4- and acetaminophen-induced liver injury models [52, 53]. Similarly, serum amyloid P has been shown to reduce CCl4-induced liver injury and the development of metabolic dysfunction-associated steatotic liver disease (MASLD) in mice [54, 55]. High serum concentrations of lipopolysaccharide binding protein (LBP) are associated with decreased survival in patients with decompensated cirrhosis, and Lbp knockdown increases liver inflammation in mice fed a methionine- and choline-deficient (MCD) diet [56, 57]. Alpha-1-acid glycoprotein (AGP) or orosomucoid 1 (ORM1) is another long-known APP secreted by hepatocytes, and its serum levels increase drastically in a number of systemic or extrahepatic inflammatory disorders, with diverse and sometimes opposing effects on immune cells [58, 59]. Its variant, ORM2, has recently attracted significant interest because its decrease is associated with poor prognosis in patients with HCC and metastasis [60, 61]. Similarly, ORM2 levels in the livers and plasma of patients with MASLD as well as in MASH model mice are lower than those in the corresponding controls [62, 63]. In those studies, ORM2 was shown to protect hepatocytes from steatosis while increasing portal inflammation in murine in vivo and in vitro models. Taken together, the secretion of APPs by hepatocytes illustrates the central role of the liver in controlling systemic inflammation and immunity, with potent anti- and proinflammatory effects depending on the disease- and time-specific context. In summary, while most effects of hepatocyte-secreted APPs are systemic, facilitating the clearance of infections and cellular debris after sterile injury, they may also play a role in liver diseases.
Immune cell mediators stimulate hepatocyte proliferation and liver regeneration following liver injury
While immune cell‒hepatocyte interactions contribute to the pathogenesis of liver injury and disease, they also drive the restoration of liver mass and function after injury ceases. Many immune‒hepatocyte interactions are likely involved in the elimination of infected, pathogenic, stressed, or dying hepatocytes while promoting the expansion of surviving healthy hepatocytes. These regeneration-promoting effects have been most clearly shown in models of liver regeneration following partial hepatectomy. Kupffer cells and bone marrow-derived macrophages and their mediators, such as TNF and IL-6, play key roles in liver regeneration, as demonstrated by knockout, depletion, and bone marrow transplantation studies [64–70]. Crosstalk between hepatocytes and macrophages, involving metabolic reprogramming of bone marrow-derived macrophages, promotes hepatocyte proliferation in a WNT3–YAP-dependent manner [71]. In addition to Kupffer cells and bone marrow-derived macrophages, peritoneal macrophages can also contribute to the removal of necrotic cells and tissue repair following heat- or CCl4-induced liver injury, which delays wound healing after macrophage depletion [72]. The absence of NK and NKT cells but not NK cells alone reduces liver regeneration [73]. Moreover, the activation of NK cells by TLR3 ligands such as poly I:C may also delay liver regeneration [74]. Furthermore, γδT cells promote liver regeneration via IL-17A [75]. Additional immune cell types, such as dendritic cells and innate lymphoid cells, may also contribute to liver regeneration [27].
Stressed hepatocytes drive steatotic liver disease-induced inflammation
As a central regulator of lipid, glucose, and protein metabolism, the liver is highly susceptible to changes in energy intake and body composition. Accordingly, obesity and diabetes are closely associated with the development of metabolic dysfunction-associated steatotic liver disease (MASLD). The increasing incidence of MASLD points toward the urgent need for a better understanding of the implications of dysregulated metabolism and lipotoxicity [76, 77]. In the liver, excess lipids lead to the development of steatosis and oxidative stress [77]. Ultimately, chronic hepatocyte injury and cell death lead to the release of DAMPs that trigger liver inflammation or carcinogenic pathways in the liver [78]. These signals directly initiate and potentiate an immune response, which is often regarded as a detrimental actor in liver diseases. A landmark study recently reported the presence of CXCR6+ CD8+ T lymphocytes, which are autoaggressive via a mechanism distinct from antigen-driven cytotoxicity, in MASH mice and patient samples, thus providing further insights into disease-driving mechanisms [79]. Interestingly, efferocytosis of hepatocyte-derived debris by SIRPα+ liver macrophages (both KC-derived and monocyte-derived) represses the onset of liver fibrosis [80, 81]. This mechanism of immune response regulation is further enhanced when the expression of the hepatocyte “don’t eat me” molecule CD47 is blocked with a specific IgG, which also leads to a downregulation of fibrosis-related gene expression and collagen deposition in a mouse model of MASLD [80]. In the present study, mice fed the HF-CDAA diet for 6 weeks and treated with an anti-SIRPα antibody for another 6 weeks presented increased efferocytosis of hepatocyte-derived necrotic bodies, decreased collagen deposition, and a reduced α-SMA+ area and Opn+ stained areas.
In patients with chronic alcohol-associated liver disease (ALD), acute-on-chronic severe liver injury or alcohol-associated hepatitis (AH) may occur in rare cases. In ALD, hepatocyte cell death results in the release of DAMPs that, together with gut-derived PAMPs, activate surrounding immune cells and serve as recruiting signals for circulating immune cells [82, 83]. In addition, in mouse models of ALD and patients with AH, hepatocytes overexpress macrophage migration inhibitory factor (MIF), and higher levels of MIF in patient suprahepatic serum are associated with increased markers of liver injury and slightly yet significantly increased mortality [84]. The authors suggested that this detrimental role of MIF may be related to its chemotactic functions. Neutrophils are also potent inducers of hepatocyte injury in AH through the release of reactive oxygen species (ROS) [85] and via degranulation [86]. Similarly, higher levels of hepatic IL-8 and CXCL5 expression are associated with poor survival in AH patients [87]. However, recent data suggest that IL-8 is produced mainly by neutrophils in AH [88]. Moreover, the hepatocyte endoplasmic reticulum (ER) stress-related transcription factor ATF4 leads to increased LECT2 expression in neutrophils, which is key in neutrophil-induced liver injury and neutrophil extracellular trap (NET) formation in ALD [89]. However, it has also been suggested that neutrophils can be restorative and promote liver regeneration, and the disease-promoting versus pathogenic roles of neutrophils [90, 91] are likely time-, context- or subpopulation-dependent.
Antigen and lipid presentation by hepatocytes drive immune cell activation
Compared with other organs, the liver is characterized by an immunotolerant environment, highlighted by a high rate of chronic viral infections [92] as well as successful transplantation despite major histocompatibility complex (MHC) mismatch [93]. Although hepatocytes can present antigens to T cells through MHC-I [94, 95] and trigger antiviral immune responses after murine hepatitis virus B infection [94, 96, 97], the efficacy of local priming in the liver for virus-specific CD8 + T-cell immunity is low, often leading to dysfunctional CD8 + T cells [98, 99]. This may be due to the absence of survival signals such as IL-2 [98, 100], Bim-mediated lymphocyte death [101], and the ability of hepatocytes to eliminate CD8 + T cells by engulfment in a process termed suicidal emperipolesis [94, 99]. Suicidal emperipolesis has been investigated primarily in the context of autoreactive T cells in autoimmune hepatitis but may also contribute to tolerogenic environments in other settings, such as viral hepatitis [94, 99]. Efficient antiviral immune responses may require priming by Kupffer cells and/or secondary lymphoid tissues by professional antigen-presenting cells (APCs) rather than hepatocytes and subsequent migration of cytotoxic effector CD8+ T cells to the liver [98, 99, 102]. While some infections with hepatotropic viruses, such as HCV, often become chronic, others can be efficiently cleared, such as infection with HBV in adulthood [103]. In addition to the induction of efficient CD8+ T-cell responses via professional APC priming, viral clearance is aided by the high sensitivity of infected hepatocytes to TNF-dependent CD8+ T-cell-induced cell death and allows the clearing of large numbers of infected hepatocytes by a relatively small number of CD8+ T cells (outnumbered 100--1000) [99, 104]. Hepatocytes also present lipid antigens via CD1d, which leads to the activation of natural killer T (NKT) cells in the context of HBV infection [105]. The absence of NKT cells, CD1d, or a defective transfer of lipids onto CD1d leads to diminished HBV-specific T and B-cell responses and delayed viral control [105]. In some settings, such as infection with the parasite Schistosoma japonicum, hepatocyte CD1d is downregulated, which may exacerbate liver disease [106]. Accordingly, AAV8-mediated Cd1d overexpression results in decreased NKT cell numbers; downregulation of IL-13, IL-4, and IFN-gamma; and reduced liver damage in this model [106]. Moreover, hepatocytes aberrantly express the major histocompatibility class II (MHC-II) transactivator, known as CIITA, when exposed to IFN-gamma and during hepatitis B virus infection as part of a protection mechanism against HBV replication [107, 108]. Hepatocytic MHC-II expression has also been detected in alcohol-associated hepatitis (AH), primary sclerosing cholangitis (PSC), and AIH patient samples [109, 110]. However, this unexpected MHC-II expression by hepatocytes has only been described in a few studies and needs further robust validation [111]. Ultimately, characterizing the peptides presented by hepatocytes and leading to an immune response holds promise for patients affected by diseases such as viral hepatitis or hepatocellular carcinoma [106]. Hepatocytes are equipped with many innate immune sensors, which represent the first line of defense against any pathogen [92]. Innate immunity is largely based on pattern recognition receptors (PRRs) [112]. Hepatocytes functionally express a wide range of PRRs that recognize pathogen-associated molecular patterns from viruses and bacteria in different cellular compartments. These include RIG-I-like receptors (RLRs), retinoic acid inducible gene-I (RIG-I), melanoma differentiation antigen 5 (MDA5), Nod-like receptors (NLRs), and Toll-like receptors [92, 113]. PRR activation leads to inflammatory and antiviral responses, particularly the expression of interferons and hundreds of interferon-stimulated genes [92]. Moreover, it drives the recruitment of adaptive immune cells and enhances adaptive immune responses [114]. While these responses are effective for many viral and bacterial infections, hepatitis C virus (HCV) often causes chronic infections. Although hepatocytes sense PAMPs from HCV and mount an initial innate immune response, HCV proteins such as NS3/4a can inhibit innate immune responses by proteolytically targeting MAVS (essential for RIG-I signaling) and TRIF (essential for TLR3 signaling) [92]. The resulting moderate activation of PRRs and innate immunity is associated with viral persistence [92]. Accordingly, a high dose of interferon alpha can lead to HCV clearance in acute or chronic HCV infection [115]. Hepatocytes also express TLRs, including TLR4, that detect bacterial PAMPs such as LPS. Using hepatocyte-specific knockout approaches, TLR4 on hepatocytes has been shown to trigger innate immune responses that contribute to MASLD and MASLD-induced liver fibrosis [116, 117]. While TLR4 signaling is implicated in the development of alcohol-related liver disease and other forms of liver fibrosis [23, 118], the specific role of hepatocyte-expressed TLR4 needs to be further investigated in these diseases.
Hepatocytes influence immune cell activation profiles in cholestatic liver disease via bile acid-derived signals
Hepatocytes represent the primary source of bile acids, which are involved in numerous interorgan and cell‒cell crosstalk pathways as well as immunity and are toxic to hepatocytes and many other cell types [119]. Hepatocyte-specific deletion of Fxr, the main receptor for bile acids, results in delayed hepatocyte proliferation following partial hepatectomy [120]. FXR has been termed a “guardian” of hepatic function, as one of its main functions is to lower the levels of bile acids by converting them into cholesterol and secretion into bile [121]. The absence of FXR leads to increased bile acid levels and the development of hepatocellular carcinoma [122]. Moreover, the interruption of bile acid reuptake by hepatocytes via pharmacological NTCP blockage alleviates acetaminophen-induced acute liver injury [123]. High bile acid levels can indirectly trigger inflammation and immune cell recruitment [124, 125]. For example, cholic acid (CA)-treated HepG2 cells generate proinflammatory signals through increased RIPK3 activity, leading to cell death and IL-8 release [126]. Defects in the bile salt export pump Bsep reduce LPS clearance in mice, suggesting the importance of bacterial compound elimination through the bile [127]. Recently, a study in cholestatic mice with hepatocyte-specific Stard1 deletion demonstrated that STARD1 is key in sustaining excessive bile acid synthesis by preventing the reduction in CYP27A1-mediated bile acid synthesis via the mitochondrial pathway, thereby sensitizing hepatocytes to cytotoxicity and ultimately potentiating liver inflammation [128]. Runt-related transcription factor-1 (RUNX1) levels are increased in mice and patients with cholestasis, and liver-specific Runx1-deficient mice subjected to bile duct ligation as well as mouse and human cell cultures revealed that RUNX1 is involved in bile acid-induced Ccl2 and Cxcl2 expression by hepatocytes through a signaling pathway that remains to be identified [129]. In addition to these proinflammatory effects, bile acids also exert anti-inflammatory effects mediated by FXR- and TGR5-mediated inhibition of the NLPR3 inflammasome [130–132].
In addition to indirectly modulating inflammation and immune cell recruitment (reviewed in [124, 125]), bile acids also direct immune cell activation profiles via specific receptors such as TGR5 (encoded by GBAR1) and FXR [130]. TGR5 and FXR are expressed by Kupffer cells, hepatic bone marrow-derived macrophages, dendritic cells, and NKT cells [130]. However, data from mice with conditional knockout of TGR5 and FXR in immune cells revealed increased inflammation, suggesting that these bile acid-activated receptors have anti-inflammatory effects on the immune system [130, 133, 134]. Moreover, secondary bile acids such as 3β-hydroxydeoxycholic acid have been shown to act directly on DCs to diminish their immunostimulatory properties and increase the differentiation of T regulatory cells [135]. In summary, the predominant direct effects of bile acids on the immune compartment appear to be suppressive.
Hepatocytes participate in shaping the immune microenvironment in liver cancer
Primary liver cancers are the 3rd leading cause of cancer-related deaths worldwide [136]. Owing to the high mortality rate, there is a critical need for a better understanding of the pathomechanisms leading to cancer initiation and progression. Current trends show a transition from chronic viral hepatitis to metabolic and alcohol-related liver cancers in many countries. Hepatocellular carcinoma (HCC) represents approximately 90% of primary liver cancers [137]. A vicious cycle of chronic inflammation, injury and inflammation, present in chronic liver diseases such as viral hepatitis, alcohol use disorders, or MASLD, is a key driver of hepatocarcinogenesis, contributing to the accumulation of mutations in hepatocytes and their transformation [138]. Studies in mice have demonstrated that chronic inflammation, e.g., driven by the overexpression of lymphotoxin in hepatocytes [139], and chronic cell death, e.g., by the deletion of MCL-1, NEMO or TAK1, are sufficient to trigger HCC development [140–142]. While chronic inflammation can contribute to hepatocarcinogenesis, the immune system also plays a key role in restraining the development of HCC by removing senescent and transformed hepatocytes and cancer cells [143–145]. Numerous checkpoint proteins, including PD-1/PD-L1, CTLA4, LAG3, and TIM3, are involved in the tumor-immune synapse and the modulation of antitumor immune responses in HCC [144]. Checkpoint molecules such as PD-L1, which are expressed by tumor-associated macrophages and cancer cells, bind to PD-1, which is expressed by T cells, and deactivate it, thus preventing antitumor adaptive immune responses [146]. Accordingly, combinations that include checkpoint inhibitors targeting PD-1/PD-L1 and CTLA4 represent current first-line therapies for HCC [144]. Growth differentiation factor-15 (GDF-15) represents another, largely hepatocyte- and tumor cell-expressed cytokine that is increased in MASLD [147] and suppresses antitumor immunity [148]. The anti-GDF-15 antibody visugromab has been evaluated in combination with anti-PD-1 therapy in patients with anti-PD-1/PD-L1 refractory tumors [148]. Erythropoietin (EPO) represents another tumor cell-derived mediator that suppresses antitumor T-cell responses in HCC by interacting with the EPO receptor on tumor-associated macrophages [149]. Accordingly, HCC patients with high expression of EPO have worse survival [149]. It is likely that additional hepatocyte- and tumor cell-expressed mediators contribute to a changed immune environment and the suppression of immunity in injured and tumor-bearing livers, promoting hepatocarcinogenesis or tumor progression, respectively.
Hepatocytes as immune cells that target autoimmune hepatitis
Autoimmune hepatitis (AIH) is a chronic liver disease primarily characterized by the detection of autoantibodies, progressive hepatocellular necrosis and inflammation, leading to liver fibrosis and, ultimately, cirrhosis. Its prevalence is three times higher in females than in males [150, 151]. The immune mechanisms leading to disease progression have been studied in detail and mostly rely on the adaptive immune response against hepatocyte-expressed antigens and defects in tolerogenic pathways [152, 153]. Recent insights have further highlighted the implications of B cells in amplifying disease-driving pathogenic immune responses [154]. The initial causes and impacts of environmental cues remain to be determined, although genetic factors have been identified in some cases [150].
Biliary epithelial cells
Biliary epithelial cells (BECs) and their derivatives, often referred to as ductular cells in liver diseases, play a key role in many liver diseases, as the transport of bile to the intestine is a central liver function that needs to be preserved to avoid toxic effects on the liver. Moreover, their microanatomic niche predisposes BECs to interact with many other cell types, including immune cells, and renders the periductular area a common site of inflammation [155–157]. Earlier studies revealed developmental and functional differences between intra- and extrahepatic BECs and between large and small BECs [15]. Moreover, BECs and ductular reactions have sparked interest as potential reservoirs of alternative liver progenitor cells, a phenomenon that also relies on the participation of innate and adaptive immune cells [158–160]. Similarly, more recent findings further elaborate on the hepatocyte-cholangiocyte phenotypical plasticity that takes place during liver regeneration [161]. Furthermore, an increasing amount of data highlights BECs as both targets and active potentiators of immune responses in the liver. Recent technological developments, such as single-cell transcriptomic and proteomic analyses along with novel algorithms to explore complex data, have provided deeper insights into rarer cell populations, such as BECs, in the context of animal models and human biliary diseases [162–166]. Monocytes and macrophages are key partners of reactive BECs, as exemplified by studies indicating that macrophage-derived TWEAK is one of the initiating signals for ductular cell proliferation via the Fn14 receptor [167, 168]. This privileged cellular crosstalk is supplemented by potent interactions between BECs and, notably, B lymphocytes and monocytes-macrophages, as detailed below.
