Abstract
The reliability of the Mycobacteria Growth Indicator Tube (MGIT [BBL]) for rapid drug susceptibility testing of Mycobacterium avium complex (MAC) isolates was evaluated. MICs of amikacin, clarithromycin, clofazimine, ethambutol, and rifabutin were determined by the MGIT system for 16 MAC strains. The results were compared with those obtained by the BACTEC broth macrodilution method. The turnaround times were 6 to 8 days (median, 7 days) for the MGIT and 5 to 7 days (median, 6 days) for the BACTEC system. Agreements with BACTEC system-determined MICs, within ±1 log2 dilution, were 100, 100, 88, 63, and 44% for amikacin, clofazimine, rifabutin, clarithromycin, and ethambutol, respectively. Within ±2 log2 dilutions, agreement with BACTEC system-determined MICs increased to 100% for all the tested drugs. In addition, if MGIT-determined MICs were evaluated according to the thresholds adopted for the interpretation of BACTEC system-determined ones, ethambutol was the only drug for which susceptible strains were frequently misclassified as resistant. It is concluded that the MGIT system is a promising, nonradiometric alternative to the BACTEC method for rapid susceptibility testing of MAC isolates; however, additional studies are required to confirm our results and to determine the optimal criteria for the interpretation of ethambutol MICs.
Despite the introduction of reasonably effective prophylaxis, Mycobacterium avium complex (MAC) infection is still a common cause of morbidity and shortened survival among AIDS patients. Once MAC disease occurs, antimycobacterial therapy with a combination of drugs is required; however, while a limited number of drugs are assumed to be active against MAC, solid clinical correlations have been achieved for clarithromycin only (6, 9). Thus, there is a need for reliable drug susceptibility testing in order to choose the drug(s) to be used with clarithromycin for preventing drug resistance (4).
At present, mycobacteriologists agree that susceptibility tests for MAC isolates should be quantitative (i.e., MICs should be determined). It has also been established that MICs determined in broth are more accurate than those obtained by the agar dilution method (2). The radiometric (BACTEC) broth macrodilution method takes into account these findings. However, it is quite expensive and generates radioactive waste which must be disposed of.
The Mycobacteria Growth Indicator Tube (MGIT [BBL]) method is a recently introduced, nonradiometric method for the isolation of mycobacteria from clinical specimens. The MGIT system consists of an oxygen-quenched fluorescent indicator embedded in silicon at the bottom of a tube filled with Middlebrook 7H9 broth. Actively growing mycobacteria consume the oxygen dissolved in the medium, thereby releasing the indicator, whose fluorescence can be detected when the tube is viewed with a 365-nm UV light.
Recent studies have focused on the rapid detection of Mycobacterium tuberculosis resistance to first-line drugs by the MGIT system (7, 12). Reliable results, showing an excellent correlation with reference methods, have been reported at a mean time of 5 to 6 days of test processing.
The purposes of the present study were to investigate the reliability of the MGIT system for the determination of MICs of various drugs for MAC isolates and to compare the results with those achieved by the radiometric method.
MATERIALS AND METHODS
Test strains.
Sixteen MAC strains isolated almost entirely from AIDS patients and identified to the species level by RNA-DNA hybridization (Accuprobe; Gen-Probe, San Diego, Calif.) were evaluated in this study. Smooth, transparent colonies growing on Middlebrook 7H10 agar were subcultured for comparative susceptibility testing.
Antimicrobial agents.
Clarithromycin (Abbott Laboratories, North Chicago, Ill.), clofazimine (Ciba-GEIGY, Basel, Switzerland), and rifabutin (Farmitalia-Carlo Erba, Milan, Italy) were kindly provided by their manufacturers; amikacin and ethambutol were purchased from the Sigma Chemical Co. (St. Louis, Mo.).
A stock solution of clarithromycin was made in methanol and then diluted with phosphate buffer at pH 6.8 made by combining 0.1 M solutions of KH2PO4 and Na2HPO4. Stock solutions of clofazimine and rifabutin were made in dimethyl sulfoxide (DMSO) and methanol, respectively. Stock solutions of amikacin and ethambutol were made in distilled water and then sterilized with a membrane filter (pore size, 0.22 μm; Millipore Corp., Bedford, Mass.). All stock solutions were kept as aliquots at −80°C except for that of clofazimine, which was stored at room temperature in the dark. Working solutions, whose concentrations were 40-fold (for the BACTEC system) or 45-fold (for the MGIT) greater than the required concentrations, were made from stock solutions in sterile distilled water, except for that of clofazimine, which was diluted in DMSO. It was also verified that DMSO did not suppress or delay the MAC strains’ growth when added undiluted into the media.
