Abstract
The mutualistic symbiosis between legume roots and soil rhizobia culminates in the formation of root nodules, where nitrogen is fixed. Root nodule symbiosis is inhibited by heavy metal stress. In this study, we investigated the relative responses of the symbiotic partners to a non-essential heavy metal cadmium (Cd) and an essential heavy metal zinc (Zn) stress and identified patterns in gene expression. We performed dual transcriptomics in nodules, using the Medicago truncatula-Sinorhizobium meliloti symbiotic system. Phenotypes were measured in the wild-type Medicago truncatula and a mutant in an ABC transporter gene (Mtabcg36), which showed compromised nodule formation in control conditions and further after heavy metal treatment. We observed that the rhizobia were particularly sensitive to Zn in mutant nodules. The greatest degree of differential gene expression in the host plant were observed under Cd and Zn treatments in wild-type nodules. Most Cd-regulated host genes were also differentially regulated by Zn, revealing little discernment between an essential and a non-essential ion under increased exposure. Furthermore, the host response to both the stresses affected auxin and iron homeostasis genes in a host genotype-dependent manner. Our results suggested impaired cadmium export from the mutant nodules. These results have potential implications in agricultural management systems and bioremediation strategies.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-17827-z.
Keywords: Heavy metal, Nodule, Symbiosis, ABC transporter, Dual transcriptomics, ICP-MS
Subject terms: Plant sciences, Plant stress responses, Abiotic
Introduction
The ionic composition of the rhizosphere is important for plant growth and development. Heavy metals such as manganese, iron, copper, and zinc (Zn) act as plant micronutrients at low concentrations but can be phytotoxic at higher concentrations. On the other hand, no function has been ascribed to heavy metals such as lead, cadmium (Cd), or mercury, and these are considered phytotoxic even at low concentrations. Geological processes such as weathering of rocks as well as anthropogenic activities such as accumulation of agrochemical and industrial waste can cause high levels of heavy metals in arable lands. These metal ions are readily taken up by the roots and often transported to the shoots, where they can enter the food web via edible plant parts such as seeds and leaves1,2. Excess levels of heavy metals can disrupt ionic homeostasis in cells and cause oxidative stress, affecting various processes in plants, including germination, photosynthesis, nutrient uptake, and their interactions with beneficial soil microorganisms3–6.
Legumes develop symbiotic relationships with nitrogen-fixing bacteria (rhizobia), culminating in symbiotic root organs called nodules, where atmospheric nitrogen is converted into a form usable by plants. Nodule formation is extremely sensitive to the abiotic environment7,8. In the model legume, Medicago truncatula, heavy metal stress (Cd, Hg, Zn) reduces the number of nodules9,10and inhibits root growth variably among genotypes11,12. In legumes, heavy metals also lower the accumulation of free amino acids, total protein and nitrogen content, and decrease the activity of nitrogenase, the bacterial enzyme that catalyzes the breakdown of atmospheric nitrogen13. Both ion and hormone homeostasis are essential for successful nodulation and maintaining symbiosis. Several metal ion transporter-encoding and hormone-responsive genes have been implicated in nodulation7.
ATP-Binding Cassette (ABC) transporter proteins are found in all forms of life. ABC transporters are membrane-bound proteins that use the energy generated from ATP hydrolysis to transport substrates across membranes. Their nucleotide-binding domains are conserved, while the transmembrane substrate-binding domains are diverse, often due to substrate specificity. ABC proteins in plants transport a variety of substrates such as hormones, pigment precursors, lipid precursors, and metal ions. In the model legume Medicago truncatula, the ABC genes have diversified into several clades, and some are known to be involved in nodule formation, including one in the G-clade, MtABCG3614–20. MtABCG36 shows variable expression patterns in various tissues during symbioses and is significantly induced during mycorrhizal symbiosis (Medicago Gene Expression Atlas)15. In Arabidopsis thaliana and rice, the expression of ABCG36 orthologs in both species was induced by Cd stress in roots more strongly than in leaves, and knockdown and knockout mutations resulted in decreased root growth and greater sensitivity to increased Cd concentrations21,22.
Dual transcriptomics is a powerful tool for simultaneously studying the responses of endosymbiotic species and their hosts in response to internal or external cues23–28. Here, we studied the effects of Cd and Zn stress on the Medicago truncatula-Sinorhizobium meliloti symbiotic partnership in the nodules of two host genotypes using dual transcriptomics. Our goals were to: (1) study the relative responses of the symbiotic partners to these stresses, (2) compare gene expressions in response to an essential and a non-essential heavy metal, and (3) assess the contribution of the host genotype in mediating heavy metal stress responses in nodules18. Because the plant host directly encountered the heavy metal treatments, we hypothesized that the host would show a stronger response than the microsymbiont. Furthermore, given the differences in the known nutritional roles between the two metals, we hypothesized that exogenous Cd would have a greater impact on the nodule transcriptome, and that there will be moderate overlap in differentially expressed genes (DEGs) in response to these treatments.
Results
Heavy metal treatments and the host genotype influence plant biomass and symbiosis with rhizobium
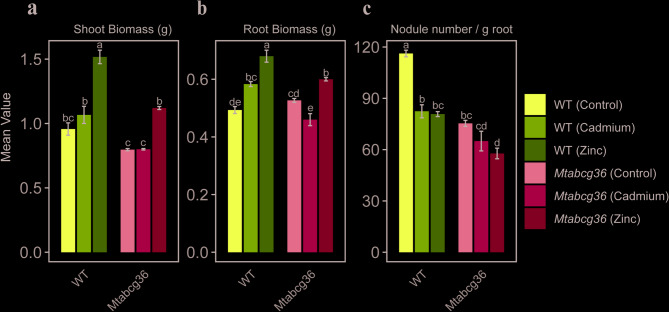
To test the effect of heavy metal stress on nodules in the wild-type (WT) and mutant host genotypes, we subjected rhizobium-inoculated plants to Cd and Zn treatments. The control treatment contained necessary macro- and micronutrients, including a basal level of Zn (see methods for details). In the WT plants, the Zn stress resulted in increased shoot and root biomass while Cd increased root biomass only (Fig. 1a, b). Nodule number per gram of root decreased in response to both treatments in the WT. Nodule number per gram of root was also lower in the Mtabcg36 mutant background compared to the WT without any heavy metal treatment (Fig. 1c), as previously observed by Curtin et al., (2017). In the Mtabcg36 mutant genotype, Cd treatment decreased root biomass compared to control treatment, but Zn treatment increased both shoot and root biomass compared to control treatment. Furthermore, in the Mtabcg36 mutant, Zn, but not Cd-treated roots had fewer nodules per gram of root (Fig. 1, S1a-c). No difference was observed in nodule biomass either between untreated genotypes or treatments within each genotype, however, nodule biomass in Zn-treated mutant nodules was significantly lower than Zn-treated wild-type nodules (Supplementary Fig. S2). These results show that both the heavy metal treatments as well as the plant genotype influenced the plant growth and nodule number, and both Cd and Zn inhibited nodule formation.
Fig. 1.
Quantification of shoot and root biomass, and normalized nodule number. Biomass measures for (a) shoot, (b) root and (c) nodule number per gram of root in wild-type (WT) and Mtabcg36 mutant plants that were inoculated with Sinorhizobium meliloti Sm2011 and treated with cadmium, zinc, or untreated (control). One week after the treatment, the shoot, root, and nodules were harvested. Biomass measurements used fresh weight. Five plants were tested in each condition, and the experiment repeated three times. The number of nodules indicates total nodules pooled from five plants. Error bars indicate standard error of the mean (SEM). Letters display the Tukey’s Honestly Significant Difference (HSD) test for multiple comparisons at α = 0.05. When common letters are shown above any bar, they are not significantly different.
Heavy metal treatments have large effects on the host but not the microsymbiont transcriptome in the nodule
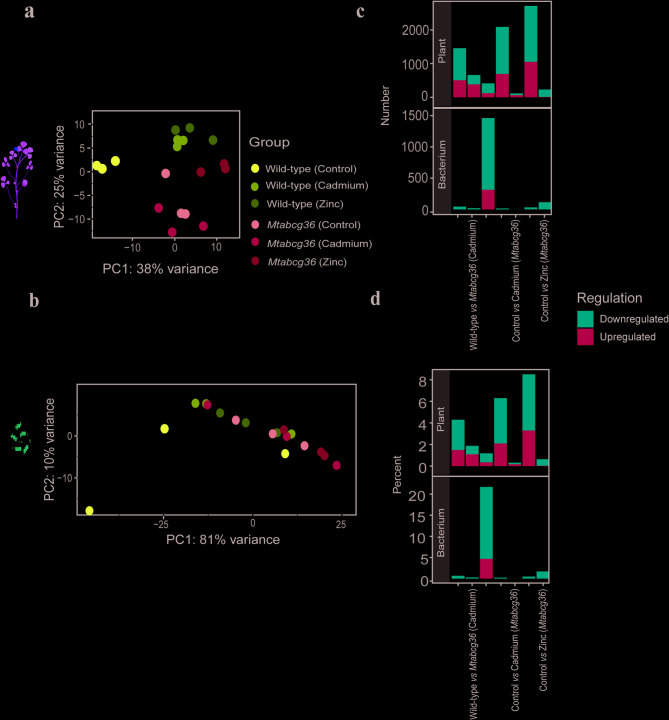
To quantify the transcriptional responses in the two plant genotypes resulting from Cd and Zn stress in the context of rhizobium-legume symbiosis, we performed dual RNA-seq from the nodules to produce genome-wide expression profiles for both symbiotic partners from the WT and Mtabcg36 mutant backgrounds (Supplementary Fig. S3). In the plant, principal component analysis (PCA) revealed distinct clustering of gene expression by plant genotype, and by stress treatments (Fig. 2a). On the bacterial side, however, no clear clustering of gene expression was observed (Fig. 2b).