There is interest in understanding the inflammatory mechanisms involved in cholangiopathies
As the archetype of BEC-centered pathologies, cholangiopathies are of distinct genetic, environmental, or idiopathic nature. Cholangiopathies include primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), liver cystic fibrosis, biliary atresia, and cholangiocarcinoma. Much remains to be discovered concerning the mechanisms driving both the initiation and progression of these relatively rare conditions. Nonetheless, in the past decade, substantial progress has been made in both understanding cholangiocyte biology and its role in liver diseases [169]. Recently, in an elegant spatially resolved transcriptome analysis of a mouse model of ductular reaction (3,5-diethoxycarbonyl-1,4-dihydrocollidine, or DDC diet), during injury, cholangiocytes represented a major cell crosstalk hub in the portal area through predicted interactions with virtually all immune and parenchymal cells in their surroundings [170]. Cholangiocytes are particularly active in expressing chemoattraction-related genes and are suggested to inhibit hepatocyte-driven liver regeneration through TGF-β2 and Atoh8 expression in the DDC model. Interestingly, this study also revealed that increased cholangiocyte stress may be attributed to a decrease in the cholangiocyte bicarbonate umbrella, which results in increased sensitivity of biliary cells to bile acids [170]. Indeed, one of the main functions of BECs lining the bile ducts is to allow for the transport of bile from the liver to the intestines. Thus, alterations in the biliary compartment may lead to either a lack of bile efflux from the liver or leakage from the bile ducts, leading to both BEC and hepatocyte injuries and liver inflammation as a consequence of toxic bile exposure. During homeostasis, BECs are protected from bile toxicity through the bicarbonate umbrella. The presence of anti-annexin A11 IgG1/IgG4 autoantibodies drives IgG4-related cholangitis pathogenesis by altering this protective HCO3− secretion [171].
The pathogenesis of PSC remains unknown, and consequently, no effective therapy has been identified at the time this review was written, although ongoing studies are exploring potential candidates, including UDCA [172]. PSC is strongly associated with inflammatory bowel disease (IBD), although the causes remain to be characterized [173]. However, PSCs exhibit some signs of an autoimmune disease directed against BECs, and evidence points toward intense crosstalk between BECs, neutrophils, macrophages, and T and B cells [174, 175]. PSC patients are usually identified at later disease stages, making identifying the disease-causing mechanisms challenging. Finally, PSC is considered a risk factor for intrahepatic cholangiocarcinoma [176]. Recently, endoscopic retrograde cholangiopancreatography (ERCP)-derived extrahepatic cholangiocyte organoids generated from PSCs and non-PSC patients were shown to be largely similar when cultured in vitro, notably in terms of their BEC marker expression and BEC landmark functions, and they displayed similar responses to inflammatory cytokine stimulation [176]. While these results open possibilities for autologous cell-based therapies in PSCs, they also propose that the causes of PSC, which remain to be identified, are not due to intrinsic changes within BECs but may be the consequences of changes within the BEC microenvironment in the liver.
PBC may represent the best-characterized type of cholangiopathy, although some characteristics, such as a female preponderance, remain to be explained. PBC is an autoimmune disease that primarily involves the targeting of intrahepatic BECs and results in chronic liver inflammation and fibrosis, in which hepatocyte injury is secondary and only manifests in later stages due to increased inflammation and cholestasis leading to oxidative stress [177]. Gene variants in HLA or IL-12 signaling have been associated with PBC, and most patients display autoreactive antibodies against mitochondrial antigens and subsequent failure of biliary transporters, e.g., anion exchange protein 2 (AE2) [177–179]. However, immunosuppression alone is insufficient for treating PBC patients [180]. Instead, ursodeoxycholic acid (UDCA), a hydrophilic bile acid, is used as a first-line therapy for PBC to reduce bile acid toxicity and, thus, liver inflammation and hepatocyte injury [177]. Interestingly, single-cell sequencing data acquired from PBC patients revealed a decreased bicarbonate umbrella and metabolism-related gene expression and increased chemokine, coagulation, and phagosome-related gene expression in cholangiocytes [181]. Importantly, variants in the 19p13.3 locus were shown to play a role in PBC pathogenesis in two Chinese cohorts through increased autophagy-related ARID3A enhancer activity in CD14+ monocytes [182]. This further points toward landmark monocyte accumulation near BECs in PBC progression, which may represent a yet unexplored target for drug development, an observation made by us and others in PBC patient liver samples [175]. Recently, live CD8+ CD69+ CD103+ E-cadherinhigh T cells were shown to be internalized by, or invade, BECs in PBC patient samples and to a lesser extent in healthy livers [183]. However, the functional implications of CD8+ T-cell invasion in BECs remain to be clarified.
BECs and B cells: evidence for a disease-driving mechanism in autoimmune cholangiopathies
Biliary atresia is a cholangiopathy that affects infants and is characterized by intra- and extrahepatic bile duct paucity, leading to bile build-up in the liver and jaundice, for which the primary treatment remains surgery with the Kasai procedure to allow bile flow to the intestines [184]. Circulating CXCL8/IL-8-expressing B cells, which are considered proinflammatory, are found in the peripheral blood of biliary atresia patients [185]. In addition, a rhesus rotavirus (RRV)-induced neonatal mouse model of biliary atresia leads to liver infiltration by B cells, and B-cell deficient mice are resistant to bile duct injury [186]. Interestingly, the authors demonstrated that B-cell-mediated bile duct injury, as well as macrophage and T-cell activation, are independent of the antigen presented by B cells in this model. B-cell infiltration and high levels of autoantibodies are also evident in Mdr2−/− mice, and B-cell depletion by an anti-CD20 antibody leads to reduced fibrosis and inflammation [187]. Liver-infiltrating antibody-secreting B cells are also detected in both PSCs and PBC, although fewer IgM-positive plasma cells are observed in PSCs than in PBC [188]. On the other hand, IgA autoantibodies have been measured in PSCs independently of their association with intestinal bowel disease (IBD) [189]. However, the association between autoantibodies and PSC remains to be fully elucidated. Recently, a study in a mouse MASH model revealed that intestinal IgA release induces the activation of CD11b+CCR2+F4/80+CD11c-FCGR1+ liver macrophages through the Fc-receptor gamma [190]. Although ductular reactions have not been investigated in the latter, it is reasonable to propose that the BEC‒macrophage axis is involved in the response to gut inflammation.
Gut-derived signals trigger portal area inflammation through BECs
Recent data point toward a direct link between cystic fibrosis transmembrane conductance regulator (CFTR)-induced gut dysbiosis and increased liver injury through ductular reactions, which include portal inflammation in CFTR-deficient mice [191]. BECs are known to express TLRs 1–6, and in particular, human BECs are particularly sensitive to poly:IC (a TLR3 ligand), which leads to their apoptosis through TRAIL [192]. Similarly, TLR2 ligands induce apoptosis in a mouse cholangiocyte cell line, along with increased Cxcl10. Interestingly, the inflammatory cytokines TNF-alpha and CCL2, as well as ICAM-1, are increased in BECs in a murine model of autoimmune cholangitis, further driving CD8+ T-cell-mediated portal inflammation. In addition, the authors proposed that TNF-alpha increases TLR2 expression on BECs and, thus, their sensitivity to gut microbiota antigen-induced cell death. In addition, microbial antigen sensing is generally attributed to liver-resident macrophages, and proinflammatory TLR signaling is inhibited by triggering receptor expressed on myeloid cells-2 (TREM-2). Accordingly, Trem2-deficient mice subjected to bile duct ligation (BDL) developed a more severe phenotype than did their wild-type counterparts, as indicated by a more prominent ductular reaction [193]. TREM-2 expression is positively correlated with both disease progression and macrophage recruitment in PSC and PBC patients and in corresponding mouse models, suggesting that TREM2 overexpression is a mechanism that responds to cholestasis by repressing the proinflammatory activation of liver macrophages, a process that is amplified by UDCA. In addition, TREM-2 overexpression reduces IL-33 expression, and IL-33 has been shown to favor mouse BEC proliferation [194]. Moreover, in vitro LPS stimulation of human BECs leads to the release of T-cell chemokines such as CCL20 and Th17-polarizing cytokines, including IL-1β and IL-6 [195]. IL-17-producing cells accumulate in the periportal area and sustain liver fibrosis while potentiating ductular cell accumulation [159, 196–198]; however, this study further suggests a direct role of BECs in driving this disease-promoting, local recruitment and polarization of Th17 cells [195]. Notably, IL-17A overexpression in the perilobular area induces the transformation of ductular cells into cancer stem cells, suggesting that the Th17-enhancing roles of BECs may be involved in liver cancer initiation [199]. In line with these findings, it is no surprise that ductular cell accumulation is associated with immune cells in a variety of chronic liver diseases. Overall, these studies support the hypothesis that intricate crosstalk between BECs and immune cells is a driving force for the liver ductular reaction and is under the influence of gut-derived signals.
Reactive BECs and monocyte–macrophage complexes closely interact in acute liver diseases and in MASLD
As with virtually all (liver) cells, our view of the range of cytokines secreted by BECs continues to expand as new results are released. In reference to “cholangiokines”, the BEC secretome is versatile, and we have just started to explore its potential for shaping portal inflammation [156]. In a mouse model of acute and targeted BEC injury and in the absence of confounders, we found that BECs engage in cell proliferation while expressing genes associated with chemotaxis, including the Ccl2 chemoattractant for monocytes [200]. This BEC CCL2-driven monocyte recruitment was also observed in mice injected with BV6, another model of acute cholangitis, and in human cholangiocytes exposed to BV6 in vitro [201]. Reciprocally, monocytes can drive a ductular reaction in the absence of additional injury [167]. Moreover, the joint response of monocytes and reactive BECs supports biliary tree repair and, during severe liver injury, supports hepatocyte regeneration [200, 202]. TRAIL knockout in myeloid cells was shown to exacerbate a proinflammatory phenotype in cholangiocytes in a DDC model [203]. This BEC proinflammatory phenotype was particularly marked by increased gene expression of Cxcl1, a typical neutrophil chemoattractant, which is associated with increased neutrophil recruitment and liver fibrosis. Notably, neutrophils drive ductular cell expansion and liver fibrosis in the same murine DDC model [204]. In patients, CCL28-expressing macrophages are observed near ductular cells but not close to large bile ducts in PSC livers [205]. Similarly, despite very distinct disease features, higher ductular cell densities have been associated with an increased extent of hepatocyte loss and with the number of monocytes accumulating in inflamed portal areas in MASLD/MASH [175, 206, 207]. Hence, more research is needed to fully decipher the intricate crosstalk between BECs and myeloid immune cells. Similarly, we recently showed in a primary mouse cell-based biliary niche-on-a-chip model that injured BECs release APP ORM2, shifting liver macrophages to a phenotype marked by increased secretion of pro- and anti-inflammatory cytokines [62]. Notably, recent insights from single-nuclei sequencing analyses of patient liver samples suggested that hepatocyte-cholangiocyte cellular plasticity is a key mechanism of liver repair in MASLD [161]. A similar mechanism of cellular plasticity has been demonstrated in mice, where concanavalin-A injections lead JAG1-expressing monocytes to induce the expression of hepatocyte Sox9, a typical ductular cell marker, near necrotic lesions. Considering, as mentioned above, that ductular and immune cells are reciprocally supportive, this finding suggests that the BEC-immune cell axis holds high potential for liver regenerative medicine, particularly in MASLD/MASH.
Insights into intrahepatic biliary tree-derived malignancies
Patients with PSC, fluke infections, and MASLD have an increased risk of cholangiocarcinoma, whereas patients with PBC have a relatively lower risk [208, 209]. Intrahepatic cholangiocarcinoma (ICC) is a biliary tree neoplasia that arises in noncirrhotic and cirrhotic livers and has a rising incidence [210, 211]. It is thought that the majority of ICCs are derived from BECs [210], but mouse and human hepatocytes also represent potential sources for ICCs [212, 213]. Reduced T and B lymphocyte intrahepatic and increased tumor-associated intrahepatic and CD14+CD16+ circulating monocyte numbers are associated with poor survival in patients with intrahepatic cholangiocarcinoma [214–217]. Moreover, high PDHA1 succinylation is associated with poor survival, and in mice, CD8+ T-cell depletion reversed the protumoral effects of PDHA1 succinylation [218]. In conclusion, much remains to be discovered, but cholangiocarcinoma is a highly desmoplastic and cold tumor with low immune cell infiltration and heterogeneous intratumor areas [219, 220].
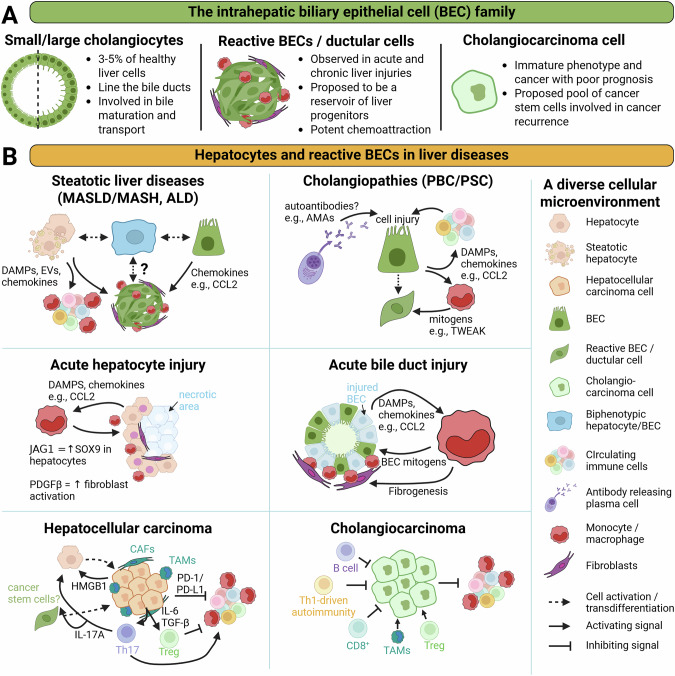
Overall, the BEC family is very diverse in its role in liver diseases, and much remains to be understood about the active roles played by ductular reactions in liver disease progression (Fig. 2).
Fig. 2.
Intrahepatic BEC diversity and implications of BECs and hepatocytes in liver diseases. A This schematic view summarizes the diverse forms that BECs can adopt in healthy and diseased livers. B Key BEC- and hepatocyte-driven mechanisms regulating liver immune cell recruitment and activation in a variety of etiologies. Abbreviations: ALD: alcohol-related liver disease; AMAs antimitochondrial antibodies, BECs biliary epithelial cells, CAFs cancer-associated fibroblasts, CCL chemokine (C-C motif) ligand, DAMPs damage-associated molecular patterns, EVs extracellular vesicles, HMGB1 high mobility group box 1 protein, ILs interleukins, JAG1 Jagged-1, MASH metabolic dysfunction–associated steatohepatitis, MASLD metabolic dysfunction–associated liver disease, PBC primary biliary cholangitis, PD-1/PD-L1 programmed cell death protein 1/programmed death-ligand 1, PDGFβ platelet-derived growth factor beta, PSC primary sclerosing cholangitis, SOX9 SRY-box transcription factor 9, TAMs tumor-associated macrophages, TGF-β transforming growth factor-beta
Hepatic stellate cells and their interactions with immune cells in health and disease
HSCs—a communication hub and mediator of homeostasis in the healthy liver
HSCs constitute the primary mesenchymal cell population of the liver [221]. They constitute approximately 5-8% of the cells in the healthy liver, where they reside in the space of Disse between the endothelial cells lining the liver sinusoids and hepatocytes [221]. This anatomical position places HSCs in close contact with endothelial cells, hepatocytes, and Kupffer cells as well as other resident and circulating or infiltrating immune cells [222]. Indeed, analyses of ligand‒receptor interactions in single-cell RNA sequencing datasets have revealed that HSCs are among the most interactive cell types in the liver [19, 223, 224]. HSCs not only engage in direct and bidirectional communication with hepatocytes, endothelial cells, macrophages, and immune cells but are also part of multicellular communication networks that control liver functions in health and disease (Fig. 3). For example, HSCs, together with LSECs, regulate the expression of hepatocyte genes, notably those required for the hepatocellular activation of the beta-catenin pathway [19]. Similarly, HSCs provide signals to endothelial cells via GDF-2 and BMP10, which in turn regulate liver macrophage specialization as well as hepatocyte function and zonation [225]. Thus, it appears that some hepatocyte-regulatory circuits in the liver involve multicellular modules with HSCs playing a central role [226]. In the healthy liver, HSCs are in a quiescent state (qHSCs), in which they store large amounts of retinoids in the form of retinyl esters [16, 221]. Nearly 50–80% of the body’s retinoids, which are potent regulators of innate and adaptive immune responses, are stored in HSCs [17]. Moreover, qHSCs are enriched in cytokines and growth factors, including hepatocyte growth factor (HGF), bone morphogenetic proteins (BMPs), and R-spondin 3, and this population has therefore also been termed cytokine- and growth factor-enriched HSCs (cyHSCs) [19, 224, 225]. qHSCs are considered homeostatic because they maintain liver zonation and function via R-spondin 3 [19] and BMPs [225] and protect hepatocytes from injury via HGF [224]. Accordingly, HSC-selective deletion of R-spondin 3 alters liver zonation, metabolism, and regeneration [19]. The HSC-selective deletion of BMP9 and BMP10 alters Kupffer cell and endothelial cell homeostasis [225]. The HSC-selective deletion of HGF predisposes the liver to increased liver injury [224]. Overall, in light of recent studies, HSCs clearly perform diverse functions in the liver under both homeostatic and pathogenic conditions; thus, further studies are needed to understand the role of qHSCs/cyHSCs in the immune homeostasis of the healthy liver.