Radiometric method.
The growth of bacteria was recorded radiometrically by using the BACTEC 460-TB system (Becton Dickinson, Sparks, Md.).
Growth in Middlebrook 7H12 liquid medium (Becton Dickinson) containing 14C-labeled palmitic acid leads to the consumption of this substrate, with subsequent release of 14CO2 into the confined atmosphere above the medium. The BACTEC instrument detects the amount of 14CO2 and records it as a growth index (GI) on a scale from 0 to 999.
MIC determination in Middlebrook 7H12 broth.
For MIC determination in broth, all the drugs were added to Middlebrook 7H12 vials in a volume of 0.1 ml per vial to achieve serial doubling concentrations. A culture was grown in a Middlebrook 7H12 broth seed vial and the results were recorded daily until the culture reached the maximum GI, and then the culture was diluted 1:100; 0.1 ml of this dilution was inoculated into each of the test vials and into one of the drug-free control vials. Such an inoculum provides an initial bacterial concentration of 104 to 105 CFU/ml.
Another drug-free control vial, containing a 1:100-diluted control, was inoculated with a bacterial suspension with a concentration 100 times lower in order to obtain 102 to 103 CFU/ml, which represents 1% of the bacterial population. The vials were incubated at 37°C, and the GI readings were recorded daily with the BACTEC 460-TB instrument. In accordance with previous studies (2, 3), the MIC determined in broth by the BACTEC system was defined as the lowest drug concentration in the presence of which the final GI reading was no greater than 50 within the maximum of 8 days of incubation. During the same period, the GI of the 1:100-diluted control was greater than 20 for three consecutive days, while the growth in the undiluted control reached the maximum GI reading of 999 no earlier than the fourth day of cultivation.
MIC determination in Middlebrook 7H9 MGIT broth. (i) Preparation of mycobacterial inoculum.
A few colonies, scraped off a 7H10 agar slant, were inoculated into an OADC-supplemented (MGIT OADC enrichment; Becton Dickinson) MGIT tube and incubated at 35 to 37°C. The tube was visually examined for growth (for approximately 3 to 5 days), and once the tube was turbid, the growth was diluted with sterile saline with a nephelometer to match a McFarland 0.5 standard. Then the McFarland 0.5 suspension was homogenized thoroughly and diluted 1:50 by adding 0.5 to 9.5 ml of BACTEC diluting fluid (Becton Dickinson Diagnostic Instrument Systems); this dilution was the preliminary suspension.
(ii) MGIT procedure.
The preliminary suspension was diluted 1:100 by adding 0.1 to 9.9 ml of diluting fluid, and 0.1 ml of this dilution (working suspension) was inoculated into the experimental tubes and into one of the drug-free control tubes (the undiluted control). Such an inoculum provides an initial bacterial concentration of 104 to 105 CFU/ml. The working suspension was then diluted 1:100, and 0.1 ml was used to inoculate another MGIT drug-free tube (containing a 1:100-diluted control), which contained 1% of the bacterial population. MGIT tubes were prepared by adding 0.5 ml of the MGIT OADC enrichment and 0.1 ml of the inoculum as described previously. Then 0.1 ml of antibiotic solution was added to each of the test tubes to obtain serial doubling concentrations. All tubes were incubated at 37°C and examined daily for fluorescence by placing them on a 365-nm UV transilluminator (UVP, Inc.). They were compared with a positive control tube which had been prepared by adding 5 ml of a 0.4% sodium sulfite solution to an empty MGIT tube. An uninoculated MGIT tube was used as a negative control. Fluorescent tubes, showing a bright orange color on the bottom and a reflection on the meniscus, that most closely resembled the positive control tube were considered positive. The MIC was considered interpretable when the 1:100-diluted control became positive. This requirement was usually fulfilled between days 6 and 8 of incubation. If the undiluted control became positive earlier than day 4, the tube was considered overinoculated. Similarly, if the 1:100-diluted control did not show fluorescence by day 8 of incubation, the tube was considered underinoculated. In both cases, the assays were repeated.