Fig. 2.
Mutation in MtABCG36 and heavy metal treatments strongly influence the host transcriptome in the nodule. Principal component analysis (PCA) of the (a) plant and (b) bacterial transcriptome in the wild-type or Mtabcg36 mutant nodules subjected to cadmium or zinc treatments. Control, cadmium or zinc treated samples were colored using purple hues for wild-type and green hues for mutant. Stacked bar plots showing the number (c) or percent (d) of differentially expressed genes (DEGs) using adjusted p-value < 0.05 (False Discovery Rate, FDR) for comparisons between control and Cd or Zn treated plants, and comparisons between WT and mutant. Red indicates downregulated DEGs and green indicates upregulated DEGs.
We questioned whether the absence of clustering on the bacterial side was a technical artifact, such as sequencing coverage, or had any potential biological explanation. We therefore compared the total mapped read counts in each condition for each biological replicate for the bacterium and the plant in the dual RNA-seq data. The plant genome has about five times the number of annotated genes than in the bacterium (Supplementary Fig. S4a) and about twice (on average across all samples) the number of mapped reads in the plant compared with the bacteria (Fig. S4b). When mapped reads were normalized to the total number of genes in either species, both the host plant and the bacteria showed comparable coverage (Supplementary Fig. S4c, d). These results corroborated the fact that the lack of clustering of gene expression on the bacterial side had a biological relevance.
To gain a deeper insight into the effect of each treatment, host genotype, and their interactions, on the dual transcriptome, we conducted pairwise differential expression analyses of all biologically meaningful combinations of conditions (Supplementary Tables S1, S2). Our analyses revealed that all treatments, genotypes, and their interactions influenced the host transcriptome to different degrees. The Cd and Zn treatments in the WT nodules resulted in the largest number of DEGs in the host plant. These treatments led to 2087 (2.1% of the total number of genes) and 2711 (3.3% of the total number of genes) plant DEGs in WT nodules, respectively (p-adj < 0.05) (Fig. 2c, d). In contrast, the mutant genotype showed dramatically small numbers of DEGs in response to Cd or Zn (113 (0.31%) and 229 (0.64%), respectively), indicating that the effect of these metals on the plant transcriptome in the nodule is largely dependent on the plant genotype. When comparing sets of genes from the post-treatment conditions in WT vs. mutant, the number of DEGs from Cd-treated plants was greater than for Zn-treated plants. This finding was supported by the PCA which showed a greater distance between the WT Cd-treated plants and mutant Cd-treated plants on the PC2 axis compared with the separation between Zn-treated WT and Zn-treated mutant plants on the PC2 axis (Fig. 2a).
The DEGs observed in S. meliloti from nodules were remarkably fewer compared with the host plant (Fig. 2c, d). The Zn-treated WT vs. Zn-treated mutant nodules was the only category that had any considerable differential expression on the rhizobial side. Because a strong response was not observed in the rhizobia induced by Zn treatment in the WT host-plant background, or between rhizobia in the WT and mutant host-plant genotypes without any treatment, or in rhizobia in the mutant host in response to Zn (i.e., control vs. treatment), the response when comparing rhizobial gene expression after Zn treatment in the WT and the mutant host-plant backgrounds, appears to be synergistic, rather than additive (Supplementary Fig. S5)29. This category showed over 20% of the rhizobial genome to be differentially expressed after Zn treatment in the WT vs. mutant backgrounds. While the clustering of the RNA-seq data in the PCA from the rhizobial samples was much less obvious compared to the host plant, the Zn-treated rhizobial samples in the WT vs. mutant was among the broadest separation on the PC1 axis (Fig. 2b, Supplementary Fig. S6). Together, these results indicate that the host and the microsymbiont behave quite differently in their response to heavy metal stresses in the nodule. At the whole transcriptome level, the host cells in the nodules appear much more sensitive to the heavy metal stresses that lead to changes in gene expression, compared to the microsymbiont.
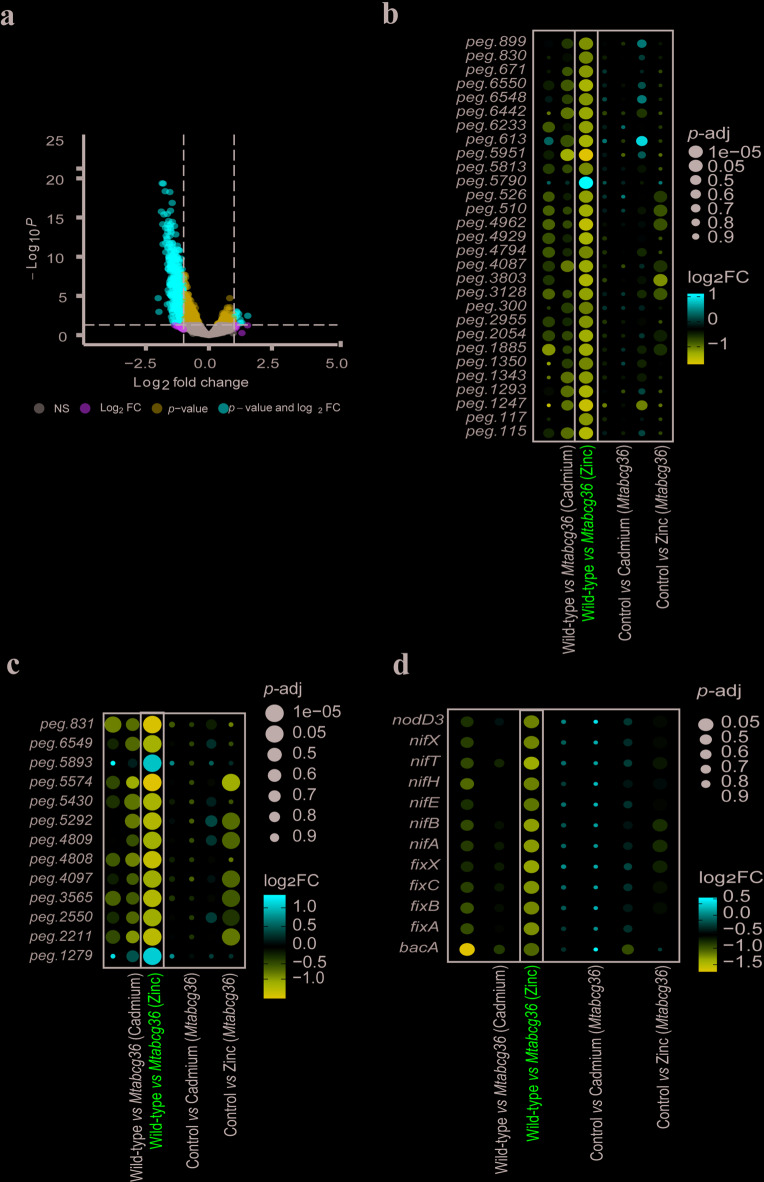
Several transcriptional regulator-, transporter-encoding-, and symbiotic rhizobial genes are downregulated in the Mtabcg36 mutant nodules under Zn treatment compared to Zn-treated WT nodules
The combination of Zn treatment and the host mutant background had a synergistic effect on the rhizobial transcriptome in the nodule, as neither Zn treatment nor the host mutant background alone, impacted gene expression in the rhizobia substantially. Together, they had a large effect on the rhizobial transcriptome after Zn treatment in WT and Mtabcg36 hosts that is much greater (based on numbers of DEGs) than the added effects of control vs. Zn treatment in either the WT or mutant (Fig. 2C, D). Because Zn-treated WT vs. mutant nodules was the only comparison in S. meliloti that showed considerable differential gene expression at the whole transcriptome level (Fig. 2c, d), we explored the DEGs in this category. Using a stringent threshold of |log2FC| >1 and p-adj < 0.05, we observed that the majority of DEGs were downregulated in the mutant nodules vs. WT after both were subjected to Zn treatment, suggesting that these genes were negatively regulated by Zn in the rhizobia in the Mtabcg36 host plant nodules (Fig. 3a, Supplementary Table S3). This category of DEGs included several genes encoding putative transcriptional regulators and transporters (Fig. 3b, c, Supplementary Tables S4,S5). Over half of the DEGs encoding transporters were putative ABC transporters (Fig. 3c). The Zn-treated WT vs. mutant category also included several symbiotic genes, including nodD3, bacA, and multiple nif and fix genes, which all had significantly lower expression in mutant vs. WT host background under Zn treatment (Fig. 3d). None of the rhizobial genes in these three groups was significantly regulated by Zn in the WT host plant (Fig. 3b-d). Combined, these findings show that host-plant genotype influences gene expression after Zn stress in the microsymbiont in the nodule, and unlike the response in the host-plant, the expression differences in the microsymbiont are much larger after Zn treatment than after Cd treatment.