Fig. 3.
HSC interactions in healthy and diseased livers, switching from homeostasis to inflammation and fibrosis. In the healthy liver, HSCs maintain homeostasis by secreting factors such as BMP9, BMP10, GDF10, ECM1, RSPO3, and HGF. BMP9 and BMP10 are important for Kupffer cell survival and LSEC identification. RSPO3 promotes hepatocyte metabolic zonation. HGF promotes hepatocyte survival. GFD10 and ECM1 maintain HSCs in a quiescent state. In chronic liver disease, these homeostatic HSC factors are progressively lost and replaced by pathogenic mediators driving interactions that keep HSCs in an activated state, promoting fibrosis. HSCs recruit bone marrow-derived monocytes, which then secrete TGF-β, IL-1β and TNF to promote HSC activation and survival. HSCs contribute to the recruitment of lymphocytes via the secretion of chemokines such as CCL5, which in turn maintain HSC activation. The secretion of ECM and the ensuing stiffness result in hepatocyte dedifferentiation, hepatocyte replacement, and an increased risk for transformation into cancer cells. NK cells may limit fibrosis by killing activated HSCs. Abbreviations: BM bone marrow, ECM extracellular matrix, HSC hepatic stellate cell, KC Kupffer cell, LSECs liver sinusoidal endothelial cell, MoMF monocyte-derived macrophage, NK natural killer
Hepatic stellate cells switch from hepatoprotection to fibrogenesis following liver injury
Following injury, HSCs undergo a well-characterized activation process and become the primary fibrogenic cell type of the liver [221, 227, 228]. HSC activation is driven by a wide range of mediators, with TGF-β and PDGF representing the two most potent fibrogenic activators of HSCs [228]. Activated HSCs are best known for producing fibrillar collagens such as type I collagen but also secrete a wide range of other extracellular matrix (ECM) components [221, 228, 229]. Continuous production of ECM by HSCs leads to the development of liver fibrosis. In more advanced disease stages, the ECM can replace functional liver parenchyma and thereby contribute to the development of liver cirrhosis, which is characterized by bridging fibrosis, regenerative nodules, and the loss of liver function [230]. In addition to ECM production, activated HSCs release fibrogenic extracellular vesicles, mainly in ECM-rich zones, to amplify liver fibrosis [231–233]. Activated HSCs also upregulate α-SMA and become contractile through protocadherin 7 (PCDH7) and may thereby contribute to portal hypertension [234, 235]. The increase in these disease-driving interactions is accompanied by a loss of the above-described homeostatic and protective mediators BMP9, BMP10, GDF10, RSPO3, and HGF [226] (Fig. 3).
Bidirectional interactions between hepatic stellate cells and immune cells
In addition to interacting with endothelial cells and hepatocytes, HSCs also engage in bidirectional interactions with a wide range of immune cells [236, 237]. For example, HSCs regulate Kupffer cell homeostasis via BMP9/10 [225]. Conversely, Kupffer cells are essential for HSC activation and survival via Kupffer cell-secreted TGF-β, TNF-α, and IL-1β [238]. Similarly, HSCs may regulate the recruitment and functions of diverse subsets of immune cells, while immune cells regulate the activation and death of HSCs, as discussed in further detail in the following sections.
Regulation of HSC death, survival, and activation by immune cells
Macrophages are key mediators of HSC activation, as shown by genetic and pharmacologic depletion [23, 239, 240]. It is believed that the profibrogenic effects of macrophages are largely mediated by TGF-β and that TGF-β derived from activated HSCs may perpetuate HSC activation in more advanced disease stages [221, 228]. Recent studies have suggested that physical interactions between macrophages and fibroblasts are needed to allow for TGF-β-mediated fibroblast activation in a mechanically soft environment [241]. Additionally, ILC2s (via IL-33), T cells (via IL-17), B cells (immunoglobulin independent), Tregs (via amphiregulin) and platelets have been suggested to directly promote HSC activation [197, 237, 242–245]. Moreover, many immune cells promote liver steatosis and MASH and, thereby, indirectly promote HSC activation and liver fibrosis [246]. In addition to the promotion of HSC activation and proliferation by immune cells, HSC survival and death are also regulated by immune cells. Macrophages secrete not only TGF-β to activate HSCs but also TNF-α and IL-1β to promote HSC survival [238] (Fig. 3). Other immune cell subsets, such as NK cells, can induce HSC death in an NKG2D- and TNF-related apoptosis-inducing ligand-dependent manner and, as a result, ameliorate liver fibrosis [247]. These intricate immune cell‒HSC interactions regulate the number of HSCs and thereby allow fine-tuning of wound healing, regeneration, and restoration in different disease stages.
Antigen presentation and the regulation of immune cell identity and function by HSCs
The liver is known to be a classical immune-privileged organ [248]. Among several cell populations, HSCs have been shown to positively and negatively regulate hepatic immunity. The release of retinoids from HSCs and their subsequent conversion to retinoic acid can maintain regulatory T (Treg) cell differentiation and thereby immune tolerance [249]. Activated HSCs can veto T cells via ICAM1- and contact-dependent mechanisms [250]. Moreover, HSC-expressed PD-L1 may contribute to the inhibition of T-cell- and B-cell-mediated immune responses [251, 252]. Similarly, HSC-expressed TGF-β may inhibit T-cell-mediated immunity [236]. On the other hand, HSCs express MHC class II molecules and have been suggested to serve as antigen-presenting cells (APCs) [253, 254]. However, the antigen-presenting role of HSCs remains controversial, as other studies have shown that HSCs do not cross-present antigens [255]. Furthermore, innate immune receptors on HSCs, such as TLR4 and TLR9, may promote hepatic inflammation [256, 257].
Hepatic stellate cells and their interactions with immune cells regulate the development and regression of liver fibrosis in chronic liver diseases, including MASLD
In patients with MASLD, liver fibrosis represents the primary determinant of mortality [258–261]. Therefore, the activation process that differentiates HSCs from quiescent and homeostatic cells to ECM-producing fibroblasts represents a key disease-driving process in MASLD [262]. Like in many other liver diseases, the macrophage pool changes in MASLD, with a loss of resident Kupffer cells and an increase in bone marrow-derived macrophages [20, 263]. While the functions of macrophage subpopulations need further clarification, they may play important but distinct roles in HSC activation and fibrosis [264–266] as well as in the repair and resolution of liver fibrosis, e.g., via TREM2 [267, 268]. Recruitment of CCR2+ monocyte-derived macrophages appears to promote fibrosis, e.g., via the release of profibrogenic mediators such as TGF-β [264, 265]. Moreover, the subpopulation of TREM2-expressing lipid-associated macrophages is important for the efferocytosis of dying hepatocytes and repair [267, 268]. Failure of TREM2-dependent efferocytosis and repair exacerbates fibrosis, most likely via increased HSC activation [268]. The upregulation of “don’t-eat-me” signals” via CD47 on hepatocytes and its receptor anti-SIRPa on macrophages can lead to a similar suppression of efferocytosis and increased fibrosis in MASLD [80]. In addition to signals from immune cells to HSCs, HSCs also provide signals to immune cells that may contribute to MASLD pathogenesis and possibly regression [236]. HSCs secrete mediators, sometimes termed “stellakines”, in MASLD, including CCL2, CCL11, CXCL10, CXCL12, and CXCL16, which may also increase their recruitment and interaction with immune cells and thereby promote liver fibrosis or repair [223, 246]. Additional proinflammatory signals in MASLD are mediated by microbe-associated metabolic patterns (MAMPs). Because of its direct connection to the intestine via the portal vein, the liver is the first target of gut-derived nutrients and mediators. In MASLD, the gut‒liver axis becomes severely disturbed, and increased exposure to intestinal MAMPs can activate TLRs on hepatic immune cells and HSCs to promote inflammation, immune cell recruitment, and fibrogenesis [269, 270]. Restorative macrophages play a key role in degrading the extracellular matrix and restoring normal liver architecture via their high expression of matrix metalloproteinases [271–274]. It remains to be investigated how HSCs or other liver cell types contribute to the recruitment of monocytes and their polarization toward a restorative phenotype during fibrosis regression. In summary, macrophage‒HSC interactions are central for the pathogenesis and resolution of MASLD, with additional roles for HSC interactions with other immune cells, such as lymphocytes and NK cells, as reviewed elsewhere [237, 246].
Hepatic stellate cells and their interactions with immune cells in liver cancer
Hepatocellular carcinoma (HCC) represents one of the deadliest complications of chronic liver disease and causes more than 800,000 deaths per year worldwide [275–277]. Almost 90% of hepatocellular carcinomas (HCCs) develop in fibrotic or cirrhotic livers [277]. Accordingly, fibrosis represents a significant risk factor for the development of HCC in patients [261]. HSCs contribute to a tumor-promoting environment in the chronically injured liver through multiple mediators. HSC-secreted type I collagen promotes a stiff environment that activates mechanosensitive signals in the liver, such as TAZ in hepatocytes, and thereby contributes to hepatocarcinogenesis [278]. The key role of type I collagen in HSCs and TAZ in hepatocytes in hepatocarcinogenesis has been demonstrated via cell-specific knockout studies [224]. A number of additional HSC mediators regulate the development of HCC. For example, the production of hyaluronic acid also promotes the development of HCC but in a less potent manner than type I collagen does [224]. Conversely, HSC-secreted HGF protects against the development of HCC by reducing hepatocyte death and the ensuing vicious cycle of regeneration and inflammation [224]. In addition to promoting HCC, HSCs also promote the growth of cholangiocarcinoma and desmoplastic liver metastases [279, 280]. However, this process is not mediated by type I collagen but rather through the secretion of growth factors such as HGF and hyaluronic acid [278]. In addition to affecting the ECM and growth factors, HSCs influence liver carcinogenesis by controlling inflammation and immunity in the liver [278]. In chronic liver disease, there may be an imbalance between increased inflammation and suppressed immunity [224]. HSCs suppress antitumor immunity in liver cancer through a wide range of mechanisms and mediators. The expression of TGFβ and PD-L1 by HSCs and HSC-derived cancer-associated fibroblasts (CAFs) may downregulate T-cell-mediated immune responses and increase regulatory T cells [236, 251, 252, 281–283]. Moreover, HSCs may participate in the mobilization of myeloid-derived suppressor cells via cyclooxygenase-2 [284].
Endothelial cells and their interactions with immune cells in health and disease
Endothelial cells play a crucial role in homeostasis in the liver [285, 286]. Indeed, angiocrine Wnt signaling establishes and maintains the pericentral phenotype of hepatocytes, as demonstrated in a mouse model in which the Wnt cargo receptor Evi (Wls), which is important for Wnt ligand exocytosis, was deleted in Stab 2+ endothelial cells [287]. In line with this study, pericentral angiocrine R-spondin 3 (Rspo3) maintains the pericentral metabolic signature of most pericentral WNThigh hepatocytes through β-catenin [288]. However, this conclusion was reached after Rspo3, which is not an endothelial cell-specific Cre driver, was deleted in CAGCreERT2 mice. A recent study using HSC-specific Rspo3 and EC-specific approaches revealed distinct roles for HSC- and EC-derived Rspo3 in zonation and assigned most hepatocyte-regulatory functions to HSC-derived Rspo3 [19]. In the adult liver, endothelial cells appear to be zonated. scRNA-seq revealed that periportal LSECs express high levels of CD36 and Adam23, which progressively diminish in the midzonal and pericentral zones. On the other hand, pericentral LSECs and central venous ECs abundantly express Wnt2, Wnt9, Lhx6, and Kit, which decrease toward midzonal and periportal zone ECs. Flt4, Lyve1, Cd32b, and Stab 2 are highly expressed by LSECs but not by vascular ECs [18, 289–293]. In addition to LSECs and venous ECs, lymphatic endothelial cells (LyECs) also play important roles in the liver, as they maintain fluid balance and immune regulation [294]. Like LSECs, liver LyECs are characterized by the appearance of fenestrae and cellular pores [295]. However, a recent study demonstrated that LyECs in the liver can be differentiated from other ECs through the expression of LyEC-specific IL7 [295], opening more possibilities for studying LyECs in health and disease.
Role of liver sinusoidal endothelial cells in hepatic immune regulation during homeostasis
LSECs are the port of entry to the liver for circulating immune cells and can function as resident antigen-presenting cells, promoting immunotolerance under physiological conditions [296]. During homeostasis, LSECs secrete TGF-β and delta-like ligand 4 (DLL4) to maintain Kupffer cell identity (Fig. 4) [297, 298]. In a transgenic model in which Kupffer cells are depleted via diphtheria toxin, LSECs and HSCs recruit circulating monocytes, which acquire Kupffer cell identity [299]. These studies demonstrate the importance of LSECs in maintaining the pool of Kupffer cells in the liver. In turn, Kupffer cells release IL-10 to induce T-cell immunotolerance [300]. CD8+ T-cell tolerance is also promoted by LSECs. Indeed, the interaction between antigen-presenting LSECs and naïve CD8+ T cells increases B7-homolog 1/programmed death-ligand 1 (B7-H1/PD-L1) expression on LSECs and PD-1 expression on T cells in vitro [301]. This leads to CD8+ T-cell tolerance, which is stable when LSEC-tolerant T cells are transferred to congenic animals in vivo [301]. The systemic effect of hepatic tolerance on controlling inflammatory or autoimmune diseases can be explained by the capacity of the liver to generate antigen-specific CD4+ CD25+ FOXP3+ regulatory T cells (Tregs). LSECs are the most efficient cells in the liver that induce Treg generation through TGFβ [302]. In addition to their role in immune tolerance, LSECs play important roles in immune surveillance. In the case of viral infection, major histocompatibility complex class I (MHC-I) molecules can be transferred from HSCs to LSECs, which cross-present soluble antigens to CD8+ T cells [303]. These studies have opened an avenue for investigating the detailed mechanisms by which LSECs regulate immunotolerance and immunosurveillance, as well as the balance between these two states.
Fig. 4.
Role of the interaction between LSECs and immune cells during liver health and disease. During homeostasis, fenestrated LSECs maintain Kupffer cell identity through TGFβ and DLL4. LSECs participate in immunotolerance through PD-1/PD-L1-mediated interactions with CD8+ T cells and TGFβ and DLL4 release toward CD4+ T cells. During liver fibrosis, LSECs lose their fenestrae and produce high levels of collagen IV and prostanoids and low levels of nitric oxide (NO) to increase portal hypertension. In addition, LSECs recruit immune cells that amplify fibrosis through multiple mechanisms. LSECs release CXCL1 to recruit neutrophils, which release NETs and subsequently amplify portal hypertension. In response to TNFα, LSECs also produce CCL2 through an NFκB/p300/BRD4-dependent mechanism to recruit monocytes/macrophages and increase liver fibrosis. During ASH, they release CXCL1, CXCL6, and CXCL8 through an NFκB/BRD4-dependent mechanism to recruit immune cells. During MASH, LSECs respond to lipotoxicity by increasing their expression of VCAM1 to recruit immune cells and activate HSCs. Lipotoxicity also increases fibrosis and inflammation through GSK3β. During viral hepatitis, LSECs interact with platelets to recruit CD8+ T cells, which send their protrusions through LSEC fenestrae to reach antigen-presenting hepatocytes. The release of LSEC-derived IL-27 promotes T-cell activation. Intrahepatic T-cell responses can also be enhanced by the natural killer (NK) cell-dependent maturation of LSECs as a beneficial process to control HBV replication and expression. During cholestatic liver disease, neutrophil infiltration in the liver promotes the formation of NETs, which induce the production of procoagulants in LSECs, which are important for the formation of thrombi. Finally, during cancer, tumor cells produce IL-8, which interacts with LSEC CXCR1, leading to the release of GARP-latent-TGFβ, which preferentially induces the polarization of Tregs and inhibits antitumor immunity. Similarly, the uptake of SV-enriched nanoparticles by LSECs leads to the release of CXCL16, recruitment of NKT cells, and suppression of tumor progression. ASH alcohol-associated steatohepatitis, KC Kupffer cells, LSEC liver sinusoidal endothelial cells, MASH metabolic dysfunction-associated steatohepatitis, NET neutrophil extracellular trap, NK cells natural killer cells, NKT cells natural killer T cells, NO nitric oxide
Endothelial cell and immune cell crosstalk in the injured and fibrotic liver
The zonation of LSECs, as well as their communication with other cell types, is altered during liver injury. In healthy livers, LSECs prevent HSC activation [304]. In cirrhotic mouse livers, LSECs become capillarized mostly in the pericentral area, where they decrease some of their markers, such as lymphatic vessel endothelial receptor 1 (Lyve1), cluster of differentiation 32b (Cd32b), and Fms-related tyrosine kinase 4 (Flt4) [293]. Capillarized LSECs (Fig. 4), characterized by high nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)-dependent collagen IV release [305], lose their HSC-suppressive properties and thereby contribute to an environment that favors HSC activation and fibrosis [304]. Moreover, they secrete vasoconstrictors such as prostanoids and reduce NO release, leading to increased portal pressure [306, 307]. This elevation of blood pressure in the portal vein, named portal hypertension, is a complication of chronic liver disease and is often characterized by high pressures in the liver sinusoids. This mechanical force exerted on LSECs has been demonstrated to increase the release of C-X-C chemokine ligand 1 (CXCL1), as shown in a mouse model of inferior vena cava ligation [308]. CXCL1 secretion, which is mediated by stiffness-dependent glycolysis [309], leads to neutrophil recruitment to the liver. Neutrophils form extracellular traps and microthrombi that, in turn, amplify portal hypertension [308]. During fibrosis, LSECs also recruit monocytes/macrophages to the liver through the release of C‒C motif chemokine ligand 2 (CCL2) [231, 310]. Indeed, in CCl4-mediated chronic liver injury, TNFα in LSECs leads to the interaction of NF-κB with histone acetyltransferase protein 300 (p300) and bromodomain containing 4 (BRD4) to epigenetically upregulate CXCL1 and CCL2, which significantly increases liver inflammation and promotes fibrosis. These studies demonstrate that LSECs are crucial for the recruitment of immune cells during liver fibrosis.