The MIC was defined as the lowest drug concentration in the presence of which the tube remained negative after the incubation period. During the same period, the 1:100-diluted control became positive, while the growth in the undiluted control showed positive fluorescence not earlier than the fourth day of cultivation. The MIC50 was defined as the minimal drug concentration to which 50% of the test isolates were found to be susceptible, whereas the MIC90 was the minimal drug concentration to which at least 90% of the isolates were found to be susceptible.
MIC validation.
Samples were plated onto 7H10 agar to establish the CFU counts from the 1:100-diluted working suspension and, for three strains, from the test tubes at the end of the test. This was done to verify that the inoculum provided an initial bacterial concentration of 102 to 103 CFU/ml and that the MIC was the lowest drug concentration in the presence of which the growth did not exceed 1% of the initial bacterial population.
RESULTS
Reading and interpretation of MGIT-determined MICs.
The turnaround times for susceptibility test results ranged from 6 to 8 days (median, 7 days) for the MGIT and from 5 to 7 days (median, 6 days) for the BACTEC system. After the incubation period, the majority of strains demonstrated clear results (presence or absence of strong fluorescence at the bottoms of the tubes) which were easy to interpret. Occasionally, when weak fluorescence was observed, it was recorded as positive.
Comparison of BACTEC system-determined and MGIT-determined MICs.
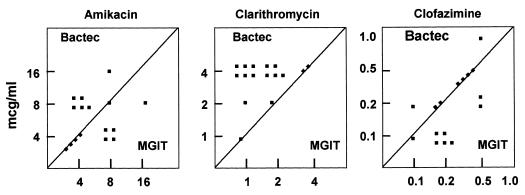
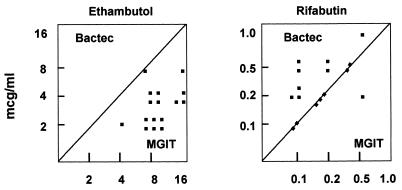
The agreements between the radiometric method and the MGIT system, within ±1 log2 dilution, were 100, 100, 88, 63, and 44% for amikacin, clofazimine, rifabutin, clarithromycin, and ethambutol, respectively (Table 1). Within ±2 log2 dilutions, agreements with BACTEC system-determined MICs increased to 100% for all the tested drugs. The MICs obtained by both methods are shown in Table 2. Excellent agreement between BACTEC system- and MGIT-determined MICs of amikacin, clofazimine, and rifabutin was demonstrated, while discrepancies of more than 1 dilution step could frequently be observed for clarithromycin and ethambutol. MGIT-determined MICs were, on average, 2 log2 dilutions lower for clarithromycin and 2 log2 dilutions higher for ethambutol than BACTEC system-determined MICs (Fig. 1 and 2).
TABLE 1.
Comparison of MGIT-determined and BACTEC system-determined MICs for 16 MAC isolates
| Antimicrobial agent | % Variation in MGIT-determined MIC from BACTEC system-determined MIC within dilution
|
% Agreement within ±1 dilution | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | +1 | +2 | ||
| Amikacin | 37 | 37 | 26 | 100 | ||
| Clarithromycin | 37 | 37 | 26 | 63 | ||
| Clofazimine | 12 | 44 | 44 | 100 | ||
| Ethambutol | 7 | 37 | 56 | 44 | ||
| Rifabutin | 12 | 37 | 44 | 7 | 88 | |
TABLE 2.
MICs of five antimicrobial agents for 16 MAC isolates by the MGIT system and a radiometric methoda
| Antimicrobial agent | BACTEC method
|
MGIT method
|
||||
|---|---|---|---|---|---|---|
| MIC50 | MIC90 | Range | MIC50 | MIC90 | Range | |
| Amikacin | 4 | 8 | 4–16 | 4 | 8 | 4–16 |
| Clarithromycin | 4 | 4 | 1–4 | 1 | 2 | 1–4 |
| Clofazimine | 0.25 | 0.5 | 0.12–1 | 0.25 | 0.5 | 0.12–0.5 |
| Ethambutol | 2 | 4 | 2–8 | 8 | 16 | 4–16 |
| Rifabutin | 0.25 | 0.5 | 0.12–1 | 0.25 | 0.5 | 0.12–0.5 |
All data are expressed in micrograms per milliliter.
FIG. 1.