Fig. 3.
Differentially expressed transcriptional regulator-, transporter-encoding and symbiotic genes in rhizobium in the nodule in the wild-type (WT) vs. Mtabcg36 mutant comparison under zinc (Zn) treatment. (a) Volcano plot showing the significant rhizobial DEGs comparing Zn treated WT vs. Mtabcg36 mutant samples. Blue points adjusted p-value < 0.05, red points are |log2FC| > 1 and adjusted p-value < 0.05, gray points are non-significant, (NS). Negative log2FC indicates lower expression in the mutant than the WT after the Zn treatment. (b-d) Dot plots representing differential expressions of (b) transcriptional regulator-, (c) transporter-encoding, and (d) symbiotic rhizobial genes. Differential expression was defined as log2FC| > 1; p-adj < 0.05. The box includes only significant DEGs for the Zn-treated WT vs. Mtabcg36 comparison. These genes may or may not be differentially regulated in any other combination of genotypes and treatments. The color of the dots represents the directionality of gene expression. The size of the dots represents the reverse of the adjusted p-value; larger dots represent a smaller adjusted p-value. Labels on y-axes of b-d are gene IDs from the RAST annotation (Tables S4-5) or known symbiosis homologs (Table S3).
Cd and Zn act through common host genotype-dependent mechanisms to influence the host transcriptome in the nodule
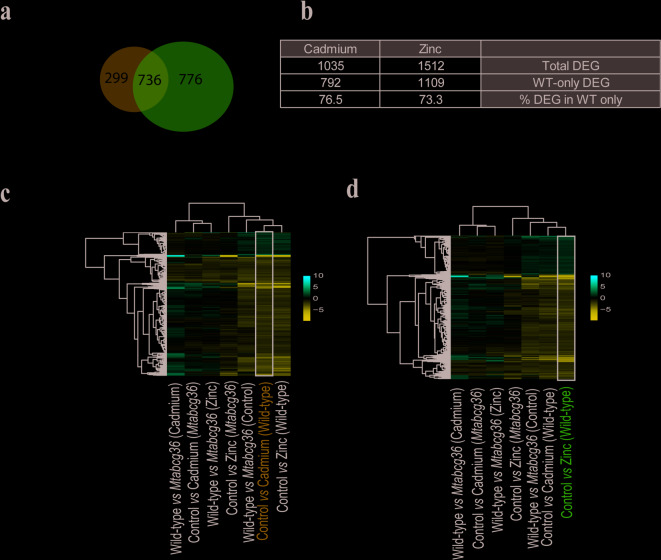
Unlike the rhizobial symbiont, the host plant showed far greater transcriptional responses in all comparisons (Fig. 2c, d). In the WT, Cd and Zn treatments resulted in the largest number of DEGs (|log2FC| >1, p-adj < 0.05) showing over 6% (of the total number of plant genes) and 8% (of the total number of plant genes) DEGs that responded to the Cd and Zn treatments, respectively (Fig. 2C, D). Together, these treatments resulted in a total of 1,811 DEGs in the WT, which may or may not be differentially regulated in the mutant (Fig. 4a). Over 70% of these genes were not differentially regulated in the mutant, corroborating the role of the host genotype in response to Cd and Zn stress (Fig. 4b, Supplementary Tables S6, S7). There was a strong overlap between the genes that were differentially regulated by Cd and Zn. The Cd treatment induced expression changes in 1035 genes, out of which 736 (71.1%) genes were also differentially expressed in response to Zn, and in both cases, more genes were downregulated (Fig. 4c, d). In the WT, the expression profiles of these genes were very similar in these two treatments, and also similar to the WT vs. mutant in control conditions based on combined cluster analysis of gene expression and plant genotype-treatment (Fig. 4c, d), suggesting that both Cd and Zn homeostasis depends on MtABCG36. Furthermore, despite being a micronutrient, Zn acts similar to Cd, though the latter does not have a known nutritional role in plants.
Fig. 4.
Differentially expressed host plant genes in the nodule in response to Cd and Zn. (a) Venn diagram showing the DEGs that were unique to either cadmium or zinc treatments, and those that overlap in wild-type R108 nodules. These genes may or may not be differentially regulated in the mutant by these treatments. (b) Table summarizing the total number of plant DEGs and the number and percentage of DEGs that were differentially regulated in the WT only, in response to Cd or Zn. “Total DEG” indicates DEGs in response to Cd or Zn in the WT that are, or are not differentially regulated in the mutant. “WT-only DEG” indicates DEGs in response to Cd or Zn in the WT but not the mutant, and “% DEG in WT only” indicates % DEGs in response to Cd or Zn in the WT but not the mutant (c) Heatmap of DEGs observed under cadmium treatment in the wild-type nodules (vertical rectangle box). (d) Heatmap of DEGs observed under zinc treatment in the wild-type nodules (vertical rectangle box). The colors represent the directionality of gene expression. For the heatmaps, the genes may or may not be differentially regulated in the other combinations of genotypes and treatments other than the ones specified in blue/pink X-axis label and enclosed in the vertical box. DEGs are defined as |log2FC| > 1; p-adj < 0.05. Clustering was using expression level change (y-axis) and sample/treatment (x-axis) on the heatmaps.
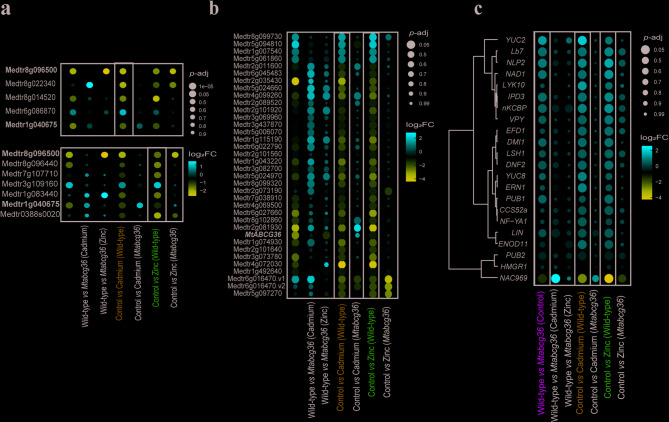
ABC transporter genes in M. truncatula have evolved into a large superfamily comprising several phylogenetic clades15. Multiple ABC transporters in the G clade (ABCG) are involved in homeostasis of the plant hormones abscisic acid, auxin, and cytokinin (Supplementary Fig. S7)16,17,30,31. Hence, we explored our transcriptome data for possible connections between MtABCG36 and hormone homeostasis, focusing on these three hormones. No abscisic acid-associated gene was differentially regulated by Cd or Zn in the WT (Supplementary Tables S8, S9). Only one cytokinin-associated gene (Medtr4g044110: putative cytokinin dehydrogenase) was regulated by Zn, and none by Cd in the WT (Supplementary Tables S8, S9). In contrast, several genes associated with auxin were differentially regulated by both heavy metal treatments in the WT. Two genes (Medtr1g040675, auxin-induced protein IAA4 and Medtr8g096500, putative small auxin-up RNA) were shared between the Cd and Zn treatments. Most of these genes were not regulated by the treatments in the mutant, suggesting a role of MtABCG36 in auxin homeostasis in nodules (Fig. 5a, Supplementary Tables S8, S9).
Fig. 5.
Cadmium or zinc-responsive host plant genes in the nodule associated with auxin, transporters, or symbiosis. (a) Dot plots representing auxin-associated DEGs under cadmium (upper panel) and zinc (lower panel) treatments in the nodule. Genes observed in both the Cd and Zn sets are shown in bold. (b) Dotplot of DEGs encoding transporters that are observed under both cadmium and zinc treatments. The MtABCG36 gene in bold is Medtr2g101090 in the M. truncatula A17 version 4.0. (c) Dotplot of symbiotic genes differentially regulated in the mutant under the control condition, or by Cd or Zn treatment in the wild-type, clustered by log2FC. The color of the dots represents the directionality of gene expression. The size of the dots represents the reverse of the adjusted p-value; larger dots represent a smaller adjusted p-value. The focal groups are enclosed within black boxes and labeled with blue (Cd) and pink (Zn). For a and b, gene IDs on y-axis are Mt4.0 gene annotations, and for c, common names of known symbiosis genes are shown. Gene IDs with v1 and v2 indicate more than one homologs between the R108 Hi-C and Mt4.0 genomes. For all dotplots, the genes may or may not be differentially regulated in combination of genotypes and treatments other than the ones specified. DEGs were defined as: (a, b) |log2FC| > 1; p-adj < 0.05; and (c) p-adj < 0.05.