Endothelial cell and immune cell crosstalk during steatotic liver disease
Liver inflammation, along with excessive lipid accumulation and liver fibrosis, are the main features of alcohol-associated liver disease (ALD) and metabolic dysfunction-associated steatohepatitis (MASH). Alcoholic hepatitis (AH) is the most severe manifestation of ALD and presents an accumulation of neutrophils in the liver [292, 311]. Indeed, tumor necrosis factor alpha (TNFα) augments the expression of several chemokines in LSECs, including CXCL1, CXCL6, and CXCL8, through NF-κB and bromodomain-containing protein 4 (BRD4) binding to a common superenhancer (Fig. 4) [312]. Pharmacological inhibition of BRD4 in a murine model of AH reduces chemokine expression and neutrophil infiltration in the liver [312]. In addition to neutrophils, other innate and adaptive immune cells infiltrate the liver during AH [313]. However, the LSEC-dependent mechanisms that attract these immune cells remain to be studied. The proinflammatory phenotype of LSECs has also been explored in MASLD and MASH, where they are characterized by a loss of fenestrae and increased expression of adhesion molecules [292]. Compared with steatohepatitis, the loss of fenestrae has been observed to occur to a greater extent in human steatotic livers [314], suggesting that LSEC capillarization might occur preferentially during the early stages of MASLD. During MASH, lipotoxic stimulation leads to increased expression of endothelial vascular cell adhesion molecule 1 (VCAM1) [315]. The inhibition of VCAM1 in a murine model of MASH leads to decreased HSC activation and fibrosis [316]. Selective deletion of VCAM1 in LSECs in a murine model of CCl4-induced liver fibrosis also reduces macrophage accumulation in the liver [316], suggesting that LSEC VCAM1 is important for immune cell infiltration and liver fibrosis. In line with these studies, LSEC injury induced by lipotoxic palmitate leads to increased phosphorylation of glycogen synthase kinase 3 beta (GSK3β). Pharmacological GSK3β inhibition reduces neutrophil and monocyte migration in vitro and ameliorates liver inflammation and fibrosis in a murine model of MASH in vivo [317]. In addition to innate immunity, a recent study demonstrated that CD8+ T-cell clonal expansion is a feature of MASH [318], indicating a possible role for CD8+ T cells during MASH progression. Nevertheless, a deeper understanding of the molecular mechanism by which LSECs recruit adaptive and innate immune cells during steatotic liver disease is needed. Finally, the accumulation of lipid droplets in the liver generates mechanical forces, and their effect on the biology of LSECs remains to be explored.
Endothelial cell and immune cell crosstalk during viral hepatitis
Crosstalk between LSECs and CD8+ T cells has also been studied in the context of viral hepatitis (Fig. 4). In a transgenic mouse model of hepatitis B virus infection, in which mouse GP-Ibα is replaced with transgenic human GP-Ibα, it has been observed that effector CD8+ T cells arrest within the sinusoids by docking on platelets, which are already adhered to LSECs. Then, effector CD8+ T cells crawl along the sinusoids and extend their cytoplasmic protrusions through LSEC fenestrae to search for antigens on hepatocytes [319], suggesting a crucial role for LSECs in the process of viral antigen detection by T cells. In addition, HBV exposure leads to a switch from a tolerogenic state to an immunogenic state in LSECs. This process involves the release of LSEC-derived cytokines, such as IL-27, which promote the activation of T cells [320]. Intrahepatic T-cell responses can also be enhanced by the natural killer (NK) cell-dependent maturation of LSECs as a beneficial process to control HBV replication and expression [321]. The modulation and leveraging of the immunogenic versus tolerogenic properties of LSECs, especially in the recruitment and activation of T cells, to treat viral hepatitis seem to be attractive strategies and deserve increased attention.
Endothelial cell and immune cell crosstalk during cholestatic liver disease
Tolerogenic LSECs have been explored in a model of autoimmune cholangitis. In a model of CD8+ T-cell-mediated autoimmunity, the administration of nanoparticles loaded with an autoantigen peptide leads to cross-presentation of the autoantigen on MHC I molecules on LSECs, subsequently reducing T-cell infiltration into the liver and ameliorating cholangitis [322]. Cholestatic liver disease is accompanied by infiltration of neutrophils into the liver, which promotes the formation of neutrophil extracellular traps (NETs) (Fig. 4) [323]. In turn, NETs induce the production of procoagulants in LSECs, which are important for the formation of thrombi [323]. The crosstalk between LSECs and immune cells in cholestatic liver disease remains largely unknown and warrants further investigation.
Endothelial cell and immune cell crosstalk during drug-induced liver injury
In addition to cholangiopathies, LSECs are also important during drug-induced liver injury (DILI), where they can be a direct target of acetaminophen toxicity, preceding hepatocyte injury [324]. More recently, the G protein-coupled receptor cysteinyl leukotriene receptor 1 (CYSLTR1) was shown to promote acetaminophen-induced injury. CYSLTR1 is expressed in LSECs and monocytes, and its inhibition in conjunction with a G protein-coupled bile acid receptor 1 (GPBAR1) agonist potently reverses LSEC/monocyte interactions and reduces liver damage [325]. It would be interesting to understand how G protein-coupled receptors affect the crosstalk not only between LSECs and monocytes but also with other immune cells.
Endothelial cell and immune cell crosstalk during liver cancer
The LSEC phenotype, expression profile, and ability to interact with other cells are affected during liver cancer (Fig. 4). Indeed, in mice, the initial steps triggering HCC correlate with LSEC capillarization [326]. During hepatocellular carcinoma (HCC) progression in humans, LSECs lose the expression of their markers, including lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), CD32b, stabilin-1, and stabilin-2 [327]. Additionally, LSECs participate in vascular invasion and local microenvironmental immune escape in HCC [328]. In this context, HBV-associated tumor cells release high amounts of interleukin 8 (IL-8), which binds to endothelial CXCR1, leading to increased LSEC permeability to facilitate tumor invasion. The IL-8/CXCR1 axis also induces glycoprotein-A repetitions predominant (GARP)-latent-TGFβ in liver sinusoidal endothelial cells to subsequently provoke preferential regulatory T-cell polarization to suppress antitumor immunity [328]. In line with these studies, a subset of tumor-associated endothelial cells secretes CXCL12, which inhibits the differentiation of CD8+ naïve T cells into CD8+ cytotoxic T cells and thus mediates immunosuppression [329]. In addition, LSECs alter the DC-mediated activation of T cells, inhibit CD8+ T-cell cytotoxicity, and promote T-cell exhaustion in a mouse model of early HCC development [330]. However, LSECs can also be modulated to increase antitumor immunity. Indeed, the upregulation of CXCL16 in LSECs through the administration of simvastatin-loaded nanoparticles in mice leads to reduced LSEC capillarization and the recruitment of NKT cells to suppress tumor progression [331], suggesting that LSECs are promising therapeutic targets. Studies to better understand the modulation of LSEC behavior and tolerogenic versus immunogenic properties, as well as how different LSEC subpopulations interact with immune cells and other cell types in health and liver diseases, are fundamental for the development of efficient therapeutic strategies.
Looking for a needle in the haystack—the power of single-cell-resolved analyses and data integration for the study of liver inflammation and immunity
Fast-paced recent advances in single-cell-resolved technologies have allowed the scientific community to dissect the liver cellular landscape with unprecedented depth, particularly highlighting the previously overlooked intricate cellular crosstalk between the liver and immune cells [332–334]. More importantly, those technologies have allowed us to “look for a needle in a haystack”, hepatocytes representing the haystack in this analogy, and needles rare populations such as BECs, endothelial cells, HSCs, and rare immune cell populations. Indeed, previous investigations using, for example, RNA bulk sequencing or even tissue microdissections often led to an overwhelming contribution of hepatocyte-derived signals in transcriptomic or proteomic analyses and often completely lacked information on rare cell populations (which often also contain much less RNA and protein than hepatocytes do). One landmark study described the fibrotic niche in healthy and cirrhotic human livers via single-cell RNA sequencing [335]. In addition to providing a first-of-its-kind liver cell atlas, this study revealed the presence of two disease-associated endothelial cell populations in the fibrotic niche, characterized as CD34+PLVAP+VWA1+ and CD34+PLVAP+ACKR1+ populations. Moreover, this same study identified PDGFRA as a marker of scar-associated fibroblasts in cirrhotic livers. The authors’ analysis also provides in-depth characterization of liver macrophages and intrahepatic cellular interactomes and remains highly valuable and illustrates the many opportunities that arose for single-cell-resolved studies [335]. Another multidimensional analysis demonstrated that in a murine model of persistent hepatitis B virus infection, LSECs increased protein kinase A activation on CD8+ T cells, which abrogated their ability to perform cytotoxic functions toward infected hepatocytes and, ultimately, failure of virus-specific immunity. This mechanism was termed “the liver immune rheostat” by the authors and further illustrates the complex cellular interplay taking place in liver diseases [336]. Similarly, a combination of single-cell transcriptomics and multiplex immunofluorescence techniques revealed that HSC depletion led to altered hepatocyte zonation and liver repair mechanisms in mice and that HSC R-spondin3 expression was greater in patients with a better prognosis in ALD and MASLD [19]. In line with high-throughput technologies, several recent studies have utilized spatial and single-cell multiomics to investigate the immune landscape of liver cancers, including hepatocellular carcinoma and cholangiocarcinoma [337–339]. These studies elucidated the tumor microenvironment, immune cell populations, and effects of immunotherapies on immune correlates in patients with liver cancer. Integration of multiomics analysis can provide a more complete landscape of the immune mechanisms of disease progression. However, recognizing the limitations of single-cell-based and spatial transcriptomic technologies is important for proper data interpretation. For example, single-cell RNA-seq relies on liver digestion and cell isolation and can lead to artifacts caused by prolonged warm digestion, complications for droplet-based RNA sequencing for large cell types (e.g., steatotic hepatocytes) and biases owing to the inability to achieve proper representation of cell types in highly fibrotic livers. As such, single-nucleus RNA sequencing provides less bias and better representation of cell populations such as hepatic stellate cells and hepatocytes from steatotic livers. Likewise, sequencing-based spatial transcriptomic studies often do not achieve single-cell resolution. In addition, bioinformatic tools are rapidly evolving; thus, the resolutions and codes used for data analysis can become obsolete and need constant updating.
In addition, technologies for multiplexed tissue imaging have become broadly accessible and, consequently, have been used. This includes high-complexity platforms such as imaging mass cytometry and sequential multiplex immunofluorescence. This has led to the identification of major disease progression-associated histological changes involving multiple cell types, such as MASLD/MASH, PSC, PBC liver cancer and neonatal infections [175, 340–342]. Multiplex immunofluorescence also holds key advantages for molecular pathway exploration in animal models, as this allows for a multidimensional evaluation of cellular phenomena on archival, formalin-fixed and paraffin-embedded tissue sections [343]. As such, multiplex immunofluorescence revealed the crucial role of Jagged-1-expressing monocyte-derived macrophages in inducing Sox9 expression in hepatocytes, allowing liver repair mechanisms to engage in a murine model of acute concanavalin-A-induced liver injury [344]. The same study also demonstrated the mobilization of activated HSCs to form a scar around the necrotic area, representing another example of how multiplexed technologies now support the exploration of distinct cellular responses occurring concomitantly. In addition to expanding the investigational toolbox, multiplex immunofluorescence dramatically increases immunostaining-based data robustness, as it allows the experimenters to identify potentially questionable antibody specificities by better visualizing positive signal distributions. This increasing dimensionality in digital images further stimulates the development of advanced tools for image analyses and data extraction that go beyond what the experimenter’s eye, which is perhaps unconsciously biased but surely biologically limited, may see [345]. Intense efforts are being made to deploy artificial intelligence-based algorithms that can facilitate large dataset analyses and data integration, with implications far beyond image analyses or data extraction [346].
Notably, some of the studies mentioned above, despite being of high quality, remain descriptive of several aspects—understandably, since not all mechanisms may be tested by a single group within the time frame of one manuscript and without leading to an overwhelmingly complex report. Nevertheless, groundbreaking advances in the field of in vitro modeling of liver diseases are also taking place, and this is to some extent supported by recent large datasets [347]. On the one hand, the community now benefits from extensive information that can be used to define transcriptomic and proteomic signatures associated with specific cell types and states, which provide essential input as to what models should be mimicked [161]. On the other hand, interactome analyses emphasized the need for multicellular culture systems. Indeed, crucial milestones have been reached within the last decade, from the isolation and in vitro expansion of bile duct-derived progenitor cells to diseased patient-derived cholangiocyte organoid cultures for detailed disease-driving mechanism investigations [161, 348]. These approaches hold promise in applications such as autologous transplant. For example, gallbladder-derived cholangiocyte organoids have been shown to engraft in the intrahepatic biliary tree after injection into deceased transplant donor livers and prevent ischemic injury while the organ is under machine perfusion, which represents a promising example of ex vivo cell-based therapy [164]. This approach using unaltered, autologous gallbladder cells combined with genetic alterations in MHC protein expression may further prevent the recurrence of autoimmune cholangitis after liver transplantation [349]. Further developments are expected in the near future, notably allowing for the generation of patient-derived multicellular and immune-competent organoid systems that will open doors for mechanistic studies of cellular crosstalk [350]. Moreover, we and others recently developed in vitro, mouse primary cell-based models for the study of immune-driving mechanisms of acute liver injuries and, potentially, drug candidate testing in a multicellular environment [351]. It is expected that such models containing primary human immune and liver cells will be developed in the near future, which could represent major advances in the field of personalized medicine.
However, this must not be underestimated, and conventional and long-term validated methods remain necessary to confirm each single brick of crucial data generated from large-scale methods. As such, in vivo experimentation, monocultures, single immunostaining with robust controls, targeted protein expression quantitation, and targeted molecular pathway interventions, to name a few, are still crucially needed despite the current enthusiasm and, obviously, the many opportunities derived from novel technologies.
Conclusions
There is increasing evidence that cell‒cell communication involving multicellular circuitries is essential for maintaining proper liver function during homeostasis. Not a single cell in the liver exists and functions properly in isolation, and disease processes in the liver or any other organ involve multiple cell types [352]. Liver disease is often associated with and potentially aggravated by dysfunction of these multicellular units that maintain proper function. Moreover, the liver is a primary example of a highly resilient organ that can efficiently repair and regenerate and deal with a wide range of often harmful or immunogenic substances and pathogens that enter through its dual vascular supply while maintaining metabolic functions that are essential for organismal survival. Given that modern diseases often do not cause massive life-threatening but rather chronic low-grade injury and inflammation, further understanding of multicellular hepatic communication circuitries may provide a deeper understanding of liver regeneration and restoration, which represent unmet needs in the field. Interactions of liver cells with immune cells are not only key for many aspects of liver pathogenesis but also particularly important and promising for restorative therapies, as demonstrated by recent trials [271]. Similarly, multicellular circuits involving HSCs, LSECs, hepatocytes and Kupffer cells maintain proper liver function in homeostasis and become deranged in liver disease [19, 225]. Promoting homeostatic and restorative properties in these multicellular circuits may hold great therapeutic promise and synergize with current therapeutic concepts that are largely aimed at curing the causative agent but often fail to restore liver architecture and function in advanced disease.
Acknowledgements
This work was supported by the German Research Foundation (Project-ID 556479455 and SFB1382, Project-ID 403224013, to AG), the Mayo Clinic Center for Biomedical Discovery, the Mayo Center for Cell Signaling in Gastroenterology (via NIH P30 DK084567), the NIH R01 DK136511 to EK, the Columbia University Digestive and Liver Disease Research Center (via NIH grant 5P30DK132710) and the NIH grants 5R01CA262424 and 5R01DK128955 (both to RFS).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Enis Kostallari, Email: kostallari.enis@mayo.edu.
Robert F. Schwabe, Email: rfs2102@cumc.columbia.edu
Adrien Guillot, Email: adrien.guillot@charite.de.