MICs of amikacin, clarithromycin, and clofazimine for 16 MAC strains as determined by the BACTEC and MGIT systems.
FIG. 2.
MICs of ethambutol and rifabutin for 16 MAC strains as determined by the BACTEC and MGIT systems.
Comparison of BACTEC system-determined and MGIT-determined MICs according to the BACTEC interpretive breakpoints.
The qualitative susceptibility results from both tests (i.e., resistance and susceptibility), based on the thresholds adopted for interpretation of BACTEC system-determined MICs (3), are given in Table 3. Excellent agreement was demonstrated for all MAC clinical isolates for clarithromycin and rifabutin (100%). For clofazimine and amikacin, discrepant results (88 and 50% agreement, respectively) occurred because most of the MICs determined by both systems were close to the resistance breakpoint concentrations. In this case, even a 1-dilution difference made some strains shift from the moderate-susceptibility category into the resistance category. With ethambutol, discrepant results were obtained with the majority of strains tested: unlike the strains treated with clarithromycin, a considerable number of strains categorized as susceptible to ethambutol by the BACTEC system were revealed to be resistant when tested by the MGIT system.
TABLE 3.
Susceptibility of 16 MAC isolates as determined by the MGIT system and a radiometric methoda
| Antimicrobial agent | No. of isolates:
|
||
|---|---|---|---|
| Susceptible or moderately susceptible by both methods | Resistant by both methods | With discrepant results | |
| Amikacin | 5 | 3 | 8 |
| Clarithromycin | 16 | 0 | 0 |
| Clofazimine | 9 | 5 | 2 |
| Ethambutol | 1 | 2 | 13 |
| Rifabutin | 16 | 0 | 0 |
Based on the thresholds proposed by Heifets (3).
MGIT-determined MIC validation.
We recorded an average count of 2,186 CFU/ml (range, 600 to 5,004 CFU/ml) by sampling and plating onto 7H10 agar from the 1:100-diluted working suspension, while the tubes at the end of the test yielded an average count of 2,498 CFU/ml (range, 20 to 6,301 CFU/ml). The MGIT-determined MICs correlated well with those based on CFU counts. The MIC was the lowest drug concentration in the presence of which the growth did not exceed 1% of the initial bacterial population.
DISCUSSION
MAC disease will remain a therapeutic challenge for AIDS-infected patients. Although a linear correlation between clinical response and MIC results has not yet been found (10), therapy still relies on in vitro drug susceptibility test results and therefore requires rapid and standardized methods (8). The MGIT system was evaluated for its ability to determine MICs of a range of antibiotics for MAC isolates, and the results were compared with those of the radiometric method. The test was technically easy to perform, and the results were easy to interpret. Inoculum preparation and susceptibility turnaround time were similar to those of the BACTEC procedure. Within ±1 log2 dilution, MICs of amikacin, clofazimine, and rifabutin showed 100, 100, and 88% agreement, respectively. In contrast, for clarithromycin and ethambutol, MIC agreements within ±1 log2 dilution were 63 and 44%, respectively. Within ±2 log2 dilutions, agreement with BACTEC system-determined MICs increased to 100% for all the tested drugs. Moreover, if MGIT-determined MICs were evaluated according to the thresholds adopted for the interpretation of BACTEC system-determined ones, ethambutol was the only drug for which susceptible strains were frequently misclassified as resistant. The cause of such a discrepancy has not been determined. Recent data on M. tuberculosis complex drug susceptibility testing by the MGIT system seem to support our findings. Major discrepancies have been reported for ethambutol by different authors (1, 5, 11) who observed that about 2 to 5% of the strains classified as resistant by the MGIT were classified as susceptible by the BACTEC system. We suggest that the new detection system, the medium’s richness, and ethambutol’s mechanism of action (bacteriostatic instead of bactericidal) could help to explain this phenomenon. Moreover, the possibility of a quenching effect on ethambutol by one or more components of the MGIT system (perhaps the fluorescent indicator or the silicon embedded at the bottom of the tube) should also be investigated.
In addition, we believe that our technique is open to further improvement as kinetic reading of the MGIT test tubes by the newly developed BACTEC MGIT 960 system (Becton Dickinson) becomes available.
It is concluded that the MGIT system shows promise as a nonradiometric alternative to the BACTEC system for quantitative susceptibility testing of MAC isolates. However, additional studies are needed to confirm our results and to determine optimal criteria for the interpretation of ethambutol MICs.