Given the overlap between Cd and Zn-responsive host plant DEGs in the WT nodules, we hypothesized that several transporter-encoding genes would be co-regulated by these treatments. Indeed, several genes, including putative ABC transporter genes were co-regulated by Cd and Zn in the WT (Fig. 5b). Among the upregulated genes in both Cd and Zn stress conditions, were MtYSL3 (Medtr1g007540; AT5G53550) and MtMRP3, an ortholog of AtABCC3 (Medtr5g094810; AT3G13080), which have both been shown to be involved in Cd transport or vacuolar sequestration in A. thaliana32,33. Loss of function mutations in MtYSL3 have also been shown to affect Fe and Zn uptake and distribution in M. truncatula R108 34. Multiple such genes were also differentially regulated in the mutant in the control condition, suggesting once again, a convergence of metal stress response and MtABCG36-regulated processes in rhizobium-legume symbiosis. As expected, MtABCG36 (Medtr2g101090) was significantly downregulated in the mutant compared to WT in the control conditions (Fig. 5b, Supplementary Table S10).
M. truncatula forms indeterminate nodules, and a single nodule encompasses all the stages of development that occurred during root nodule symbiosis8. We focused on well-characterized host symbiotic genes and studied their expression patterns in response to the metal stresses. Because of the longitudinal gradient of differentiation in nodules, we did not set a cut-off for log2 fold-change because we sampled entire nodules, and genes with small changes in expression could still be meaningful. We did not see clear developmental stage/zone-wise expression dynamics (Fig. 5c), where the dendrogram on the Y-axis does not suggest clustering of symbiosis genes by developmental stage. The DEGs include early signaling/infection-associated genes (e.g.: HMGR1, PUB1,2, VPY etc.), as well as those that function much later (e.g.: NAD1, DNF2, NAC969). Moreover, contrary to the whole transcriptome where the majority of DEGs were downregulated in response to both metals, most of the symbiotic genes were upregulated under one or both metal stresses, a trend previously observed under sodium chloride stress (Fig. 5c, Fig. S8)35. Among these genes were EARLY NODULIN 11 (ENOD11), LYSM RECEPTOR-LIKE KINASE 10/EXOPOLYSACCHARIDE RECEPTOR 3 (LYK10/EPR3), VAPYRIN (VPY), and ETHYLENE RESPONSE FACTOR IN NODULATION 1 (ERN1). Unlike most other genes discussed earlier, these genes were not differentially expressed in the mutant compared to WT under the control condition. Auxin-synthetic YUCCA 2 and 8 (YUC2, YUC8) were upregulated by Cd in the WT but not in the mutant, in line with the differential regulation of several auxin-associated genes discussed above (Fig. 5a, c, Supplementary Tables S11-15).
Transcription factors play critical roles in different stages of rhizobium-legume symbiosis8,36. Multiple transcription factor-encoding genes were upregulated by both Cd and Zn in the WT nodules but not in the mutant nodules under the same treatments. In addition to ERN1, this set included INTERACTING PROTEIN OF DMI3 (IPD3), NUCLEAR FACTOR YA-1 (NF-YA1), NIN-LIKE PROTEIN 2 (NLP2), ETHYLENE-RESPONSE FACTOR REQUIRED FOR NODULE DIFFERENTIATION 1 (EFD1), and, LIGHT-SENSITIVE SHORT HYPOCOTYL 1 (LSH1). NLP2 directly induces the expression of leghemoglobin (Lb) genes37. In our dataset, Lb1/Lb7 (Medtr5g081000) mirrored the expression profiles of NLP2 in all conditions and their expression profiles clustered closely, corroborating this transcriptional regulation, and pointing towards a possible interaction between Cd, Zn, and iron (Fe) homeostasis in a host genotype-dependent manner (Fig. 5c).
The transcription factor-encoding NAC969 (acronym for NAM, ATAF1/2, and CUC2-969) is induced in senescing nodules and repressed by sodic salt stress in the nodules38. In our dataset, this gene was strongly downregulated under both metal treatments in the WT but not mutant nodules. On the other hand, NAC969 was strongly induced by Cd treatment in the mutant compared to the Cd-treated wild-type. These results suggest complex transcriptional regulation of NAC969 under metal stresses as a function of the plant genotype (Fig. 5c, Supplementary Tables S11-15).
Heavy metal treatments regulate ion homeostasis in the nodule in a host genotype-dependent manner
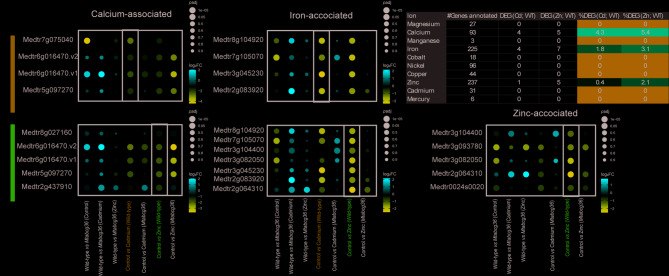
Given the role of ABCG transporters in the transport of bivalent metal ions, we searched the set of genes differentially expressed in response to Cd or Zn for associations with these ions based on annotations containing text that includes the following ions: magnesium, (Mg), calcium (Ca), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), zinc (Zn), Cd, or mercury (Hg). No DEG (|log2FC| >1, p-adj < 0.05) was observed to be associated with Mg, Mn, Co, Ni, Cu, Cd, or Hg. Only one DEG (Medtr3g467150) was found to be associated with Zn in response to Cd (Fig. 6, Supplementary Tables S16, 17). Consistent with our Zn treatment, five DEGs with Zn-related functional annotations were observed in the WT. Multiple Ca and Fe associated genes were differentially regulated by Cd and Zn in the WT. No Fe-associated DEGs under any treatment in the WT was differentially expressed in the mutant. These results suggest a role of MtABCG36 in Fe homeostasis in the nodule, in line with the differential regulation of leghemoglobin genes such as Lb1/Lb7 in the mutant (Figs. 5c and 6, Supplementary Tables S16, 17).
Fig. 6.
Effect of metal treatments and genotypes on ion homeostasis in the nodule. Dot plots showing plant DEGs that had gene annotations associated with bivalent ions. The upper panels show cadmium-induced DEGs, and lower panels show zinc-induced DEGs in the WT nodule. Gene IDs on the y-axes are Mt4.0 gene annotations and gene IDs with v1 and v2 indicate more than one homolog between the R108 Hi-C and Mt4.0 genomes. Only one zinc-associated gene (Medtr3g467150) was differentially regulated by cadmium. The color of the dots represents the directionality of gene expression. The size of the dots represents the reverse of the adjusted p-value; larger dots represent a smaller adjusted p-value. Vertical black boxes contain only significant DEGs for those comparisons. For all dotplots, the genes may or may not be differentially regulated in combination of genotypes and treatments other than the ones specified. |log2FC| > 1; p-adj < 0.05.
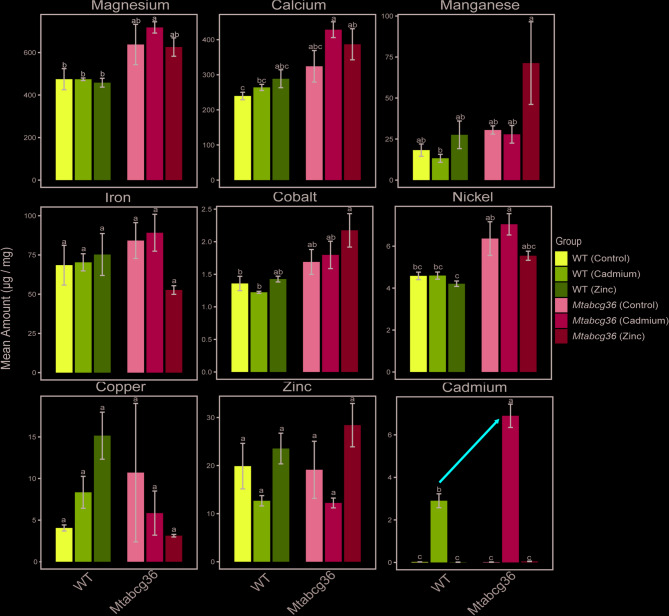
Taking a deeper look into the connection between MtABCG36 and homeostasis of bivalent ions, we quantified these ions in the WT and mutant nodules in the presence of Cd or Zn, or under the control conditions using inductively coupled plasma mass spectrometry (ICP-MS) (Fig. 7). We observed that overall, Mg ion concentration was the highest in nodules amongst all others, congruent with the fact that it is the bivalent cation with the highest concentration in cells39. Using tests for multiple comparisons, we did not observe any significant differences in the concentrations of any ion, either between the two genotypes, or in response to Cd and Zn treatments, except Cd. Cd ions showed an expected, significant increase in response to Cd treatment in the WT nodules. This concentration was significantly higher in the mutant under the same condition, suggesting that MtABCG36 could act as a Cd exporter, as previously shown in Arabidopsis (Fig. 7)21.
Fig. 7.
Quantification of elements in the nodules using inductively coupled plasma mass spectrometry (ICP-MS). Bar plots show metal ion accumulation (in microgram per milligram nodule tissue) in the wild-type (WT) and Mtabcg36 mutant nodules treated with cadmium, zinc or control conditions. Error bars indicate standard error of the mean (SEM). The red arrow in the Cadmium panel highlights the increased accumulation of Cd in the mutant nodules compared to the WT under Cd treatment. Letters display the Tukey’s Honestly Significant Difference (HSD) test for multiple comparisons at α = 0.05. When common letters are shown above any bar, they are not significantly different.