References
- 1.Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–51. [DOI] [PubMed] [Google Scholar]
- 2.Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. [DOI] [PubMed] [Google Scholar]
- 3.Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Yatskievych TA, Baker RK, Antin PB. Regulation of Hex gene expression and initial stages of avian hepatogenesis by Bmp and Fgf signaling. Dev Biol. 2004;268:312–26. [DOI] [PubMed] [Google Scholar]
- 5.McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–17. [DOI] [PubMed] [Google Scholar]
- 6.Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell. 2010;18:175–89. [DOI] [PubMed] [Google Scholar]
- 7.Yang L, Wang X, Yu XX, Yang L, Zhou BC, Yang J, et al. The default and directed pathways of hepatoblast differentiation involve distinct epigenomic mechanisms. Dev Cell. 2023;58:1688–700.e6. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–63. [DOI] [PubMed] [Google Scholar]
- 9.Han S, Dziedzic N, Gadue P, Keller GM, Gouon-Evans V. An endothelial cell niche induces hepatic specification through dual repression of Wnt and Notch signaling. Stem Cells. 2011;29:217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maretti-Mira AC, DeLeve LD. Sinusoids as drivers of liver development: more than simple chemistry. Hepatology. 2019;70:737–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miah M, Goh I, Haniffa M. Prenatal development and function of human mononuclear phagocytes. Front Cell Dev Biol. 2021;9:649937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou S, Liu C, Yao Y, Bai Z, Gong Y, Wang C, et al. Hematopoietic stem cell development in mammalian embryos. Adv Exp Med Biol. 2023;1442:1–16. [DOI] [PubMed] [Google Scholar]
- 13.Liang Y, Kaneko K, Xin B, Lee J, Sun X, Zhang K, et al. Temporal analyses of postnatal liver development and maturation by single-cell transcriptomics. Dev Cell. 2022;57:398–414.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol. 2017;27:R1147–R51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabibian JH, Masyuk AI, Masyuk TV, O’Hara SP, LaRusso NF. Physiology of cholangiocytes. Compr Physiol. 2013;3:541–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001;21:311–35. [DOI] [PubMed] [Google Scholar]
- 17.Blaner WS, O’Byrne SM, Wongsiriroj N, Kluwe J, D’Ambrosio DM, Jiang H, et al. Hepatic stellate cell lipid droplets: a specialized lipid droplet for retinoid storage. Biochim Biophys Acta. 2009;1791:467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu S, Liu S, Bian Y, Poddar M, Singh S, Cao C, et al. Single-cell spatial transcriptomics reveals a dynamic control of metabolic zonation and liver regeneration by endothelial cell Wnt2 and Wnt9b. Cell Rep Med. 2022;3:100754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugimoto A, Saito Y, Wang G, Sun Q, Yin C, Lee KH, et al. Hepatic stellate cells control liver zonation, size and functions via R-spondin 3. Nature. 2025;640:752–61. [DOI] [PMC free article] [PubMed]
- 20.Guilliams M, Scott CL. Liver macrophages in health and disease. Immunity. 2022;55:1515–29. [DOI] [PubMed] [Google Scholar]
- 21.Balmer ML, Slack E, de Gottardi A, Lawson MA, Hapfelmeier S, Miele L, et al. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci Transl Med. 2014;6:237ra66. [DOI] [PubMed] [Google Scholar]
- 22.Fox ES, Thomas P, Broitman SA. Clearance of gut-derived endotoxins by the liver. Release and modification of 3H, 14C-lipopolysaccharide by isolated rat Kupffer cells. Gastroenterology. 1989;96:456–61. [DOI] [PubMed] [Google Scholar]
- 23.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–32. [DOI] [PubMed] [Google Scholar]
- 24.Jagavelu K, Routray C, Shergill U, O’Hara SP, Faubion W, Shah VH. Endothelial cell toll-like receptor 4 regulates fibrosis-associated angiogenesis in the liver. Hepatology. 2010;52:590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao H, Jiang Y, Zeng G, Huda N, Thoudam T, Yang Z, et al. Cell-to-cell and organ-to-organ crosstalk in the pathogenesis of alcohol-associated liver disease. eGastroenterology. 2024;2:e100104. [DOI] [PMC free article] [PubMed]
- 26.Ronca V, Gerussi A, Collins P, Parente A, Oo YH, Invernizzi P. The liver as a central “hub” of the immune system: pathophysiological implications. Physiol Rev. 2025;105:493–539. [DOI] [PubMed] [Google Scholar]
- 27.Li N, Hua J. Immune cells in liver regeneration. Oncotarget. 2017;8:3628–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strnad P, Tacke F, Koch A, Trautwein C. Liver—guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14:55–66. [DOI] [PubMed] [Google Scholar]
- 29.Bode JG, Albrecht U, Haussinger D, Heinrich PC, Schaper F. Hepatic acute phase proteins-regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-kappaB-dependent signaling. Eur J Cell Biol. 2012;91:496–505. [DOI] [PubMed] [Google Scholar]
- 30.Quinton LJ, Blahna MT, Jones MR, Allen E, Ferrari JD, Hilliard KL, et al. Hepatocyte-specific mutation of both NF-kappaB RelA and STAT3 abrogates the acute phase response in mice. J Clin Invest. 2012;122:1758–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schumacher N, Yan K, Gandrass M, Muller M, Krisp C, Hasler R, et al. Cell-autonomous hepatocyte-specific GP130 signaling is sufficient to trigger a robust innate immune response in mice. J Hepatol. 2021;74:407–18. [DOI] [PubMed] [Google Scholar]
- 32.Ehlting C, Wolf SD, Bode JG. Acute-phase protein synthesis: a key feature of innate immune functions of the liver. Biol Chem. 2021;402:1129–45. [DOI] [PubMed] [Google Scholar]
- 33.Thorgersen EB, Barratt-Due A, Haugaa H, Harboe M, Pischke SE, Nilsson PH, et al. The Role of Complement in Liver Injury, Regeneration, and Transplantation. Hepatology. 2019;70:725–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sack GH Jr. Serum amyloid A (SAA) proteins. Subcell Biochem. 2020;94:421–36. [DOI] [PubMed] [Google Scholar]
- 35.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Investig. 2003;111:1805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou HH, Tang YL, Xu TH, Cheng B. C-reactive protein: structure, function, regulation, and role in clinical diseases. Front Immunol. 2024;15:1425168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segers FM, Verdam FJ, de Jonge C, Boonen B, Driessen A, Shiri-Sverdlov R, et al. Complement alternative pathway activation in human nonalcoholic steatohepatitis. PLoS One. 2014;9:e110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haugaa H, Thorgersen EB, Pharo A, Boberg KM, Foss A, Line PD, et al. Inflammatory markers sampled by microdialysis catheters distinguish rejection from ischemia in liver grafts. Liver Transplant. 2012;18:1421–9. [DOI] [PubMed] [Google Scholar]
- 40.Hussein MH, Hashimoto T, Suzuki T, Abdel-Hamid Daoud G, Kato T, Hibi M, et al. Liver Transplantation from Female Donors Provokes Higher Complement Component C5a Activity. Ann Transpl. 2017;22:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tajima T, Hata K, Kusakabe J, Miyauchi H, Badshah JS, Kageyama S, et al. Anti-complement 5 antibody ameliorates antibody-mediated rejection after liver transplantation in rats. Front Immunol. 2023;14:1186653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waelgaard L, Thorgersen EB, Line PD, Foss A, Mollnes TE, Tonnessen TI. Microdialysis monitoring of liver grafts by metabolic parameters, cytokine production, and complement activation. Transplantation. 2008;86:1096–103. [DOI] [PubMed] [Google Scholar]
- 43.Kusakabe J, Hata K, Miyauchi H, Tajima T, Wang Y, Tamaki I, et al. Complement-5 inhibition deters progression of fulminant hepatitis to acute liver failure in murine models. Cell Mol Gastroenterol Hepatol. 2021;11:1351–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marshall KM, He S, Zhong Z, Atkinson C, Tomlinson S. Dissecting the complement pathway in hepatic injury and regeneration with a novel protective strategy. J Exp Med. 2014;211:1793–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M, Kim YM, Koh JH, Park J, Kwon HM, Park JH, et al. Serum amyloid A expression in liver promotes synovial macrophage activation and chronic arthritis via NFAT5. J Clin Investig. 2024;134:e167835. [DOI] [PMC free article] [PubMed]
- 46.You K, Wang Y, Chen X, Yang Z, Chen Y, Tan S, et al. Neutralizing serum amyloid a protects against sinusoidal endothelial cell damage and platelet aggregation during acetaminophen-induced liver injury. Biochem Biophys Res Commun. 2023;639:20–8. [DOI] [PubMed] [Google Scholar]
- 47.Ji YR, Kim HJ, Bae KB, Lee S, Kim MO, Ryoo ZY. Hepatic serum amyloid A1 aggravates T-cell-mediated hepatitis by inducing chemokines via Toll-like receptor 2 in mice. J Biol Chem. 2015;290:12804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stone ML, Lee J, Lee JW, Coho H, Tariveranmoshabad M, Wattenberg MM, et al. Hepatocytes coordinate immune evasion in cancer via release of serum amyloid A proteins. Nat Immunol. 2024;25:755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JW, Stone ML, Porrett PM, Thomas SK, Komar CA, Li JH, et al. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature. 2019;567:249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sander LE, Sackett SD, Dierssen U, Beraza N, Linke RP, Muller M, et al. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med. 2010;207:1453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bickerstaff MC, Botto M, Hutchinson WL, Herbert J, Tennent GA, Bybee A, et al. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat Med. 1999;5:694–7. [DOI] [PubMed] [Google Scholar]
- 52.Tang ZM, Yuan P, Gao N, Lei JG, Ahmed M, Hua YX, et al. C-reactive protein attenuates CCl(4)-induced acute liver injury by regulating complement system activation. Mol Immunol. 2025;180:44–54. [DOI] [PubMed] [Google Scholar]
- 53.Li HY, Tang ZM, Wang Z, Lv JM, Liu XL, Liang YL, et al. C-reactive protein protects against acetaminophen-induced liver injury by preventing complement overactivation. Cell Mol Gastroenterol Hepatol. 2022;13:289–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cong M, Zhao W, Liu T, Wang P, Fan X, Zhai Q, et al. Protective effect of human serum amyloid P on CCl4-induced acute liver injury in mice. Int J Mol Med. 2017;40:454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pilling D, Cox N, Thomson MA, Karhadkar TR, Gomer RH. Serum amyloid P and a dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin ligand inhibit high-fat diet-induced adipose tissue and liver inflammation and steatosis in mice. Am J Pathol. 2019;189:2400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agiasotelli D, Alexopoulou A, Vasilieva L, Hadziyannis E, Goukos D, Daikos GL, et al. High serum lipopolysaccharide binding protein is associated with increased mortality in patients with decompensated cirrhosis. Liver Int. 2017;37:576–82. [DOI] [PubMed] [Google Scholar]
- 57.Milbank E, Diaz-Trelles R, Dragano N, Latorre J, Mukthavaram R, Mayneris-Perxachs J, et al. Liver lipopolysaccharide binding protein prevents hepatic inflammation in physiological and pathological nonobesogenic conditions. Pharm Res. 2023;187:106562. [DOI] [PubMed] [Google Scholar]
- 58.Fournier T, Medjoubi NN, Porquet D. Alpha-1-acid glycoprotein. Biochim Biophys Acta. 2000;1482:157–71. [DOI] [PubMed] [Google Scholar]
- 59.Ruiz M. Into the labyrinth of the lipocalin alpha1-acid glycoprotein. Front Physiol. 2021;12:686251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elpek GO. Orosomucoid in liver diseases. World J Gastroenterol. 2021;27:7739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu HZ, Zhou WJ, Wan YF, Ge K, Lu J, Jia CK. Downregulation of orosomucoid 2 acts as a prognostic factor associated with cancer-promoting pathways in liver cancer. World J Gastroenterol. 2020;26:804–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu H, Yin G, Franco Leonardi B, Lan T, Ait Ahmed Y, Berger H, et al. Reactive cholangiocyte-derived ORM2 drives a pathogenic modulation of the injured biliary niche through macrophage reprogramming. Gut. 2025. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 63.Zhou B, Luo Y, Ji N, Hu C, Lu Y. Orosomucoid 2 maintains hepatic lipid homeostasis through suppression of de novo lipogenesis. Nat Metab. 2022;4:1185–201. [DOI] [PubMed] [Google Scholar]
- 64.Abshagen K, Eipel C, Kalff JC, Menger MD, Vollmar B. Loss of NF-kappaB activation in Kupffer cell-depleted mice impairs liver regeneration after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1570–7. [DOI] [PubMed] [Google Scholar]
- 65.Aldeguer X, Debonera F, Shaked A, Krasinkas AM, Gelman AE, Que X, et al. Interleukin-6 from intrahepatic cells of bone marrow origin is required for normal murine liver regeneration. Hepatology. 2002;35:40–8. [DOI] [PubMed] [Google Scholar]
- 66.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–83. [DOI] [PubMed] [Google Scholar]
- 67.Nishiyama K, Nakashima H, Ikarashi M, Kinoshita M, Nakashima M, Aosasa S, et al. Mouse CD11b+Kupffer cells recruited from bone marrow accelerate liver regeneration after partial hepatectomy. PLoS One. 2015;10:e0136774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Selzner N, Selzner M, Odermatt B, Tian Y, Van Rooijen N, Clavien PA. ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNF-alpha/IL-6 in mice. Gastroenterology. 2003;124:692–700. [DOI] [PubMed] [Google Scholar]
- 69.Shiratori Y, Hongo S, Hikiba Y, Ohmura K, Nagura T, Okano K, et al. Role of macrophages in regeneration of liver. Dig Dis Sci. 1996;41:1939–46. [DOI] [PubMed] [Google Scholar]
- 70.Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA. 1997;94:1441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rigual MDM, Angulo-Aguado M, Zagorac S, Alvarez-Diaz R, Benitez-Mondejar M, Yi F, et al. Macrophages harness hepatocyte glutamate to boost liver regeneration. Nature. 2025;641:1005–16 . [DOI] [PubMed]
- 72.Wang J, Kubes P. A reservoir of mature cavity macrophages that can rapidly invade visceral organs to affect tissue repair. Cell. 2016;165:668–78. [DOI] [PubMed] [Google Scholar]
- 73.Hosoya S, Ikejima K, Takeda K, Arai K, Ishikawa S, Yamagata H, et al. Innate immune responses involving natural killer and natural killer T cells promote liver regeneration after partial hepatectomy in mice. Am J Physiol Gastrointest Liver Physiol. 2013;304:G293–9. [DOI] [PubMed] [Google Scholar]
- 74.Sun R, Gao B. Negative regulation of liver regeneration by innate immunity (natural killer cells/interferon-gamma). Gastroenterology. 2004;127:1525–39. [DOI] [PubMed] [Google Scholar]
- 75.Rao R, Graffeo CS, Gulati R, Jamal M, Narayan S, Zambirinis CP, et al. Interleukin 17-producing gammadeltaT cells promote hepatic regeneration in mice. Gastroenterology. 2014;147:473–84 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.European Association for the Study of the L, European Association for the Study of D, European Association for the Study of O. EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81:492–542. [DOI] [PubMed] [Google Scholar]
- 78.Hernandez C, Huebener P, Pradere JP, Antoine DJ, Friedman RA, Schwabe RF. HMGB1 links chronic liver injury to progenitor responses and hepatocarcinogenesis. J Clin Investig. 2018;128:2436–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dudek M, Pfister D, Donakonda S, Filpe P, Schneider A, Laschinger M, et al. Autoaggressive CXCR6(+) CD8 T cells cause liver immune pathology in NASH. Nature. 2021;592:444–9. [DOI] [PubMed] [Google Scholar]
- 80.Shi H, Wang X, Li F, Gerlach BD, Yurdagul A Jr, Moore MP, et al. CD47-SIRPalpha axis blockade in NASH promotes necroptotic hepatocyte clearance by liver macrophages and decreases hepatic fibrosis. Sci Transl Med. 2022;14:eabp8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang M, Xu S, Han Y, Cao X. Apoptotic cells attenuate fulminant hepatitis by priming Kupffer cells to produce interleukin-10 through membrane-bound TGF-b. eta Hepatol. 2011;53:306–16. [DOI] [PubMed] [Google Scholar]
- 82.Mackowiak B, Fu Y, Maccioni L, Gao B. Alcohol-associated liver disease. J Clin Investig. 2024;134. [DOI] [PMC free article] [PubMed]
- 83.Feng D, Hwang S, Guillot A, Wang Y, Guan Y, Chen C, et al. Inflammation in alcohol-associated hepatitis: pathogenesis and therapeutic targets. Cell Mol Gastroenterol Hepatol. 2024;18:101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marin V, Poulsen K, Odena G, McMullen MR, Altamirano J, Sancho-Bru P, et al. Hepatocyte-derived macrophage migration inhibitory factor mediates alcohol-induced liver injury in mice and patients. J Hepatol. 2017;67:1018–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jaeschke H. Neutrophil-mediated tissue injury in alcoholic hepatitis. Alcohol. 2002;27:23–7. [DOI] [PubMed] [Google Scholar]
- 86.Kreimeyer H, Gonzalez CG, Fondevila MF, Hsu CL, Hartmann P, Zhang X, et al. Fecal proteomics links neutrophil degranulation with mortality in patients with alcohol-associated hepatitis. Gut. 2024;74:103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dominguez M, Miquel R, Colmenero J, Moreno M, Garcia-Pagan JC, Bosch J, et al. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639–50. [DOI] [PubMed] [Google Scholar]
- 88.Guan Y, Feng D, Maccioni L, Wang Y, Gao B. New therapeutic target for alcohol-associated hepatitis (AH): AH-associated IL-8(+) neutrophils. eGastroenterology. 2024;2:e100166. [DOI] [PMC free article] [PubMed]
- 89.Xu H, Wu Z, Qin J, Li X, Xu F, Wang W, et al. Stressed hepatocyte sustains alcohol-associated hepatitis progression by producing leukocyte cell-derived chemotaxin 2. Gut. 2025. Online ahead of print. [DOI] [PubMed]
- 90.Taieb J, Delarche C, Paradis V, Mathurin P, Grenier A, Crestani B, et al. Polymorphonuclear neutrophils are a source of hepatocyte growth factor in patients with severe alcoholic hepatitis. J Hepatol. 2002;36:342–8. [DOI] [PubMed] [Google Scholar]