ACKNOWLEDGMENT
We thank Daniele Silvestri of Becton Dickinson Co., Milan, Italy, for supplying the MGIT system and for his technical assistance.
REFERENCES
- 1.Aller A I, Nogales M C, Lopez-Prieto M D, Tocòn G, Pujante M M, Bernal S, Pablos C. Abstracts of the 18th Annual Congress of the European Society of Mycobacteriology 1997. 1997. Evaluation of the radiometric Bactec system and the Mycobacteria Growth Indicator Tube (MGIT) for susceptibility testing of Mycobacterium tuberculosis; p. 124. [Google Scholar]
- 2.Heifets L. Dilemmas and realities in drug susceptibility testing of Mycobacterium avium-Mycobacterium intracellulare and other slowly growing nontuberculous mycobacteria. In: Heifets L, editor. Drug susceptibility in the chemotherapy of mycobacterial infections. Boca Raton, Fla: CRC Press; 1991. pp. 123–146. [Google Scholar]
- 3.Heifets L. Susceptibility testing of Mycobacterium avium complex isolates. Antimicrob Agents Chemother. 1996;40:1759–1767. doi: 10.1128/aac.40.8.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masur H the US Public Health Service Task Force on Prophylaxis and Therapy for Mycobacterium avium complex. Recommendations on prophylaxis and therapy for Mycobacterium avium complex disease in patients infected with the HIV. N Engl J Med. 1993;329:898–904. doi: 10.1056/NEJM199309163291228. [DOI] [PubMed] [Google Scholar]
- 5.Nardi G, Gismondo M R, Frisenda L, Polato R. Abstracts of the 8th European Congress of Clinical Microbiology & Infectious Diseases 1997. 1997. Validation of the new Mycobacteria Growth Indicator Tube (MGIT) drug susceptibility system on Mycobacterium tuberculosis strains, abstr. P-1084; p. 266. [Google Scholar]
- 6.Peloquin C A. Mycobacterium avium complex infection. Pharmacokinetic and pharmacodynamic considerations that may improve clinical outcomes. Pharmacokinetics. 1997;32:132–144. doi: 10.2165/00003088-199732020-00004. [DOI] [PubMed] [Google Scholar]
- 7.Reisner B A, Gatson M A, Wood G L. Evaluation of Mycobacteria Growth Indicator Tubes for susceptibility testing of Mycobacterium tuberculosis to isoniazid and rifampin. Diagn Microbiol Infect Dis. 1995;22:325–329. doi: 10.1016/0732-8893(95)00147-7. [DOI] [PubMed] [Google Scholar]
- 8.Salfinger M, Wallace R J., Jr Susceptibility testing for nontuberculous mycobacteria: should it be performed? Clin Microbiol Newsl. 1997;19:68–71. [Google Scholar]
- 9.Shafran S D, Singer J, Zarowny D P, Philliphs P, Salit I, Wamsley S L, et al. A comparison of two regimens for the treatment of Mycobacterium avium complex bacteremia in AIDS: rifabutin, ethambutol, and clarithromycin versus rifampin, ethambutol, clofazimine, and ciprofloxacin. N Engl J Med. 1996;335:377–383. doi: 10.1056/NEJM199608083350602. [DOI] [PubMed] [Google Scholar]
- 10.Sison J P, Yao Y, Kemper C A, Hamilton J R, Brunner E, Stevens D A, Deresinsky S C. Treatment of Mycobacterium avium complex infection: do the results of in vitro susceptibility tests predict therapeutic outcome in humans? J Infect Dis. 1996;173:677–683. doi: 10.1093/infdis/173.3.677. [DOI] [PubMed] [Google Scholar]
- 11.Van Elderle J, Moons V, Standaert K, Verbist L. Abstracts of the 8th European Congress of Clinical Microbiology & Infectious Diseases 1997. 1997. Evaluation of the Mycobacteria Growth Indicator Tube for drug susceptibility testing of Mycobacterium tuberculosis, abstr. P-1083; p. 266. [Google Scholar]
- 12.Walters S B, Hanna B A. Testing of susceptibility of Mycobacterium tuberculosis to isoniazid and rifampin by mycobacterium growth indicator tube method. J Clin Microbiol. 1996;34:1565–1567. doi: 10.1128/jcm.34.6.1565-1567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]