Discussion
Leveraging the power of dual transcriptomics to simultaneously compare gene expression dynamics in both symbiotic partners, we observed patterns of responses to these stresses in both partners that were largely contingent on the plant genotype. We harvested nodules for dual transcriptomics under conditions that had clear effects on the shoot and root biomass, as well as nodulation. The Zn treatment resulted in increased shoot biomass, reflecting the nutritional role of Zn in plants, while Cd did not have this effect on shoot biomass. Root biomass and the number of nodules/root biomass were negatively correlated under both metal treatments, as expected due to tradeoffs for resource allocation in root tissues. Thus, corroborating previous findings that nodulation is more sensitive to ionic imbalances compared to general plant growth35. Both Cd and Zn increased root biomass and inhibited nodulation, likely reflecting decreased carbon flux towards sustaining symbiosis under a stressed environment40. The plant genotype was critical to nodulation, and nodulation was severely impacted in the mutant (Fig. 1). Together, we observed a strong effect of both the heavy metal treatments and the plant genotype on nodule development and symbiosis with rhizobia.
Heavy metal stress response is absorbed by plant cells in nodules
Nodules are comprised of both plant and bacterial cells at various stages of differentiation. These cells possess their own genomic mechanisms for processing nutrients or toxic ion transport. If the plant cells are unable to maintain homeostasis in the root environment during ionic stress, then symbiosis with rhizobia, and nitrogen fixation will be impaired10. The PCA of the nodule dual transcriptome data revealed the plant clearly clustered by treatment and genotype, while the bacteria showed very little clustering (Fig. 2a, b). We found that the rhizobia inside mutant nodules showed considerable differences in gene expression compared with rhizobia in WT plants after the Zn treatment, but such differences were not observed after Cd treatment. A previous study exposing free-living S. meliloti to Cd and Zn stress demonstrated changes in gene expression under both treatments and highlighted a role of exopolysaccharides in Zn, but not Cd stress response41. We did not observe any exo genes differentially expressed in the mutant nodules under Zn treatment. Furthermore, the genes previously identified in heavy metal tolerant Mesorhizobium metallidurans and S. meliloti such as CadA and other ATPase membrane transporter-encoding genes42–44 did not show expression changes in S. meliloti Sm2011 in the nodules in our study. The differences in these studies raise two primary possibilities: (a) the mechanism of Zn stress tolerance between free-living and symbiotic conditions are different, and/or (b) the host genotype has a role to play, as previously observed45. In our study, because we found that very few rhizobial genes responded to the Cd or Zn treatment in both WT and mutant nodules when compared with control conditions, it appears that the host absorbs most of the stress caused by Cd or Zn inside nodules. When comparing the post Zn treatment conditions, the majority of DEGs following Zn treatment in the rhizobia occurred only when the host was a mutant, which suggests that the WT host is better able to absorb more of the stress caused specifically by Zn.
Because a nodule is a symbiotic organ that is comprised of both plant and rhizobia cells, in principle the stress response could equally affect both types of cells at the whole organ level. However, because the rhizobial cells comprise a symbiosome that is surrounded by plant cells, there is a strong potential for the cell types to show very different responses to an external stress. As such, it is not difficult to conceive that these partners will respond to environmental changes differently. Moreover, the nodules provide a unique niche for nitrogen fixation, where the host and symbiont physiologies are substantially different from their non-symbiotic states, which enables them to play their respective roles in this symbiosis, in addition to compartmentalizing stress to maintain homeostasis necessary for symbiosis. In Medicago nodules, the host and the symbiont respond differently to drought stress46. Similarly, heat stress causes symbiont-specific transcriptional changes in photosymbiodemes47. We find that not only do the host and the microsymbiont respond differently to the metal stresses, where the host shows large numbers of DEGs, and the microsymbiont showed minimal changes in gene expression (control vs. Cd or Zn in the WT), but the host genotype (WT or Mtabcg36 mutant) and the environment (Cd or Zn treatment) interact to uniquely influence the symbiont. In addition, while large differences in gene expression were observed in the symbiont in WT vs. mutant nodules after Zn treatment, that is not the case for Cd treatment.
The large number of rhizobial DEGs observed between Zn-treated WT and Zn-treated mutant nodules were not observed in response to Zn treatment alone in either genotype, nor between the genotypes without any treatment. This observation suggests that the Zn-genotype interaction is synergistic, and their combined impact is much stronger than their added individual impacts29. In the fields of ecology or toxicology, synergy is a contextual phenomenon, and in our case, the overall directionality is negative, as observed by the downregulation of the majority of genes (Fig. 3a) which we interpret is the result of the combined stress of the Mtabcg36 mutant and the Zn stress. The combination of the mutation and Zn stress interaction is further supported by significantly lower nodule number in the mutant Zn-treated plants compared with WT while the root biomass in both WT and mutant is high (Fig. 1), due to the resource allocation tradeoff previously mentioned. Therefore, despite the Zn treatment providing a nutrient supplement for plant biomass, in the mutant plants, this comes at the expense of nodulation. These data suggest that the mutant nodules become especially Zn starved due to increased transport to roots and shoots, resulting in a negative impact on the rhizobia in mutant nodules. Cd stress does not have this strong effect on root and shoot biomass, and Cd is not a requirement for normal nodule formation.
Shared mechanisms of cd and Zn stress responses in the host
Metal ions participate in various cellular functions and act as macro-, or micronutrients. Zn is an essential micronutrient for plants that acts as an enzyme cofactor, participates in hormone synthesis, photosynthesis, and protection against oxidative cellular damage48. However, excess Zn can be toxic to plants49–51. During root nodule symbiosis, Zn triggers nodule senescence52. On the other hand, Cd is not known to play any nutritional role in plants and is phytotoxic even at low concentrations, although natural variation in tolerance to toxic heavy metals does exist in plants, including Medicago11,12,53,54. It is widely known that Zn transport mechanisms have been co-opted in plants to transport toxic heavy metals, particularly Cd55–57. We observed an increase in root growth and a decrease in the number of nodules per unit root weight under both Cd and Zn treatments (Fig. 1). Because root nodule symbiosis in M. truncatula evolved from the lateral root developmental pathway58our results suggest that both Cd and Zn treatments act as environmental cues that shift the balance between root and nodule development. The similarities in root and nodule phenotypes under these treatments were reflected in our nodule RNA-seq data. Over 70% of plant DEGs observed under Cd treatment were also observed under Zn treatment in WT nodules (Fig. 4). These genes included several transporter-encoding, metal and auxin-associated genes, suggesting perturbed ion and auxin homeostasis (Fig. 5a, b). Given that our experimental set-up comprised of nutrient-replete growth conditions, the overlap of gene expression profiles between Cd and Zn treatments suggests that these treatments affect the plant using several shared mechanisms (Fig. 4c, d).
The majority of the host genes commonly regulated by Cd and Zn in the nodule were downregulated by both treatments, which could imply slowing down of basal metabolism and development as previously reported under drought stress (Fig. 4c, d)46. However, most plant genes known to be involved in root nodule symbiosis were upregulated under these treatments in the WT nodules (Fig. 5c). Multiple genes such as ENOD11, LYK10/EPR3, VPY, and ERN1 that were upregulated by the heavy metal salt treatments in our study have been previously shown to be upregulated in rhizobium-inoculated roots under sodic salt treatment compared to untreated, inoculated roots35. Such similar results could imply that under these stresses, the nodules do not reach maturity, and the pool of RNA harvested from these tissues contain the markers of early symbiotic genes in abundance compared to the unstressed nodules. These studies used two different genotypes of M. truncatula, R108 and A17. R108 is more sensitive to environmental fluctuations59and yet similar genes were upregulated in both genotypes. In field conditions, extreme physical environments often constitute multifactorial stress combinations60. These studies identified a subset of symbiotic genes that are sensitive to abiotic stresses, in particular, stresses caused by excess salts of sodium and heavy metals. Manipulation of these genes could be useful in engineering environmentally resilient rhizobium-legume symbiosis.
An ABC protein in metal stress response during trans-kingdom nutritional symbiosis
ABC proteins are present in all domains of life. While they operate using the conserved mechanism of ATP hydrolysis, flexibility in their substrate-binding ability has made them an integral component of various cellular mechanisms, either via direct transport, or through indirect regulation of downstream processes. Their roles range from multidrug resistance in humans to cellular detoxification in plants that is critical to agriculture61,62.
While we expected that the mutation in the host plant would result in the disruption of coregulated transport mechanisms, we also found that the microsymbiont is more sensitive to Zn exposure and accumulation when the expression of the host MtABCG36 gene is compromised, where over 20% of the rhizobial genes were expressed lower in the mutant compared with WT after Zn treatment. Under the same conditions, less than 2% of DEGs were observed in the host (Fig. 2d). Moreover, multiple nod, nif, and, fix genes in the rhizobia are downregulated under these conditions, where the host symbiotic genes are either not differentially expressed, or when expressed, they were typically upregulated (Fig. 5c, Supplementary Tables S1, S3). Technical complications compelled us to base our analyses on a single insertional mutant. Future studies using CRISPR or similar methods will allow us to more specifically identify the role of MtABCG36 in mediating response to heavy metals during symbiosis. Nonetheless, our current findings clearly suggest that the host genotype influences ion homeostasis in the microsymbiont inside the nodule.