- 91.Yang W, Tao Y, Wu Y, Zhao X, Ye W, Zhao D, et al. Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat Commun. 2019;10:1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Horner SM, Gale M Jr. Regulation of hepatic innate immunity by hepatitis C virus. Nat Med. 2013;19:879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Donaldson P, Underhill J, Doherty D, Hayllar K, Calne R, Tan KC, et al. Influence of human leukocyte antigen matching on liver allograft survival and rejection: “the dualistic effect. Hepatology. 1993;17:1008–15. [PubMed] [Google Scholar]
- 94.Benseler, Warren V, Vo A, Holz LE M, Tay SS, Le Couteur DG, et al. Hepatocyte entry leads to degradation of autoreactive CD8 T cells. Proc Natl Acad Sci USA. 2011;108:16735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Warren A, Le Couteur DG, Fraser R, Bowen DG, McCaughan GW, Bertolino P. T lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology. 2006;44:1182–90. [DOI] [PubMed] [Google Scholar]
- 96.Burwitz BJ, Hashiguchi PK, Mansouri M, Meyer C, Gilbride RM, Biswas S, et al. MHC-E-restricted CD8(+) T cells target hepatitis B virus-infected human hepatocytes. J Immunol. 2020;204:2169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu HC, Huang J, Pandyra AA, Pandey P, Wang R, Zhang Z, et al. Single MHC-I expression promotes virus-induced liver immunopathology. Hepatol Commun. 2022;6:1620–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Benechet AP, De Simone G, Di Lucia P, Cilenti F, Barbiera G, Le Bert N, et al. Dynamics and genomic landscape of CD8(+) T cells undergoing hepatic priming. Nature. 2019;574:200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wohlleber D, Knolle PA. Tissue determinants of antiviral immunity in the liver. Z Gastroenterol. 2025;63:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bertolino P, Trescol-Biemont MC, Rabourdin-Combe C. Hepatocytes induce functional activation of naïve CD8+ T lymphocytes but fail to promote survival. Eur J Immunol. 1998;28:221–36. [DOI] [PubMed] [Google Scholar]
- 101.Holz LE, Benseler V, Bowen DG, Bouillet P, Strasser A, O’Reilly L, et al. Intrahepatic murine CD8 T-cell activation associates with a distinct phenotype leading to Bim-dependent death. Gastroenterology. 2008;135:989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.De Simone G, Andreata F, Bleriot C, Fumagalli V, Laura C, Garcia-Manteiga JM, et al. Identification of a Kupffer cell subset capable of reverting the T-cell dysfunction induced by hepatocellular priming. Immunity. 2021;54:2089–100.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shin EC, Sung PS, Park SH. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat Rev Immunol. 2016;16:509–23. [DOI] [PubMed] [Google Scholar]
- 104.Wohlleber D, Kashkar H, Gartner K, Frings MK, Odenthal M, Hegenbarth S, et al. TNF-induced target cell killing by CTL activated through cross-presentation. Cell Rep. 2012;2:478–87. [DOI] [PubMed] [Google Scholar]
- 105.Zeissig S, Murata K, Sweet L, Publicover J, Hu Z, Kaser A, et al. Hepatitis B virus-induced lipid alterations contribute to natural killer T-cell-dependent protective immunity. Nat Med. 2012;18:1060–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lei Z, Tang R, Qi Q, Gu P, Wang J, Xu L, et al. Hepatocyte CD1d protects against liver immunopathology in mice with schistosomiasis japonica. Immunology. 2021;162:328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lu JG, Ji P, French SW. The major histocompatibility complex class II-CD4 immunologic synapse in alcoholic hepatitis and autoimmune liver pathology: the role of aberrant major histocompatibility complex class II in hepatocytes. Am J Pathol. 2020;190:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dezhbord M, Kim SH, Park S, Lee DR, Kim N, Won J, et al. Novel role of MHC class II transactivator in hepatitis B virus replication and viral counteraction. Clin Mol Hepatol. 2024;30:539–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lu JG, Iyasu A, French B, Tillman B, French SW. Overexpression of MHCII by hepatocytes in alcoholic hepatitis (AH) compared to nonalcoholic steatohepatitis (NASH) and normal controls. Alcohol. 2020;84:27–32. [DOI] [PubMed] [Google Scholar]
- 110.Lobo-Yeo A, Senaldi G, Portmann B, Mowat AP, Mieli-Vergani G, Vergani D. Class I and class II major histocompatibility complex antigen expression on hepatocytes: a study in children with liver disease. Hepatology. 1990;12:224–32. [DOI] [PubMed] [Google Scholar]
- 111.de Beijer MTA, Bezstarosti K, Luijten R, Doff WAS, Boor PPC, Pieterman RFA, et al. Immunopeptidome of hepatocytes isolated from patients with HBV infection and hepatocellular carcinoma. JHEP Rep. 2022;4:100576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. [DOI] [PubMed] [Google Scholar]
- 113.Zhang WJ, Li KY, Huang BH, Wang H, Wan SG, Zhou SC. The hepatocyte in the innate immunity. Virology. 2022;576:111–6. [DOI] [PubMed] [Google Scholar]
- 114.Carroll SL, Pasare C, Barton GM. Control of adaptive immunity by pattern recognition receptors. Immunity. 2024;57:632–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368:1907–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mercado-Gomez M, Goikoetxea-Usandizaga N, Kerbert AJC, Gracianteparaluceta LU, Serrano-Macia M, Lachiondo-Ortega S, et al. The lipopolysaccharide-TLR4 axis regulates hepatic glutaminase 1 expression promoting liver ammonia build-up as steatotic liver disease progresses to steatohepatitis. Metabolism. 2024;158:155952. [DOI] [PubMed] [Google Scholar]
- 117.Yu J, Zhu C, Wang X, Kim K, Bartolome A, Dongiovanni P, et al. Hepatocyte TLR4 triggers interhepatocyte Jagged1/Notch signaling to determine NASH-induced fibrosis. Sci Transl Med. 2021;13:eabe1692. [DOI] [PMC free article] [PubMed]
- 118.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–8. [DOI] [PubMed] [Google Scholar]
- 119.Fiorucci S, Marchiano S, Urbani G, Di Giorgio C, Distrutti E, Zampella A, et al. Immunology of bile acids regulated receptors. Prog Lipid Res. 2024;95:101291. [DOI] [PubMed] [Google Scholar]
- 120.Borude P, Edwards G, Walesky C, Li F, Ma X, Kong B, et al. Hepatocyte-specific deletion of farnesoid X receptor delays but does not inhibit liver regeneration after partial hepatectomy in mice. Hepatology. 2012;56:2344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Henry Z, Meadows V, Guo GL FXR and NASH: an avenue for tissue-specific regulation. Hepatol Commun. 2023;7. [DOI] [PMC free article] [PubMed]
- 122.Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–7. [DOI] [PubMed] [Google Scholar]
- 123.Ghallab A, Hassan R, Hofmann U, Friebel A, Hobloss Z, Brackhagen L, et al. Interruption of bile acid uptake by hepatocytes after acetaminophen overdose ameliorates hepatotoxicity. J Hepatol. 2022;77:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ye X, Zhang T, Han H. PPARalpha: a potential therapeutic target of cholestasis. Front Pharm. 2022;13:916866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li T, Hasan MN, Gu L. Bile acids regulation of cellular stress responses in liver physiology and diseases. eGastroenterology. 2024;2:e100074. [DOI] [PMC free article] [PubMed]
- 126.Hoff J, Xiong L, Kammann T, Neugebauer S, Micheel JM, Gassler N, et al. RIPK3 promoter hypermethylation in hepatocytes protects from bile acid-induced inflammation and necroptosis. Cell Death Dis. 2023;14:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Remetic J, Ghallab A, Hobloss Z, Brackhagen L, Hassan R, Myllys M, et al. Loss of bile salt export pump aggravates lipopolysaccharide-induced liver injury in mice due to impaired hepatic endotoxin clearance. Hepatology. 2022;75:1095–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Conde de la Rosa L, Fabrega L, Torres S, Nunez S, Ribas V, Segales P, et al. Stard1 promotes cholestatic liver injury and disease progression by sensitizing to bile acid hepatotoxicity. Hepatology. 2024. Online ahead of print. [DOI] [PubMed]
- 129.Zhang L, Pan Q, Zhang L, Xia H, Liao J, Zhang X, et al. Runt-related transcription factor-1 ameliorates bile acid-induced hepatic inflammation in cholestasis through JAK/STAT3 signaling. Hepatology. 2023;77:1866–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fiorucci S, Biagioli M, Zampella A, Distrutti E. Bile acids activated receptors regulate innate immunity. Front Immunol. 2018;9:1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity. 2016;45:802–16. [DOI] [PubMed] [Google Scholar]
- 132.Hao H, Cao L, Jiang C, Che Y, Zhang S, Takahashi S, et al. Farnesoid X receptor regulation of the NLRP3 inflammasome underlies cholestasis-associated sepsis. Cell Metab. 2017;25:856–67.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Perino A, Demagny H, Velazquez-Villegas L, Schoonjans K. Molecular physiology of bile acid signaling in health, disease, and aging. Physiol Rev. 2021;101:683–731. [DOI] [PubMed] [Google Scholar]
- 134.Perino A, Pols TW, Nomura M, Stein S, Pellicciari R, Schoonjans K. TGR5 reduces macrophage migration through mTOR-induced C/EBPbeta differential translation. J Clin Investig. 2014;124:5424–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Campbell C, McKenney PT, Konstantinovsky D, Isaeva OI, Schizas M, Verter J, et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature. 2020;581:475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Singh SP, Madke T, Chand P. Global epidemiology of hepatocellular carcinoma. J Clin Exp Hepatol. 2025;15:102446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Prim. 2021;7:6. [DOI] [PubMed] [Google Scholar]
- 138.Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147:765–83.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Haybaeck J, Zeller N, Wolf MJ, Weber A, Wagner U, Kurrer MO, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell. 2009;16:295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bettermann K, Vucur M, Haybaeck J, Koppe C, Janssen J, Heymann F, et al. TAK1 suppresses a NEMO-dependent but NF-kappaB-independent pathway to liver cancer. Cancer Cell. 2010;17:481–96. [DOI] [PubMed] [Google Scholar]
- 141.Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–32. [DOI] [PubMed] [Google Scholar]
- 142.Weber A, Boger R, Vick B, Urbanik T, Haybaeck J, Zoller S, et al. Hepatocyte-specific deletion of the antiapoptotic protein myeloid cell leukemia-1 triggers proliferation and hepatocarcinogenesis in mice. Hepatology. 2010;51:1226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, et al. Senescence surveillance of premalignant hepatocytes limits liver cancer development. Nature. 2011;479:547–51. [DOI] [PubMed] [Google Scholar]
- 144.Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19:151–72. [DOI] [PubMed] [Google Scholar]
- 145.Ringelhan M, Pfister D, O’Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222–32. [DOI] [PubMed] [Google Scholar]
- 146.Lemaitre L, Adeniji N, Suresh A, Reguram R, Zhang J, Park J, et al. Spatial analysis reveals targetable macrophage-mediated mechanisms of immune evasion in hepatocellular carcinoma minimal residual disease. Nat Cancer. 2024;5:1534–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li Y, Zhang J, Chen S, Ke Y, Li Y, Chen Y. Growth differentiation factor 15: Emerging role in liver diseases. Cytokine. 2024;182:156727. [DOI] [PubMed] [Google Scholar]
- 148.Melero I, de Miguel Luken M, de Velasco G, Garralda E, Martin-Liberal J, Joerger M, et al. Neutralizing GDF-15 can overcome anti-PD-1 and anti-PD-L1 resistance in solid tumors. Nature. 2025;637:1218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chiu DK, Zhang X, Cheng BY, Liu Q, Hayashi K, Yu B, et al. Tumor-derived erythropoietin acts as an immunosuppressive switch in cancer immunity. Science. 2025;388:eadr3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sirbe C, Simu G, Szabo I, Grama A, Pop TL. Pathogenesis of autoimmune hepatitis-cellular and molecular mechanisms. Int J Mol Sci. 2021;22:13578. [DOI] [PMC free article] [PubMed]
- 151.Engel B, Assis DN, Bhat M, Clusmann J, Drenth JP, Gerussi A, et al. Quo vadis autoimmune hepatitis? - Summary of the 5(th) international autoimmune hepatitis group research workshop 2024. JHEP Rep. 2025;7:101265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schultheiss C, Simnica D, Willscher E, Oberle A, Fanchi L, Bonzanni N, et al. Next-generation immunosequencing reveals pathological T-cell architecture in autoimmune hepatitis. Hepatology. 2021;73:1436–48. [DOI] [PubMed] [Google Scholar]
- 153.Renand A, Cervera-Marzal I, Gil L, Dong C, Garcia A, Kervagoret E, et al. Integrative molecular profiling of autoreactive CD4 T cells in autoimmune hepatitis. J Hepatol. 2020;73:1379–90. [DOI] [PubMed] [Google Scholar]
- 154.Heneghan MA, Lohse AW. Update in clinical science: autoimmune hepatitis. J Hepatol. 2025;82:926–37. [DOI] [PubMed]
- 155.Banales JM, Huebert RC, Karlsen T, Strazzabosco M, LaRusso NF, Gores GJ. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol. 2019;16:269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cai X, Tacke F, Guillot A, Liu H. Cholangiokines: undervalued modulators in the hepatic microenvironment. Front Immunol. 2023;14:1192840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jalan-Sakrikar N, Guicciardi ME, O’Hara SP, Azad A, LaRusso NF, Gores GJ, et al. Central role for cholangiocyte pathobiology in cholestatic liver diseases. Hepatology. 2024. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 158.Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Guillot A, Gasmi I, Brouillet A, Ait-Ahmed Y, Calderaro J, Ruiz I, et al. Interleukins-17 and 27 promote liver regeneration by sequentially inducing progenitor cell expansion and differentiation. Hepatol Commun. 2018;2:329–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Manco R, Clerbaux LA, Verhulst S, Bou Nader M, Sempoux C, Ambroise J, et al. Reactive cholangiocytes differentiate into proliferative hepatocytes with efficient DNA repair in mice with chronic liver injury. J Hepatol. 2019;70:1180–91. [DOI] [PubMed] [Google Scholar]
- 161.Gribben C, Galanakis V, Calderwood A, Williams EC, Chazarra-Gil R, Larraz M, et al. Acquisition of epithelial plasticity in human chronic liver disease. Nature. 2024;630:166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Andrews TS, Atif J, Liu JC, Perciani CT, Ma XZ, Thoeni C, et al. Single-cell, single-nucleus, and spatial RNA sequencing of the human liver identifies cholangiocyte and mesenchymal heterogeneity. Hepatol Commun. 2022;6:821–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Roos FJM, van Tienderen GS, Wu H, Bordeu I, Vinke D, Albarinos LM, et al. Human branching cholangiocyte organoids recapitulate functional bile duct formation. Cell Stem Cell. 2022;29:776–94.e13. [DOI] [PubMed] [Google Scholar]
- 164.Sampaziotis F, Muraro D, Tysoe OC, Sawiak S, Beach TE, Godfrey EM, et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science. 2021;371:839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang J, Xu Y, Chen Z, Liang J, Lin Z, Liang H, et al. Liver immune profiling reveals pathogenesis and therapeutics for biliary atresia. Cell. 2020;183:1867–83.e26. [DOI] [PubMed] [Google Scholar]
- 166.Xiao MH, Ma D, Wu S, Huang Z, Liang P, Chen H, et al. Integrative single-cell and spatial transcriptomic analyses identify a pathogenic cholangiocyte niche and TNFRSF12A as therapeutic target for biliary atresia. Hepatology. 2025;81:1146–63. [DOI] [PubMed] [Google Scholar]
- 167.Bird TG, Lu WY, Boulter L, Gordon-Keylock S, Ridgway RA, Williams MJ, et al. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc Natl Acad Sci USA. 2013;110:6542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jakubowski A, Ambrose C, Parr M, Lincecum JM, Wang MZ, Zheng TS, et al. TWEAK induces liver progenitor cell proliferation. J Clin Investig. 2005;115:2330–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lazaridis KN, LaRusso NF. The cholangiopathies. Mayo Clin Proc. 2015;90:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wu B, Shentu X, Nan H, Guo P, Hao S, Xu J, et al. A spatiotemporal atlas of cholestatic injury and repair in mice. Nat Genet. 2024;56:938–52. [DOI] [PubMed] [Google Scholar]
- 171.Herta T, Kersten R, Chang JC, Hubers L, Go S, Tolenaars D, et al. Role of the IgG4-related cholangitis autoantigen annexin A11 in cholangiocyte protection. J Hepatol. 2022;76:319–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.European Association for the Study of the L. EASL Clinical Practice Guidelines on sclerosing cholangitis. J Hepatol. 2022;77:761–806. [DOI] [PubMed] [Google Scholar]
- 173.Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis - a comprehensive review. J Hepatol. 2017;67:1298–323. [DOI] [PubMed] [Google Scholar]
- 174.Cornillet M, Geanon D, Bergquist A, Bjorkstrom NK. Immunobiology of primary sclerosing cholangitis. Hepatology. 2024. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 175.Guillot A, Winkler M, Silva Afonso M, Aggarwal A, Lopez D, Berger H, et al. Mapping the hepatic immune landscape identifies monocytic macrophages as key drivers of steatohepatitis and cholangiopathy progression. Hepatology. 2023;78:150–66. [DOI] [PubMed] [Google Scholar]
- 176.Frank AK, Chung BK, De Novales MLL, Engesaeter LK, Hoyle HW, Ogaard J, et al. Single-cell transcriptomic profiling of cholangiocyte organoids derived from bile ducts of primary sclerosing cholangitis patients. Dig Dis Sci. 2024;69:3810–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hirschfield GM, Dyson JK, Alexander GJM, Chapman MH, Collier J, Hubscher S, et al. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut. 2018;67:1568–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hirschfield GM, Liu X, Xu C, Lu Y, Xie G, Lu Y, et al. Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. N Engl J Med. 2009;360:2544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ronca V, Davies SP, Oo YH, Lleo A. The immunological landscape of primary biliary cholangitis: Mechanisms and therapeutic prospects. Hepatology. 2025. Online ahead of print. [DOI] [PubMed]
- 180.Leuschner M, Maier KP, Schlichting J, Strahl S, Herrmann G, Dahm HH, et al. Oral budesonide and ursodeoxycholic acid for treatment of primary biliary cirrhosis: results of a prospective double-blind trial. Gastroenterology. 1999;117:918–25. [DOI] [PubMed] [Google Scholar]
- 181.Jin C, Jiang P, Zhang Z, Han Y, Wen X, Zheng L, et al. Single-cell RNA sequencing reveals the pro-inflammatory roles of liver-resident Th1-like cells in primary biliary cholangitis. Nat Commun. 2024;15:8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li Y, Li Z, Chen R, Lian M, Wang H, Wei Y, et al. A regulatory variant at 19p13.3 is associated with primary biliary cholangitis risk and ARID3A expression. Nat Commun. 2023;14:1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Davies SP, Ronca V, Wootton GE, Krajewska NM, Bozward AG, Fiancette R, et al. Expression of E-cadherin by CD8(+) T cells promotes their invasion into biliary epithelial cells. Nat Commun. 2024;15:853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Miller PN, Baskaran S, Nijagal A. Immunology of biliary atresia. Semin Pediatr Surg. 2024;33:151474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang Y, Zhou L, Gu G, Feng M, Ding X, Xia Q, et al. CXCL8(high) inflammatory B cells in the peripheral blood of patients with biliary atresia are involved in disease progression. Immunol Cell Biol. 2020;98:682–92. [DOI] [PubMed] [Google Scholar]
- 186.Bednarek J, Traxinger B, Brigham D, Roach J, Orlicky D, Wang D, et al. Cytokine-producing B cells promote immune-mediated bile duct injury in murine biliary atresia. Hepatology. 2018;68:1890–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Thapa M, Tedesco D, Gumber S, Elrod EJ, Han JH, Kitchens WH, et al. Blockade of BAFF reshapes the hepatic B-cell receptor repertoire and attenuates autoantibody production in cholestatic liver disease. J Immunol. 2020;204:3117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chung BK, Guevel BT, Reynolds GM, Gupta Udatha DB, Henriksen EK, Stamataki Z, et al. Phenotyping and autoantibody production by liver-infiltrating B cells in primary sclerosing cholangitis and primary biliary cholangitis. J Autoimmun. 2017;77:45–54. [DOI] [PubMed] [Google Scholar]
- 189.Berglin L, Bjorkstrom NK, Bergquist A. Primary sclerosing cholangitis is associated with autoreactive IgA antibodies against biliary epithelial cells. Scand J Gastroenterol. 2013;48:719–28. [DOI] [PubMed] [Google Scholar]
- 190.Kotsiliti E, Leone V, Schuehle S, Govaere O, Li H, Wolf MJ, et al. Intestinal B cells license metabolic T-cell activation in NASH microbiota/antigen-independently and contribute to fibrosis by IgA-FcR signaling. J Hepatol. 2023;79:296–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bertolini A, Nguyen M, Zehra SA, Taleb SA, Bauer-Pisani T, Palm N, et al. Prominent role of gut dysbiosis in the pathogenesis of cystic fibrosis-related liver disease in mice. J Hepatol. 2024;81:429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Harada K. Sclerosing and obstructive cholangiopathy in biliary atresia: mechanisms and association with biliary innate immunity. Pediatr Surg Int. 2017;33:1243–8. [DOI] [PubMed] [Google Scholar]
- 193.Labiano I, Agirre-Lizaso A, Olaizola P, Echebarria A, Huici-Izagirre M, Olaizola I, et al. TREM-2 plays a protective role in cholestasis by acting as a negative regulator of inflammation. J Hepatol. 2022;77:991–1004. [DOI] [PubMed] [Google Scholar]
- 194.Li J, Razumilava N, Gores GJ, Walters S, Mizuochi T, Mourya R, et al. Biliary repair and carcinogenesis are mediated by IL-33-dependent cholangiocyte proliferation. J Clin Invest. 2014;124:3241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jeffery HC, Hunter S, Humphreys EH, Bhogal R, Wawman RE, Birtwistle J, et al. Bidirectional cross-talk between biliary epithelium and Th17 cells promotes local Th17 expansion and bile duct proliferation in biliary liver diseases. J Immunol. 2019;203:1151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Guillot A, Hamdaoui N, Bizy A, Zoltani K, Souktani R, Zafrani ES, et al. Cannabinoid receptor 2 counteracts interleukin-17-induced immune and fibrogenic responses in mouse liver. Hepatology. 2014;59:296–306. [DOI] [PubMed] [Google Scholar]
- 197.Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143:765–76.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yang CY, Ma X, Tsuneyama K, Huang S, Takahashi T, Chalasani NP, et al. IL-12/Th1 and IL-23/Th17 biliary microenvironment in primary biliary cirrhosis: implications for therapy. Hepatology. 2014;59:1944–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gasmi I, Machou C, Rodrigues A, Brouillet A, Nguyen TC, Rousseau B, et al. Interleukin-17 programs liver progenitor cell transformation into cancer stem cells through miR-122 downregulation with increased risk of primary liver cancer initiation. Int J Biol Sci. 2022;18:1944–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Guillot A, Guerri L, Feng D, Kim SJ, Ahmed YA, Paloczi J, et al. Bile acid-activated macrophages promote biliary epithelial cell proliferation through integrin alphavbeta6 upregulation following liver injury. J Clin Investig. 2021;131:e132305. [DOI] [PMC free article] [PubMed]
- 201.Guicciardi ME, Trussoni CE, Krishnan A, Bronk SF, Lorenzo Pisarello MJ, O’Hara SP, et al. Macrophages contribute to the pathogenesis of sclerosing cholangitis in mice. J Hepatol. 2018;69:676–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lu WY, Bird TG, Boulter L, Tsuchiya A, Cole AM, Hay T, et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat Cell Biol. 2015;17:971–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Krishnan A, Ozturk NB, Cutshaw KA, Guicciardi ME, Kitagataya T, Olson KE, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) deletion in myeloid cells augments cholestatic liver injury. Sci Rep. 2024;14:2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Arino S, Aguilar-Bravo B, Coll M, Lee WY, Peiseler M, Cantallops-Vila P, et al. Ductular reaction-associated neutrophils promote biliary epithelium proliferation in chronic liver disease. J Hepatol. 2023;79:1025–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Govaere O, Cockell S, Van Haele M, Wouters J, Van Delm W, Van den Eynde K, et al. High-throughput sequencing identifies etiology-dependent differences in ductular reaction in human chronic liver disease. J Pathol. 2019;248:66–76. [DOI] [PubMed] [Google Scholar]
- 206.Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L, et al. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014;59:1393–405. [DOI] [PubMed] [Google Scholar]
- 207.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. [DOI] [PubMed] [Google Scholar]
- 208.Paillet J, Plantureux C, Levesque S, Le Naour J, Stoll G, Sauvat A, et al. Autoimmunity affecting the biliary tract fuels the immunosurveillance of cholangiocarcinoma. J Exp Med. 2021;218:e20200853. [DOI] [PMC free article] [PubMed]
- 209.Petrick JL, Yang B, Altekruse SF, Van Dyke AL, Koshiol J, Graubard BI, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based study in SEER-Medicare. PLoS One. 2017;12:e0186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vithayathil M, Khan SA. Current epidemiology of cholangiocarcinoma in Western countries. J Hepatol. 2022;77:1690–8. [DOI] [PubMed] [Google Scholar]
- 212.Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, et al. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Investig. 2012;122:2911–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hsu BY, Driscoll J, Human Cholangiocarcinogenesis P, Tateno C, Mattis AN, Kelley RK, et al. Human hepatocytes can give rise to intrahepatic cholangiocarcinomas. Gastroenterology. 2024;167:1029–32.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fabris L, Perugorria MJ, Mertens J, Bjorkstrom NK, Cramer T, Lleo A, et al. The tumor microenvironment and immune milieu of cholangiocarcinoma. Liver Int. 2019;39:63–78. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Goeppert B, Roessler S, Renner M, Singer S, Mehrabi A, Vogel MN, et al. Mismatch repair deficiency is a rare but putative therapeutically relevant finding in nonliver fluke associated cholangiocarcinoma. Br J Cancer. 2019;120:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Subimerb C, Pinlaor S, Lulitanond V, Khuntikeo N, Okada S, McGrath MS, et al. Circulating CD14(+) CD16(+) monocyte levels predict tissue invasive character of cholangiocarcinoma. Clin Exp Immunol. 2010;161:471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang Z, Dai Y, Zhou Y, Wang Y, Chen P, Li Y, et al. Research progress of T cells in cholangiocarcinoma. Front Immunol. 2025;16:1453344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhang N, Sun L, Zhou S, Ji C, Cui T, Chu Q, et al. Cholangiocarcinoma PDHA1 succinylation suppresses macrophage antigen presentation via alpha-ketoglutaric acid accumulation. Nat Commun. 2025;16:3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Charbel A, Tavernar L, Albrecht T, Brinkmann F, Verheij J, Roos E, et al. Spatiotemporal analysis of tumor-infiltrating immune cells in biliary carcinogenesis. Br J Cancer. 2022;127:1603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Xiang X, Liu Z, Zhang C, Li Z, Gao J, Zhang C, et al. IDH mutation subgroup status associates with intratumor heterogeneity and the tumor microenvironment in intrahepatic cholangiocarcinoma. Adv Sci. 2021;8:e2101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wake K. Hepatic stellate cells: Three-dimensional structure, localization, heterogeneity and development. Proc Jpn Acad Ser B Phys Biol Sci. 2006;82:155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Xiong X, Kuang H, Ansari S, Liu T, Gong J, Wang S, et al. Landscape of Intercellular Crosstalk in Healthy and NASH Liver Revealed by Single-Cell Secretome Gene Analysis. Mol Cell. 2019;75:644–60.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Filliol A, Saito Y, Nair A, Dapito DH, Yu LX, Ravichandra A, et al. Opposing roles of hepatic stellate cell subpopulations in hepatocarcinogenesis. Nature. 2022;610:356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhao D, Huang Z, Li X, Wang H, Hou Q, Wang Y, et al. GDF2 and BMP10 coordinate liver cellular crosstalk to maintain liver health. Elife. 2024;13:RP95811. [DOI] [PMC free article] [PubMed]
- 226.Schwabe RF, Brenner DA. Hepatic stellate cells: balancing homeostasis, hepatoprotection and fibrogenesis in health and disease. Nat Rev Gastroenterol Hepatol. 2025. Online ahead of print. [DOI] [PubMed]
- 227.Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its etiology. Nat Commun. 2013;4:2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. [DOI] [PubMed] [Google Scholar]
- 229.Karsdal MA, Nielsen SH, Leeming DJ, Langholm LL, Nielsen MJ, Manon-Jensen T, et al. The good and the bad collagens of fibrosis - Their role in signaling and organ function. Adv Drug Deliv Rev. 2017;121:43–56. [DOI] [PubMed] [Google Scholar]
- 230.Bataller R, Brenner DA. Liver fibrosis. J Clin Investig. 2005;115:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gao J, Wei B, de Assuncao TM, Liu Z, Hu X, Ibrahim S, et al. Hepatic stellate cell autophagy inhibits extracellular vesicle release to attenuate liver fibrosis. J Hepatol. 2020;73:1144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Khanal S, Liu Y, Bamidele AO, Wixom AQ, Washington AM, Jalan-Sakrikar N, et al. Glycolysis in hepatic stellate cells coordinates fibrogenic extracellular vesicle release spatially to amplify liver fibrosis. Sci Adv. 2024;10:eadn5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kostallari E, Hirsova P, Prasnicka A, Verma VK, Yaqoob U, Wongjarupong N, et al. Hepatic stellate cell-derived platelet-derived growth factor receptor-alpha-enriched extracellular vesicles promote liver fibrosis in mice through SHP2. Hepatology. 2018;68:333–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Carter JK, Tsai MC, Venturini N, Hu J, Lemasters JJ, Torres Martin M, et al. Stellate cell-specific adhesion molecule protocadherin 7 regulates sinusoidal contraction. Hepatology. 2024;80:566–77. [DOI] [PMC free article] [PubMed]
- 235.Rockey DC. Hepatic fibrosis, stellate cells, and portal hypertension. Clin Liver Dis. 2006;10:459–79. vii-viii. [DOI] [PubMed] [Google Scholar]
- 236.Carter JK, Friedman SL. Hepatic stellate cell-immune interactions in NASH. Front Endocrinol. 2022;13:867940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61:1066–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pradere JP, Kluwe J, De Minicis S, Jiao JJ, Gwak GY, Dapito DH, et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58:1461–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rivera CA, Bradford BU, Hunt KJ, Adachi Y, Schrum LW, Koop DR, et al. Attenuation of CCl(4)-induced hepatic fibrosis by GdCl(3) treatment or dietary glycine. Am J Physiol Gastrointest Liver Physiol. 2001;281:G200–7. [DOI] [PubMed] [Google Scholar]
- 241.Ezzo M, Spindler K, Wang JB, Lee D, Pecoraro G, Cowen J, et al. Acute contact with profibrotic macrophages mechanically activates fibroblasts via alphavbeta3 integrin-mediated engagement of Piezo1. Sci Adv. 2024;10:eadp4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.McHedlidze T, Waldner M, Zopf S, Walker J, Rankin AL, Schuchmann M, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Novobrantseva TI, Majeau GR, Amatucci A, Kogan S, Brenner I, Casola S, et al. Attenuated liver fibrosis in the absence of B cells. J Clin Investig. 2005;115:3072–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Faggioli F, Palagano E, Di Tommaso L, Donadon M, Marrella V, Recordati C, et al. B lymphocytes limit senescence-driven fibrosis resolution and favor hepatocarcinogenesis in mouse liver injury. Hepatology. 2018;67:1970–85. [DOI] [PubMed] [Google Scholar]
- 245.Savage TM, Fortson KT, de Los Santos-Alexis K, Oliveras-Alsina A, Rouanne M, Rae SS, et al. Amphiregulin from regulatory T cells promotes liver fibrosis and insulin resistance in nonalcoholic steatohepatitis. Immunity. 2024;57:303–18.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Peiseler M, Schwabe R, Hampe J, Kubes P, Heikenwalder M, Tacke F. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease—novel insights into cellular communication circuits. J Hepatol. 2022;77:1136–60. [DOI] [PubMed] [Google Scholar]
- 247.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–52. [DOI] [PubMed] [Google Scholar]
- 248.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–63. [DOI] [PubMed] [Google Scholar]
- 249.Erkelens MN, Mebius RE. Retinoic acid and immune homeostasis: a balancing act. Trends Immunol. 2017;38:168–80. [DOI] [PubMed] [Google Scholar]
- 250.Schildberg FA, Wojtalla A, Siegmund SV, Endl E, Diehl L, Abdullah Z, et al. Murine hepatic stellate cells veto CD8 T-cell activation by a CD54-dependent mechanism. Hepatology. 2011;54:262–72. [DOI] [PubMed] [Google Scholar]
- 251.Li Y, Lu L, Qian S, Fung JJ, Lin F. Hepatic stellate cells directly inhibit B cells via programmed death-ligand 1. J Immunol. 2016;196:1617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ, et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40:1312–21. [DOI] [PubMed] [Google Scholar]
- 253.Crispe IN. Liver antigen-presenting cells. J Hepatol. 2011;54:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, et al. Ito cells are liver-resident antigen-presenting cells for activating T-cell responses. Immunity. 2007;26:117–29. [DOI] [PubMed] [Google Scholar]
- 255.Ebrahimkhani MR, Mohar I, Crispe IN. Cross-presentation of antigen by diverse subsets of murine liver cells. Hepatology. 2011;54:1379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Watanabe A, Hashmi A, Gomes DA, Town T, Badou A, Flavell RA, et al. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. 2007;46:1509–18. [DOI] [PubMed] [Google Scholar]
- 257.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322–35. [DOI] [PubMed] [Google Scholar]
- 258.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–97 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65:1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hagstrom H, Nasr P, Ekstedt M, Hammar U, Stal P, Hultcrantz R, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67:1265–73. [DOI] [PubMed] [Google Scholar]
- 261.Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021;385:1559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mehal W. Mechanisms of liver fibrosis in metabolic syndrome. eGastroenterology. 2023;1:e100015. [DOI] [PMC free article] [PubMed]
- 263.Guilliams M, Bonnardel J, Haest B, Vanderborght B, Wagner C, Remmerie A, et al. Spatial proteogenomics reveals distinct and evolutionarily conserved hepatic macrophage niches. Cell. 2022;185:379–96.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Vonderlin J, Chavakis T, Sieweke M, Tacke F. The multifaceted roles of macrophages in NAFLD pathogenesis. Cell Mol Gastroenterol Hepatol. 2023;15:1311–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Hammerich L, Tacke F. Hepatic inflammatory responses in liver fibrosis. Nat Rev Gastroenterol Hepatol. 2023;20:633–46. [DOI] [PubMed] [Google Scholar]
- 267.Ganguly S, Rosenthal SB, Ishizuka K, Troutman TD, Rohm TV, Khader N, et al. Lipid-associated macrophages’ promotion of fibrosis resolution during MASH regression requires TREM2. Proc Natl Acad Sci USA. 2024;121:e2405746121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.De Ponti FF, Bujko A, Liu Z, Collins PJ, Schuermans S, Maueroder C, et al. Spatially restricted and ontogenically distinct hepatic macrophages are required for tissue repair. Immunity. 2025;58:362–80.e10. [DOI] [PubMed] [Google Scholar]
- 269.Kang GG, Trevaskis NL, Murphy AJ, Febbraio MA. Diet-induced gut dysbiosis and inflammation: Key drivers of obesity-driven NASH. iScience. 2023;26:105905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412–25. [DOI] [PubMed] [Google Scholar]