In Arabidopsis thaliana, the ABC protein AtPDR8 (ABCG36) acts as an exporter of Cd2+21. We also discovered greater accumulation of Cd in the Mtabcg36 mutant nodules under Cd stress compared to the WT nodules (Fig. 7), suggesting a potential role of MtABCG36 as a Cd2+ exporter. Moreover, AtABCG36 acts in Cd stress response through a mechanism that appears to be conserved between A. thaliana, rice, and poplar where loss of function mutations or RNAi in the ABCG36 orthologs decrease Cd tolerance, increase Cd accumulation in root tissues, and reduce root growth21,22,63. Using our phenotypic responses as metrics for changes in Cd resilience, we found significantly reduced shoot biomass, root biomass, and nodule number in the Cd treated Mtabcg36 mutant plants compared with Cd treated WT plants, indicating a reduction in Cd tolerance in the Mtabcg36 mutant. Because nodules are an extension of root tissue, our results suggest that the role of MtABCG36 in Cd stress tolerance in nodules likely has a similar function as a Cd2+ efflux protein in roots, but also affects rhizobium-legume symbiosis indicated by even poorer nodulation in the mutant than in the control conditions.
Expression of transporters in the WT in response to both Cd and Zn showed highly similar log2FC, significance, and directionality, with mostly downregulation of the transporters. In the Mtabcg36 genotype under the same two stress treatments, the expression level, directionality and significance changed dramatically (Fig. 5b), which showed a shutdown of differential expression of most these transporters. For host plant symbiosis genes, the upregulation of nearly all of the genes (Fig. 5c) showed parallel expression patterns in all WT conditions, and loss of significant expression changes in all of the Mtabcg36 conditions. These findings suggest that MtABCG36 can have strong pleiotropic effects on gene expression affecting nodule development and ion homeostasis, although other genes could be contributing to the observed phenotypes as well. This included two potentially important transporters that are known to regulate Cd2+ transport, MtABCC3 which was strongly upregulated in response to Cd in WT, but downregulated in response to Cd in the Mtabdg36 mutant, and MtYSL3 which showed no expression change in the Mtabcg36 mutant, but was significantly upregulated in response to Cd in WT. Finally, our findings also point towards possible involvement of iron and auxin downstream of MtABCG36 in heavy metal stress tolerance in nodules30,64 (Figs. 5a and 6). Because nodule formation is so heavily dependent on iron homeostasis, the combined effects of the MtABCG36 mutant and Cd stress can affect plant resilience due to poor nitrogen fixation. A schematic highlighting our primary findings is shown in Fig. 8.
Fig. 8.
Schematic depicting response of the symbiotic partners to cadmium and zinc treatments as observed by dual transcriptomics and ionomics from nodules. Both Cd and Zn treatments strongly influence the plant transcriptome in the nodule (black arrows). Only Zn treatment shows considerable influence on the bacterial transcriptome in the nodule in a host genotype-dependent manner, when the host plant harbors a mutation in MtABCG36 (teal arrow). On the plant side, both treatments affect auxin and iron homeostasis, likely mediated by MtABCG36. Potential involvement of MtABCG36 is depicted by dashed arrows. Plant (green boxes) and bacterial (pink box) DEGs are shown. Genes in red and blue fonts are upregulated and downregulated, respectively. The green boxes contain keyenes regulated either by Cd or Zn, or by both treatments. Cd and Zn treatments altered the expressions of the largest sets of plant genes in the wild-type nodules. The complete gene list can be found in Fig. 5c and Table S15. Auxin-associated genes other than auxin biosynthetic YUC2 and 8 are listed in Fig. 5a and Tables S8 and S9. The pink box shows key bacterial symbiotic DEGs in WT vs. mutant nodules under Zn treatment. This was the only comparison on the bacterial side that showed a considerable number of DEGs. The complete list is in Table S3. MtABCG36 is potentially involved in Cd export, as suggested by our ionomics data and existing literature from other species.
We have shown that dual transcriptomics can be a powerful approach for studying plant-microbe interactions by simultaneously analyzing the gene expression profiles of both the plant and the microbe in the context of environmental stress. This approach helped to identify specific genes that are differentially regulated in both the plant and the microbe during symbiotic interactions, and highlighted genes involved in nutrient exchange and symbiotic development. While it appeared that the host plant cells in the nodule tissue buffered much of the heavy metal stress in the S. meliloti strain, we nevertheless found important synergistic effects in the rhizobial response to Zn. Further studies using combined ion stresses could be applied to dual transcriptomics to quantify synergistic or antagonistic effects29,65 in both symbiotic partners. In addition, most studies that have used loss of function mutations in known ion transporters that result in deleterious effects on nodulation have focused mostly on the phenotypic effects of the host plant10,34 with little consideration of rhizobial responses. Therefore, incorporating experimental designs that include genetic variation for tolerance to ion stress in both symbiotic partners, would further advance our understanding of the combined effects of stress tolerance in both partners on the dual transcriptome.
Methods
Plant growth conditions and treatments
Medicago truncatula R108 was used as the wild-type genotype for all experiments. The Mtabcg36 mutant is the Tnt1 insertion line NF2826 previously characterized as pen3-like, which was previously shown to have produced fewer nodules compared to wild-type19. The annotation for MtABCG36 is Medtr2g101090 in the M. truncatula A17 version 4.0 and MtrunA17_Chr2g0331081 A17 v5.0 reference genome. In the M. truncatula R108 Hi-C whole chromosome genome assembly, MtABCG6 is MedtrR108_hic.HiC_scaffold_2.4355. The A. thaliana ortholog of ABCG36/PDR8 is AT1G59870. To germinate seeds, the seeds were scarified for three minutes in sulfuric acid, surface sterilized with 50% bleach and 0.1% tween-20 for three minutes, imbibed for two hours at room temperature, stratified overnight at 4 °C, and germinated in dark at 16/8 h light/dark, at 21 °C and 40% relative humidity.
Germinated seedlings were transplanted to 4” pots containing autoclaved Turface®: vermiculite (2:1) with half-strength, nitrogen-free Broughton and Dilworth (B&D) medium66 containing necessary macro-, and micronutrients. Zinc concentration in the half-strength medium was 0.25 µM. Four to five days after potting, the plants were inoculated with Sinorhizobium meliloti strain Sm2011 at OD600 0.7-1, and replenished with half-strength B&D medium, supplemented with 0.5mM KNO3 as required. We assume this nutrient status to be a nutrient-replete condition. Three weeks post inoculation, the plants were treated with 100µM CdCl2 or ZnSO4. To the best of our knowledge, no ABC transporter has yet been reported in plants in association with chloride or sulfate transport61. The heavy metal stress treatments were based on previous studies67–69. Because the goal was to study nodules, the treatments were applied at an advanced stage of symbiosis.
ICP-MS methods
Nodule samples were collected after one week of stress treatment, i.e., after four weeks of inoculation. To wash off the excess metals from root samples, they were sequentially immersed in 5 mM CaCl2, 1 mM MES-KOH, and ultra-pure H2O (UPW) for 30 min each. Subsequently, the samples were dried by placing them in a 50 °C oven for a minimum of 48 h. Once the samples were dried and homogenized by crushing, approximately 75 mg was weighed and transferred into tubes for digestion by adding ICP-MS grade 69% HNO3 (1017992500, Sigma-Aldrich). They were allowed to sit overnight (to avoid boiling over) and then transferred on a Digiprep heat-block set at 95 °C for 4–5 h. Finally, the samples were diluted 4-fold with UPW and were ready for analysis. We used ICP-MS (NexION 350D Model, Perkin Elmer) to determine the concentrations of metals in the samples. The instrument was calibrated using an environmental standard mix (N9307805, Perkin Elmer), and 89Y and 115In were used as internal standards (M1-ISMS-25, Elemental Scientific). The concentrations were converted from µL/g to µg/g by normalizing to the dry weights of the samples.
Dual RNA-seq from nodule samples
Total RNA from the nodules were extracted using the Qiagen RNeasy kit and treated with DNase to remove genomic DNA contamination. The samples were then run on a Fragment Analyzer (Agilent) to evaluate RNA integrity. Construction of the dual RNA-seq libraries and sequencing on the Illumina NovaSeq 6000 were performed at the Roy J. Carver Biotechnology Center at the University of Illinois at Urbana-Champaign. The total RNAs were converted into individually barcoded RNAseq libraries with the Universal Plus mRNA-Seq Library Preparation kit from Tecan, using custom probes against M. truncatula and S. meliloti rRNA designed by Tecan. Libraries were barcoded with Unique Dual Indexes (UDIs) which have been developed to prevent index switching. The adaptor-ligated double-stranded cDNAs were amplified by PCR for 10 cycles. The final libraries were quantified with Qubit (ThermoFisher) and the average cDNA fragment sizes were determined on a Fragment Analyzer. The libraries were diluted to 10nM and further quantified by qPCR on a CFX Connect Real-Time qPCR system (Biorad) for accurate pooling of barcoded libraries and maximization of number of clusters in the flowcell.
RNA-seq data analysis
Paired end Illumina reads (150 bp) were trimmed using trimmomatic70. Before mapping, the M. truncatula R108 Hi-C full chromosome reference genome assemblyand annotation (Kaur et al., 2021) was concatenated with the S. meliloti Sm2011 genome72. The Sm2011 genome was annotated using using RASTtk (https://rast.nmpdr.org)73to update previously released gene annotations of this strain, using a larger collection of wild S. medicae strains. This included using the RASTtk annotation pipeline and Orthofinder analysis45. The concatenated genome was indexed using STAR74. Trimmed reads were then mapped to the concatenated M. truncatula R108-Sm2011 reference genome using STAR. EdgeR75 was used to quantify weighted trimmed mean of the log expression ratios (trimmed mean of M values (TMM))76 as a normalized read count for quantifying and visualizing relative gene expression values in Cd-, or Zn-treated samples compared with control samples. The R108 gene annotations were merged with Mt4.0 gene annotations using Table S8 from71to utilize and identify previously reported genes in the M. truncatula literature. Subsequently, functional annotation from Phytozome v13 for M. truncaulta v4.0 was incorporated with each of the R108 Hi-C gene models. The Phytozome annotation included KOG, KEGG, ENZYME, pathway, and InterPro annotations as well as the best BLAST hit to the A. thaliana genome. Statistical analysis of read counts for each gene was done using DESeq277, separately for the R108 plant genome and the Sm2011 rhizobial genome to avoid normalization of the count data on the concatenated genome. RNA-seq data was analyzed on RStudio (2023.06.2 + 561 “Mountain Hydrangea”). “pheatmap” (https://cran.r-project.org/web/packages/pheatmap/pheatmap.pdf) and “EnhancedVolcano” were used (https://github.com/kevinblighe/EnhancedVolcano) to generate the heatmaps and volcano plots, respectively.
Statistical analysis
Plant phenotype and ICP-MS data were analyzed using JMP® Pro 17.1.0 (671353).
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding for this project came from the Department of Energy Quantitative Plant Science Initiative (QPSI) SFA and U.S. Department of Agriculture, Agricultural Research Service Project Number 3093-53000-001-000D & 5062-21000-035-000D. We thank Peter Tiffin and Brendan Epstein for discussions about dual-transcriptomics analyses and Katy Heath, Alvaro Hernandez and Chris Wight at the University of Illinois at Urbana-Champaign for consulting, oligo design, and rRNA cleanup and sequencing for the nodule samples. We thank Michael Clear for consultation with dual-transcriptomics and assistance with pipeline construction for assigning Illumina reads to each species. We thank faculty at the Children’s Nutrition Research Center and Baylor College of Medicine for useful discussions.
Author contributions
T.P. conceived the project, T.P., R.S., and A.B. designed the experiments, R.S. and A.B. performed the experiments, J.W. and K.M. generated and maintained the mutant, S.J.C. characterized the mutant, S.C., T.P., and A.B. analyzed the data, S.C. and T.P. wrote the manuscript, all authors reviewed the manuscript.
Funding
for this project came from the Department of Energy Quantitative Plant Science Initiative (QPSI) SFA and U.S. Department of Agriculture, Agricultural Research Service Project Number 3093-53000-001-000D & 5062-21000-035-000D. We thank Peter Tiffin and Brendan Epstein for discussions about dual-transcriptomics analyses and Katy Heath, Alvaro Hernandez and Chris Wight at the University of Illinois at Urbana-Champaign for consulting, oligo design, and rRNA cleanup and sequencing for the nodule samples. We thank Michael Clear for consultation with dual-transcriptomics and assistance with pipeline construction for assigning Illumina reads to each species. We thank faculty at the Children’s Nutrition Research Center and Baylor College of Medicine for useful discussions.
Data availability
Raw Illumina data is available at the NCBI Bioproject: PRJNA1137930 : Medicago truncatula nodule RNA sequencing under cadmium and zinc stresshttps://dataview.ncbi.nlm.nih.gov/object/PRJNA1137930?reviewer=m8a3a28hn7imlub6an1vaai2m.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shaffique, S. et al. Recent progress on the microbial mitigation of heavy metal stress in soybean: overview and implications. Front Plant. Sci.14, 1188856 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang, S., Yamaji, N. & Ma, J. F. Metal transport systems in plants. Annu Rev. Plant. Biol75, (2024). [DOI] [PubMed]
- 3.Carpena, R. O. et al. Cadmium-stress in white lupin: effects on nodule structure and functioning. Plant Physiol. Biochem.41, 911–919 (2003). [Google Scholar]
- 4.Shvaleva, A. et al. Flavodoxin overexpression reduces cadmium-induced damage in alfalfa root nodules. Plant. Soil.326, 109–121 (2010). [Google Scholar]
- 5.Tiwari, S. & Lata, C. Heavy metal stress, signaling, and tolerance due to plant-associated microbes: an overview. Front Plant. Sci.9, 452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali, B. & Gill, R. A. Heavy metal toxicity in plants: Recent insights on physiological and molecular aspects, II. Front Plant. Sci.13, 1016257 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy, S. et al. Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. Plant. Cell.32, 15–41 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborty, S., Valdés-López, O., Stonoha-Arther, C. & Ané, J. M. Transcription factors controlling the Rhizobium–Legume symbiosis: integrating infection, organogenesis and the abiotic environment. Plant. Cell. Physiol.10.1093/pcp/pcac063 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Sharma, R. et al. Plant genotype and microbial strain combinations strongly influence the transcriptome under heavy metal stress conditions. Preprint At.10.1101/2024.10.06.616885 (2024). [Google Scholar]
- 10.León-Mediavilla, J. et al. MtMTP2-facilitated zinc transport into intracellular compartments is essential for nodule development in medicago truncatula. Front Plant. Sci9, 990 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la García, V. S., de la Coba, T., Pueyo, J. J. & Lucas, M. M. Cadmium-tolerant and -sensitive cultivars identified by screening of medicago truncatula germplasm display contrasting responses to cadmium stress. Front Plant. Sci.12, 595001 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paape, T. et al. Genome-wide association study reveals complex genetic architecture of cadmium and mercury accumulation and tolerance traits in medicago truncatula. Front Plant. Sci.12, 806949 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pal, S. C. Effect of heavy metals on legume-Rhizobium symbiosis. in Biological Nitrogen Fixation Associated with Rice Production 21–29Springer Netherlands, Dordrecht, (1996). 10.1007/978-94-015-8670-2_3
- 14.Hwang, J. U. et al. Plant ABC transporters enable many unique aspects of a terrestrial plant’s lifestyle. Mol. Plant.9, 338–355 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Roy, S. et al. Three common symbiotic ABC subfamily B transporters in Medicago truncatula are regulated by a NIN-Independent branch of the symbiosis signaling pathway. Mol. Plant-Microbe Interactions34, 939–951 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Jarzyniak, K. et al. Early stages of legume–rhizobia symbiosis are controlled by ABCG-mediated transport of active cytokinins. Nat. Plants. 7, 428–436 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Jamruszka, T. et al. Medicago truncatula ABCG40 is a cytokinin importer that negatively regulates lateral root density and nodule number. BioRxiv Preprint bioRxiv. 2022.11.10.516000v210.1101/2022.11.10.516000 (2024).