- 271.Brennan PN, MacMillan M, Manship T, Moroni F, Glover A, Troland D, et al. Autologous macrophage therapy for liver cirrhosis: a phase 2 open-label randomized controlled trial. Nat Med. 2025;31:979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci USA. 2012;109:E3186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ramachandran P, Iredale JP. Macrophages: central regulators of hepatic fibrogenesis and fibrosis resolution. J Hepatol. 2012;56:1417–9. [DOI] [PubMed] [Google Scholar]
- 274.Fallowfield JA, Mizuno M, Kendall TJ, Constandinou CM, Benyon RC, Duffield JS, et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol. 2007;178:5288–95. [DOI] [PubMed] [Google Scholar]
- 275.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 276.Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–62. [DOI] [PubMed] [Google Scholar]
- 278.Affo S, Filliol A, Gores GJ, Schwabe RF. Fibroblasts in liver cancer: functions and therapeutic translation. Lancet Gastroenterol Hepatol. 2023;8:748–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Affo S, Nair A, Brundu F, Ravichandra A, Bhattacharjee S, Matsuda M, et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell. 2021;39:866–82.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Bhattacharjee S, Hamberger F, Ravichandra A, Miller M, Nair A, Affo S, et al. Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts. J Clin Investig. 2021;131:e146987. [DOI] [PMC free article] [PubMed]
- 281.Zhao W, Su W, Kuang P, Zhang L, Liu J, Yin Z, et al. The role of hepatic stellate cells in the regulation of T-cell function and the promotion of hepatocellular carcinoma. Int J Oncol. 2012;41:457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zhao W, Zhang L, Yin Z, Su W, Ren G, Zhou C, et al. Activated hepatic stellate cells promote hepatocellular carcinoma development in immunocompetent mice. Int J Cancer. 2011;129:2651–61. [DOI] [PubMed] [Google Scholar]
- 283.Zhao W, Zhang L, Xu Y, Zhang Z, Ren G, Tang K, et al. Hepatic stellate cells promote tumor progression by enhancement of immunosuppressive cells in an orthotopic liver tumor mouse model. Lab Invest. 2014;94:182–91. [DOI] [PubMed] [Google Scholar]
- 284.Xu Y, Zhao W, Xu J, Li J, Hong Z, Yin Z, et al. Activated hepatic stellate cells promote liver cancer by induction of myeloid-derived suppressor cells through cyclooxygenase-2. Oncotarget. 2016;7:8866–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Gao J, Lan T, Kostallari E, Guo Y, Lai E, Guillot A, et al. Angiocrine signaling in sinusoidal homeostasis and liver diseases. J Hepatol. 2024;81:543–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kostallari E, Shah VH. Angiocrine signaling in the hepatic sinusoids in health and disease. Am J Physiol Gastrointest Liver Physiol. 2016;311:G246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Leibing T, Geraud C, Augustin I, Boutros M, Augustin HG, Okun JG, et al. Angiocrine Wnt signaling controls liver growth and metabolic maturation in mice. Hepatology. 2018;68:707–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Rocha AS, Vidal V, Mertz M, Kendall TJ, Charlet A, Okamoto H, et al. The angiocrine factor Rspondin3 is a key determinant of liver zonation. Cell Rep. 2015;13:1757–64. [DOI] [PubMed] [Google Scholar]
- 289.Cooper SA, Kostallari E, Shah VH. Angiocrine signaling in sinusoidal health and disease. Semin Liver Dis. 2023;43:245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Iwakiri Y. Unlocking the role of liver sinusoidal endothelial cells: Key players in liver fibrosis: Editorial on “Liver sinusoidal endothelial cell: an important yet often overlooked player in the liver fibrosis. Clin Mol Hepatol. 2024;30:673–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.MacParland SA, Liu JC, Ma XZ, Innes BT, Bartczak AM, Gage BK, et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun. 2018;9:4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.McConnell MJ, Kostallari E, Ibrahim SH, Iwakiri Y. The evolving role of liver sinusoidal endothelial cells in liver health and disease. Hepatology. 2023;78:649–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Su T, Yang Y, Lai S, Jeong J, Jung Y, McConnell M, et al. Single-cell transcriptomics reveals zone-specific alterations of liver sinusoidal endothelial cells in cirrhosis. Cell Mol Gastroenterol Hepatol. 2021;11:1139–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kondo R, Iwakiri Y. The lymphatic system in alcohol-associated liver disease. Clin Mol Hepatol. 2020;26:633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Yang Y, Jeong J, Su T, Lai S, Zhang P, Garcia-Milian R, et al. Interleukin-7-based identification of liver lymphatic endothelial cells reveals their unique structural features. JHEP Rep. 2024;6:101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. [DOI] [PubMed] [Google Scholar]
- 297.Gibert-Ramos A, Andres-Rozas M, Pasto R, Alfaro-Retamero P, Guixe-Muntet S, Gracia-Sancho J. Sinusoidal communication in chronic liver disease. Clin Mol Hepatol. 2025;31:32–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Sakai M, Troutman TD, Seidman JS, Ouyang Z, Spann NJ, Abe Y, et al. Liver-derived signals sequentially reprogram myeloid enhancers to initiate and maintain kupffer cell identity. Immunity. 2019;51:655–70.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Bonnardel J, T’Jonck W, Gaublomme D, Browaeys R, Scott CL, Martens L, et al. Stellate cells, hepatocytes, and endothelial cells imprint the Kupffer cell identity on monocytes colonizing the liver macrophage niche. Immunity. 2019;51:638–54.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Liu J, Yu Q, Wu W, Huang X, Broering R, Werner M, et al. TLR2 Stimulation Strengthens Intrahepatic Myeloid-Derived Cell-Mediated T-Cell Tolerance through Inducing Kupffer Cell Expansion and IL-10 Production. J Immunol. 2018;200:2341–51. [DOI] [PubMed] [Google Scholar]
- 301.Diehl L, Schurich A, Grochtmann R, Hegenbarth S, Chen L, Knolle PA. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T-cell tolerance. Hepatology. 2008;47:296–305. [DOI] [PubMed] [Google Scholar]
- 302.Carambia A, Freund B, Schwinge D, Heine M, Laschtowitz A, Huber S, et al. TGF-beta-dependent induction of CD4(+)CD25(+)Foxp3(+) Tregs by liver sinusoidal endothelial cells. J Hepatol. 2014;61:594–9. [DOI] [PubMed] [Google Scholar]
- 303.Scholzel K, Schildberg FA, Welz M, Borner C, Geiger S, Kurts C, et al. Transfer of MHC-class-I molecules among liver sinusoidal cells facilitates hepatic immune surveillance. J Hepatol. 2014;61:600–8. [DOI] [PubMed] [Google Scholar]
- 304.DeLeve LD. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology. 2015;61:1740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Gan C, Yaqoob U, Lu J, Xie M, Anwar A, Jalan-Sakrikar N, et al. Liver sinusoidal endothelial cells contribute to portal hypertension through collagen type IV-driven sinusoidal remodeling. JCI Insight. 2024;9:e174775. [DOI] [PMC free article] [PubMed]
- 306.Gracia-Sancho J, Lavina B, Rodriguez-Vilarrupla A, Garcia-Caldero H, Bosch J, Garcia-Pagan JC. Enhanced vasoconstrictor prostanoid production by sinusoidal endothelial cells increases portal perfusion pressure in cirrhotic rat livers. J Hepatol. 2007;47:220–7. [DOI] [PubMed] [Google Scholar]
- 307.Popper H, Barka T, Goldfarb S, Hutterer F, Paronetto F, Rubin E, et al. Factors determining chronicity of liver disease. a progress report. J Mt Sinai Hosp N Y. 1963;30:336–48. [PubMed] [Google Scholar]
- 308.Hilscher MB, Sehrawat T, Arab JP, Zeng Z, Gao J, Liu M, et al. Mechanical stretch increases expression of CXCL1 in liver sinusoidal endothelial cells to recruit neutrophils, generate sinusoidal microthombi, and promote portal hypertension. Gastroenterology. 2019;157:193–209.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Greuter T, Yaqoob U, Gan C, Jalan-Sakrikar N, Kostallari E, Lu J, et al. Mechanotransduction-induced glycolysis epigenetically regulates a CXCL1-dominant angiocrine signaling program in liver sinusoidal endothelial cells in vitro and in vivo. J Hepatol. 2022;77:723–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Gao J, Wei B, Liu M, Hirsova P, Sehrawat TS, Cao S, et al. Endothelial p300 promotes portal hypertension and hepatic fibrosis through C-C motif chemokine ligand 2-mediated angiocrine signaling. Hepatology. 2021;73:2468–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Guan Y, Peiffer B, Feng D, Parra MA, Wang Y, Fu Y, et al. IL-8+ neutrophils drive inexorable inflammation in severe alcohol-associated hepatitis. J Clin Investig. 2024;134. [DOI] [PMC free article] [PubMed]
- 312.Liu M, Cao S, He L, Gao J, Arab JP, Cui H, et al. Super enhancer regulation of cytokine-induced chemokine production in alcoholic hepatitis. Nat Commun. 2021;12:4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Mandrekar P, Mandal A. Pathogenesis of alcohol-associated liver disease. Clin Liver Dis. 2024;28:647–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Verhaegh P, Wisse E, de Munck T, Greve JW, Verheij J, Riedl R, et al. Electron microscopic observations in perfusion-fixed human nonalcoholic fatty liver disease biopsies. Pathology. 2021;53:220–8. [DOI] [PubMed] [Google Scholar]
- 315.Furuta K, Guo Q, Pavelko KD, Lee JH, Robertson KD, Nakao Y, et al. Lipid-induced endothelial vascular cell adhesion molecule 1 promotes nonalcoholic steatohepatitis pathogenesis. J Clin Investig. 2021;131:e143690. [DOI] [PMC free article] [PubMed]
- 316.Guo Q, Furuta K, Islam S, Caporarello N, Kostallari E, Dielis K, et al. Liver sinusoidal endothelial cell expressed vascular cell adhesion molecule 1 promotes liver fibrosis. Front Immunol. 2022;13:983255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Khoury M, Guo Q, Furuta K, Correia C, Meroueh C, Kim Lee HS, et al. Glycogen synthase kinase 3 activity enhances liver inflammation in MASH. JHEP Rep. 2024;6:101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Burtis AEC, DeNicola DMC, Ferguson ME, Santos RG, Pinilla C, Kriss MS, et al. Ag-driven CD8 + T-cell clonal expansion is a prominent feature of MASH in humans and mice. Hepatology. 2025;81:591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Guidotti LG, Inverso D, Sironi L, Di Lucia P, Fioravanti J, Ganzer L, et al. Immunosurveillance of the liver by intravascular effector CD8(+) T cells. Cell. 2015;161:486–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Xie X, Luo J, Broering R, Zhu D, Zhou W, Lu M, et al. HBeAg induces liver sinusoidal endothelial cell activation to promote intrahepatic CD8 T-cell immunity and HBV clearance. Cell Mol Immunol. 2021;18:2572–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Du Y, Yan H, Zou S, Khera T, Li J, Han M, et al. Natural killer cells regulate the maturation of liver sinusoidal endothelial cells thereby promoting intrahepatic T-cell responses in a mouse model. Hepatol Commun. 2021;5:865–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Carambia A, Gottwick C, Schwinge D, Stein S, Digigow R, Seleci M, et al. Nanoparticle-mediated targeting of autoantigen peptide to cross-presenting liver sinusoidal endothelial cells protects from CD8 T-cell-driven autoimmune cholangitis. Immunology. 2021;162:452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Yu M, Li X, Xu L, Zheng C, Pan W, Chen H, et al. Neutrophil extracellular traps induce intrahepatic thrombotic tendency and liver damage in cholestatic liver disease. Hepatol Commun. 2024;8:e0513. [DOI] [PMC free article] [PubMed]
- 324.Ito Y, Bethea NW, Abril ER, McCuskey RS. Early hepatic microvascular injury in response to acetaminophen toxicity. Microcirculation. 2003;10:391–400. [DOI] [PubMed] [Google Scholar]
- 325.Biagioli M, Marchiano S, di Giorgio C, Roselli R, Bordoni M, Bellini R, et al. Combinatorial targeting of G-protein-coupled bile acid receptor 1 and cysteinyl leukotriene receptor 1 reveals a mechanistic role for bile acids and leukotrienes in drug-induced liver injury. Hepatology. 2023;78:26–44. [DOI] [PubMed] [Google Scholar]
- 326.Kawai H, Osawa Y, Matsuda M, Tsunoda T, Yanagida K, Hishikawa D, et al. Sphingosine-1-phosphate promotes tumor development and liver fibrosis in mouse model of congestive hepatopathy. Hepatology. 2022;76:112–25. [DOI] [PubMed] [Google Scholar]
- 327.Geraud C, Mogler C, Runge A, Evdokimov K, Lu S, Schledzewski K, et al. Endothelial transdifferentiation in hepatocellular carcinoma: loss of Stabilin-2 expression in peri-tumorous liver correlates with increased survival. Liver Int. 2013;33:1428–40. [DOI] [PubMed] [Google Scholar]
- 328.Zhang C, Gao Y, Du C, Markowitz GJ, Fu J, Zhang Z, et al. Hepatitis B-induced IL8 promotes hepatocellular carcinoma venous metastasis and intrahepatic treg accumulation. Cancer Res. 2021;81:2386–98. [DOI] [PubMed] [Google Scholar]
- 329.Lu Y, Liu Y, Zuo X, Li G, Wang J, Liu J, et al. CXCL12(+) tumor-associated endothelial cells promote immune resistance in hepatocellular carcinoma. J Hepatol. 2025;82:634–48. [DOI] [PubMed] [Google Scholar]
- 330.Kremer KN, Khammash HA, Miranda AM, Rutt LN, Twardy SM, Anton PE, et al. Liver sinusoidal endothelial cells regulate the balance between hepatic immunosuppression and immunosurveillance. Front Immunol. 2024;15:1497788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Yu Z, Guo J, Liu Y, Wang M, Liu Z, Gao Y, et al. Nano delivery of simvastatin targets liver sinusoidal endothelial cells to remodel tumor microenvironment for hepatocellular carcinoma. J Nanobiotechnol. 2022;20:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Wallace SJ, Tacke F, Schwabe RF, Henderson NC. Understanding the cellular interactome of nonalcoholic fatty liver disease. JHEP Rep. 2022;4:100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Guillot A, Tacke F. Spatial dimension of macrophage heterogeneity in liver diseases. eGastroenterology. 2023;1:e000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Wen Y, Ma L, Ju C. Recent insights into the pathogenesis and therapeutic targets of chronic liver diseases. eGastroenterology. 2023;1:e100020. [DOI] [PMC free article] [PubMed]
- 335.Ramachandran P, Dobie R, Wilson-Kanamori JR, Dora EF, Henderson BEP, Luu NT, et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019;575:512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Bosch M, Kallin N, Donakonda S, Zhang JD, Wintersteller H, Hegenbarth S, et al. A liver immune rheostat regulates CD8 T-cell immunity in chronic HBV infection. Nature. 2024;631:867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Hong L, Mei J, Sun X, Wu Y, Dong Z, Jin Y, et al. Spatial single-cell proteomics landscape decodes the tumor microenvironmental ecosystem of intrahepatic cholangiocarcinoma. Hepatology. 2025. Online ahead of print. [DOI] [PubMed]
- 338.Li Z, Ma L, Chen M, Chen X, Sha M, Hang H. Single-cell analyses reveal metastasis mechanism and microenvironment remodeling of lymph node in intrahepatic cholangiocarcinoma. JHEP Rep. 2025;7:101275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Myojin Y, Babaei S, Trehan R, Hoffman C, Kedei N, Ruf B, et al. Multiomics analysis of immune correlatives in hepatocellular carcinoma patients treated with tremelimumab plus durvalumab. Gut. 2025. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 340.Araujo David B, Atif J, Vargas ESCF, Yasmin T, Guillot A, Ait Ahmed Y, et al. Kupffer cell reverse migration into the liver sinusoids mitigates neonatal sepsis and meningitis. Sci Immunol. 2024;9:eadq9704. [DOI] [PubMed] [Google Scholar]
- 341.Jia G, He P, Dai T, Goh D, Wang J, Sun M, et al. Spatial immune scoring system predicts hepatocellular carcinoma recurrence. Nature. 2025. Online ahead of print. [DOI] [PubMed]
- 342.Peiseler M, Araujo David B, Zindel J, Surewaard BGJ, Lee WY, Heymann F, et al. Kupffer cell-like syncytia replenish resident macrophage function in the fibrotic liver. Science. 2023;381:eabq5202. [DOI] [PubMed] [Google Scholar]
- 343.Guillot A, Kohlhepp MS, Bruneau A, Heymann F, Tacke F. Deciphering the immune microenvironment on A single archival formalin-fixed paraffin-embedded tissue section by an immediately implementable multiplex fluorescence immunostaining protocol. Cancers. 2020;12:2449. [DOI] [PMC free article] [PubMed]
- 344.Feng D, Xiang X, Guan Y, Guillot A, Lu H, Chang C, et al. Monocyte-derived macrophages orchestrate multiple cell-type interactions to repair necrotic liver lesions in disease models. J Clin Investig. 2023;133:e166954. [DOI] [PMC free article] [PubMed]
- 345.Bolognesi MM, Dall’Olio L, Maerten A, Borghesi S, Castellani G, Cattoretti G. Seeing or believing in hyperplexed spatial proteomics via antibodies: new and old biases for an image-based technology. Biol Imaging. 2024;4:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Zigutyte L, Sorz-Nechay T, Clusmann J, Kather JN. Use of artificial intelligence for liver diseases: a survey from the EASL congress 2024. JHEP Rep. 2024;6:101209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Rezvani M, Vallier L, Guillot A. Modeling nonalcoholic fatty liver disease in the dish using human-specific platforms: strategies and limitations. Cell Mol Gastroenterol Hepatol. 2023;15:1135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Frau C, Vallier L. Exploiting the plasticity of cholangiocytes to repair the biliary tree. Curr Opin Genet Dev. 2024;89:102257. [DOI] [PubMed] [Google Scholar]
- 350.Rezvani M, Lewis K, Quach S, Iwasawa K, Weihs J, Reza H, et al. Fetal liver-like organoids recapitulate blood-liver niche development and multipotent hematopoiesis from human pluripotent stem cells. bioRxiv. 2024. Online ahead of print.
- 351.Liu H, Yin G, Kohlhepp MS, Schumacher F, Hundertmark J, Hassan MIA, et al. Dissecting acute drug-induced hepatotoxicity and therapeutic responses of steatotic liver disease using primary mouse liver and blood cells in a liver-on-a-chip model. Adv Sci. 2024;11:e2403516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Adler M, Chavan AR, Medzhitov R. Tissue biology: in search of a new paradigm. Annu Rev Cell Dev Biol. 2023;39:67–89. [DOI] [PubMed] [Google Scholar]