- 18.Banasiak, J. & Jasiński, M. ATP-binding cassette transporters in nonmodel plants. New Phytol.233, 1597–1612 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Curtin, S. J. et al. Validating genome-wide association candidates controlling quantitative variation in nodulation. Plant. Physiol.173, 921–931 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chao, L., He, L., Xianduo, Z. & Zhimin, Y. A. Pleiotropic drug resistance family protein gene is required for rice growth, seed development and zinc homeostasis. Rice Sci.30, 127–137 (2023). [Google Scholar]
- 21.Kim, D., Bovet, L., Maeshima, M., Martinoia, E. & Lee, Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J.50, 207–218 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Fu, S. et al. The ABC transporter ABCG36 is required for cadmium tolerance in rice. J. Exp. Bot.70, 5909–5918 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang, D. et al. Dual RNA-Seq analysis pinpoints a balanced regulation between symbiosis and immunity in medicago truncatula-Sinorhizobium meliloti symbiotic nodules. Int. J. Mol. Sci.24, 16178 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sauviac, L. et al. A dual legume-rhizobium transcriptome of symbiotic nodule senescence reveals coordinated plant and bacterial responses. Plant. Cell. Environ.45, 3100–3121 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Keller-Pearson, M. et al. A dual transcriptomic approach reveals contrasting patterns of differential gene expression during drought in arbuscular mycorrhizal fungus and Carrot. Mol. Plant-Microbe Interactions36, 821–832 (2023). [DOI] [PubMed] [Google Scholar]
- 26.Li, X. et al. A dual RNA-seq analyses revealed dynamic arms race during the invasion of walnut by Colletotrichum gloeosporioides. BMC Plant. Biol.24, 653 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Aubel, G. et al. Dual transcriptomic and metabolomic analysis of elicited flax sheds light on the kinetics of immune defense activation against the biotrophic pathogen oidium Lini. Phytopathology114, 1904–1916 (2024). [DOI] [PubMed] [Google Scholar]
- 28.Bhagat, N., Mansotra, R., Patel, K., Ambardar, S. & Vakhlu, J. Molecular warfare between pathogenic fusarium oxysporum R1 and host crocus sativus L. unraveled by dual transcriptomics. Plant. Cell. Rep.43, 42 (2024). [DOI] [PubMed] [Google Scholar]
- 29.Piggott, J. J., Townsend, C. R. & Matthaei, C. D. Reconceptualizing synergism and antagonism among multiple stressors. Ecol. Evol.5, 1538–1547 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aryal, B. et al. ABCG36/PEN3/PDR8 Is an exporter of the auxin precursor, indole-3-butyric acid, and involved in auxin-controlled development. Front Plant. Sci.10, 889 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou, Y., Wang, Y., Zhang, D. & Liang, J. Endomembrane-biased dimerization of ABCG16 and ABCG25 transporters determines their substrate selectivity in ABA-regulated plant growth and stress responses. Mol. Plant.17, 478–495 (2024). [DOI] [PubMed] [Google Scholar]
- 32.Curie, C. et al. Metal movement within the plant: contribution of nicotianamine and yellow Stripe 1-like transporters. Ann. Bot.103, 1–11 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunetti, P. et al. Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis. J. Exp. Bot.66, 3815–3829 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castro-Rodríguez, R. et al. The Medicago truncatula yellow Stripe1-Like3 gene is involved in vascular delivery of transition metals to root nodules. J. Exp. Bot.71, 7257–7269 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Chakraborty, S. et al. Salt stress enhances early symbiotic gene expression in medicago truncatula and induces a stress-specific set of rhizobium-responsive genes. Mol. Plant. Microbe Interact.10.1094/MPMI-01-21-0019-R (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, T. et al. Light-sensitive short hypocotyl genes confer symbiotic nodule identity in the legume medicago truncatula. Curr. Biol.34, 825–840e7 (2024). [DOI] [PubMed] [Google Scholar]
- 37.Jiang, S. et al. NIN-like protein transcription factors regulate leghemoglobin genes in legume nodules. Sci. (1979). 374, 625–628 (2021). [DOI] [PubMed] [Google Scholar]
- 38.De Zélicourt, A. et al. Dual involvement of a medicago truncatula NAC transcription factor in root abiotic stress response and symbiotic nodule senescence. Plant J.70, 220–230 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Takeda, H. et al. Structural basis for ion selectivity revealed by high-resolution crystal structure of Mg2 + channel MgtE. Nat. Commun.5, 5374 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishida, H. & Suzaki, T. Two negative regulatory systems of root nodule symbiosis: how are symbiotic benefits and costs balanced?? Plant. Cell. Physiol.59, 1733–1738 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Rossbach, S. et al. Response of Sinorhizobium meliloti to elevated concentrations of cadmium and zinc. Appl. Environ. Microbiol.74, 4218–4221 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maynaud, G. et al. CadA of mesorhizobium metallidurans isolated from a zinc-rich mining soil is a PIB-2-type ATPase involved in cadmium and zinc resistance. Res. Microbiol.165, 175–189 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Maynaud, G. et al. Genome-wide transcriptional responses of two metal-tolerant symbiotic mesorhizobium isolates to zinc and cadmium exposure. BMC Genom.14, 292 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu, M. et al. Zinc resistance mechanisms of P1B-type ATPases in Sinorhizobium meliloti CCNWSX0020. Sci. Rep.6, 29355 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhat, A. et al. Horizontal gene transfer of the Mer Operon is associated with large effects on the transcriptome and increased tolerance to mercury in nitrogen-fixing bacteria. BMC Microbiol.24, 247 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larrainzar, E. et al. Medicago truncatula root nodule proteome analysis reveals differential plant and bacteroid responses to drought stress. Plant. Physiol.144, 1495–1507 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Almer, J. et al. Symbiont-specific responses to environmental cues in a threesome lichen symbiosis. Mol. Ecol.32, 1045–1061 (2023). [DOI] [PubMed] [Google Scholar]
- 48.Hamzah Saleem, M., Usman, K., Rizwan, M., Al Jabri, H. & Alsafran, M. Functions and strategies for enhancing zinc availability in plants for sustainable agriculture. Front Plant. Sci13, 1033092 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaur, H. & Garg, N. Zinc toxicity in plants: a review. Planta253, 129 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Zhong, K. et al. Natural variation of TBR confers plant zinc toxicity tolerance through root cell wall pectin methylesterification. Nat. Commun.15, 1–14 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meng, Y. et al. Toxicity effects of zinc supply on growth revealed by physiological and transcriptomic evidences in sweet potato (Ipomoea Batatas (L.) Lam). Sci. Rep.13, 19203 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin, J. et al. Zinc mediates control of nitrogen fixation via transcription factor filamentation. Nature631(8019), 164–169 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El Rasafi, T. et al. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol.52, 675–726 (2022). [Google Scholar]
- 54.de la García, V. S., de la Coba, T., Lucas, M. M. & Pueyo, J. J. Rapid screening of medicago truncatula germplasm for mercury tolerance at the seedling stage. Environ. Exp. Bot.91, 90–96 (2013). [Google Scholar]
- 55.Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie88, 1707–1719 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Clemens, S., Aarts, M. G. M., Thomine, S. & Verbruggen, N. Plant science: the key to preventing slow cadmium poisoning. Trends Plant. Sci.18, 92–99 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Tan, L. et al. ZINC TRANSPORTER5 and ZINC TRANSPORTER9 function synergistically in zinc/cadmium uptake. Plant. Physiol.183, 1235–1249 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schiessl, K. et al. NODULE INCEPTION recruits the lateral root developmental program for symbiotic nodule organogenesis in medicago truncatula. Curr. Biol.29, 3657–3668 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Lorenzo, L. et al. Differential expression of the TFIIIA regulatory pathway in response to salt stress between medicago truncatula genotypes. Plant. Physiol.145, 1521–1532 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zandalinas, S. I., Fritschi, F. B., Mittler, R. G. & Warming Climate change, and environmental pollution: recipe for a multifactorial stress combination disaster. Trends Plant. Sci.26, 588–599 (2021). [DOI] [PubMed] [Google Scholar]
- 61.Do, T. H. T., Martinoia, E., Lee, Y. & Hwang, J. U. Update on ATP-binding cassette (ABC) transporters: how they meet the needs of plants. Plant. Physiol.187, 1876–1892 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alam, A. & Locher, K. P. Structure and mechanism of human ABC transporters. Annu. Rev. Biophys.52, 275–300 (2023). [DOI] [PubMed] [Google Scholar]
- 63.Wang, H. et al. Ectopic expression of Poplar ABC transporter PtoABCG36 confers cd tolerance in Arabidopsis Thaliana. Int. J. Mol. Sci.20, 3293 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strader, L. C. & Bartel, B. Pleiotropic drug resistance8/ABCG36 ATP binding cassette transporter modulates sensitivity to the auxin precursor Indole-3-Butyric acid. Plant. Cell.21, 1992–2007 (2009). The Arabidopsis. [DOI] [PMC free article] [PubMed]
- 65.Li, Y. et al. Synergistic regulation at physiological, transcriptional and metabolic levels in tomato plants subjected to a combination of salt and heat stress. Plant J.117, 1656–1675 (2024). [DOI] [PubMed] [Google Scholar]
- 66.Broughton, W. J. & Dilworth, M. J. Control of Leghaemoglobin synthesis in snake beans. Biochem. J.125, 1075–1080 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nonnoi, F. et al. Metal tolerance of rhizobial strains isolated from nodules of herbaceous legumes (Medicago spp. And trifolium spp.) growing in mercury-contaminated soils. Appl. Soil. Ecol.61, 49–59 (2012). [Google Scholar]
- 68.Song, W. Y. et al. Arabidopsis PCR2 is a zinc exporter involved in both zinc extrusion and Long-Distance zinc transport. Plant. Cell.22, 2237–2252 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Talke, I. N., Hanikenne, M. & Krämer, U. Zinc-Dependent global transcriptional control, transcriptional deregulation, and higher gene copy number for genes in metal homeostasis of the hyperaccumulator Arabidopsis Halleri. Plant. Physiol.142, 148–167 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics30, 2114–2120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaur, P. et al. Delineating the Tnt1 insertion landscape of the model legume medicago truncatula cv. R108 at the Hi-C resolution using a Chromosome-Length genome assembly. Int. J. Mol. Sci.22, 4326 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sallet, E. et al. Next-Generation annotation of prokaryotic genomes with EuGene-P: application to Sinorhizobium meliloti 2011. DNA Res.20, 339–354 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brettin, T. et al. RASTtk: A modular and extensible implementation of the RAST algorithm for Building custom annotation pipelines and annotating batches of genomes. Sci. Rep.5(1), 8365 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robinson, M. D., McCarthy, D. J. & Smyth, G. K. <tt > edgeR: a bioconductor package for differential expression analysis of digital gene expression data</tt >. Bioinformatics26, 139–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol.11, R25 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol.15(12), 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw Illumina data is available at the NCBI Bioproject: PRJNA1137930 : Medicago truncatula nodule RNA sequencing under cadmium and zinc stresshttps://dataview.ncbi.nlm.nih.gov/object/PRJNA1137930?reviewer=m8a3a28hn7imlub6an1vaai2m.