Abstract
In order to determine whether dogs in the subclinical phase of canine monocytic ehrlichiosis (CME) are carriers of Ehrlichia canis and to determine the significance of persistent indirect immunofluorescent anti-E. canis antibody titers during this phase, PCR was performed with blood, bone marrow, and splenic aspirates collected 34 months postinoculation from six clinically healthy beagle dogs experimentally infected with E. canis. At least one of the three samples (spleen, bone marrow, and blood) from four of the six dogs was PCR positive. The spleens of all four of these dogs were PCR positive, and the bone marrow and blood of two of the four dogs were PCR positive. Indirect immunofluorescent-antibody titers increased progressively during the first 5 months postinfection, remained high for an additional period of more than 11 months, and declined thereafter, suggesting that the dogs were recovering from the disease. Five of the dogs remained seropositive 34 months postinfection. The data obtained in this study demonstrate for the first time that clinically healthy dogs in the subclinical phase of CME are carriers of the rickettsia. It was shown that dogs can harbor E. canis for years without developing the chronic clinical disease and that dogs can eliminate the parasite and recover from CME without medical treatment. Our findings suggest that the spleen is the organ most likely to harbor E. canis parasites during the subclinical phase and the last organ to accommodate the parasite before elimination. It was concluded that PCR of DNA extracted from splenic aspirates is a reliable method for determining the carrier state of CME.
Canine monocytic ehrlichiosis (CME), a tick-borne disease caused by the rickettsia Ehrlichia canis, was first recognized as a distinct clinical entity in Algeria in 1935 (9). Since then, it has been acknowledged worldwide as an important infectious disease of dogs and other canids (17).
The pathogenesis of CME involves an incubation period of 8 to 20 days, followed by acute, subclinical, and sometimes chronic phases (17). The acute phase of the disease has been investigated extensively, whereas the subclinical and the chronic phases have not. To date, only two publications characterizing the subclinical phase of CME have been published (7, 20). Many questions regarding this phase remain to be addressed, and the following are the most pertinent: Do animals in the subclinical phase remain carriers of the rickettsia? If so, where is the parasite harbored, and for how long may they remain in this phase without developing the chronic disease? Are immunocompetent dogs able to eliminate the parasite without medical therapy? What is the significance of the persistence of anti-E. canis immunoglobulin G antibody titers during the subclinical phase? To date, presumptive answers to these questions are proposed in the literature; however, in this study we attempted for the first time to address these questions experimentally through the use of nested PCR of DNA extracted from blood, bone marrow, and splenic aspirates collected from clinically healthy dogs 34 months after experimental infection with E. canis.
The nested PCR was proposed as a useful test for laboratory diagnosis and assessment of the efficacy of antibiotic therapy for E. canis infection, especially in combination with the indirect immunofluorescent-antibody (IFA) test. It was concluded that if the results of PCR and IFA tests are both positive or negative, dogs are either infected or not infected, respectively (21). The nested PCR with primers specific for E. canis was shown to be highly specific and sensitive for the detection of E. canis. It has shown that it could detect as little as 0.2 pg of purified E. canis DNA (21). A previous study concluded that 14 days of treatment with doxycycline eliminated acute experimental E. canis infection, because dogs became PCR and culture negative after treatment and remained PCR and culture negative thereafter (5). These results suggest that ehrlichial DNA (detected by nested PCR) does not persist in dogs after elimination of the intact organisms, and therefore, we chose to use the nested PCR in this study.
MATERIALS AND METHODS
Experimental infection of dogs.
Six clinically healthy beagle dogs (Harlan Laboratories, Indianapolis, Ind.) ranging in age from 8 to 12 months were used in this study. The dogs were fed a commercially available dog ration, provided water automatically, and regularly dipped in Paramite powder (Vetkem) to eliminate ectoparasites. All dogs were seronegative for E. canis antibodies, as determined by IFA testing before artificial infection, and their hematological and biochemical parameters fell within the normal ranges. The dogs were inoculated intravenously with 5 ml of heparinized blood from a beagle infected with the Israeli strain of E. canis (strain 611) which has previously been isolated and genetically characterized (13). Rectal temperature, food consumption, and clinical signs were monitored every alternate day. Blood samples for hematology and serology were collected twice weekly for the first 60 days postinoculation; thereafter, the dogs underwent monthly examinations for an additional 6 months. Clinical signs and hematological, biochemistry, and serological test results for these dogs during the acute and subclinical phases of the disease have been reported previously (13, 20). In the present study, blood samples, bone marrow, and splenic aspirates were collected from each dog 34 months postinoculation for hematological and serological tests and PCR.
Serological tests.
Blood for serological tests was collected in plastic tubes with no anticoagulant at 15 and 30 days and 2, 5, 16, 22, and 34 months postinoculation. Serum was separated by centrifugation 2 h after the blood was drawn, and samples were stored at −70°C until serological tests were carried out. The IFA test was performed as described previously (13, 18).
Collection of samples for hematological tests and PCR.
At 34 months postinoculation, blood samples for hematological tests and PCR were collected from the jugular vein in plastic tubes containing EDTA. Hematological assays were performed within 2 h of blood collection by using an automated cell counter (Minos ST-Vet, Montpelier, France) calibrated for canine blood. The following parameters were measured: hematocrit, hemoglobin concentration, total erythrocyte count, mean corpuscular volume, mean corpuscular hemoglobin concentration, leukocyte count, platelet count, and mean platelet volume. The dogs were anesthetized by intravenous injection of ketamine and xylazine (2 and 2 mg/kg of body weight, respectively), and ultrasound-guided needle aspirates were taken from the spleen. Bone marrow aspirates were taken from the iliac crest. Samples were collected in plastic tubes containing EDTA and were kept frozen at −20°C until DNA was extracted. Giemsa-stained smears prepared from the blood, bone marrow, and splenic aspirates were examined microscopically for the presence of E. canis morulae.
DNA extraction.
DNA was extracted as described previously (3), with slight modifications. After thawing, 0.25 ml of blood, bone marrow aspirate, or splenic aspirate was mixed with 0.6 ml of sterile, filtered 0.2% NaCl, vortexed thoroughly, and inverted several times over 5 to 10 min in order to lyse the erythrocytes. The isotonicity of the cell mixture was restored by adding 0.6 ml of sterile, filtered 1.2% NaCl. After adequate mixing, the tubes were centrifuged at 700 × g for 5 min. The pellet was washed three times by repeated suspension in 0.6 ml of sterile phosphate-buffered saline and centrifugation in order to remove the hemoglobin (a PCR inhibitor). The cells were then spun at high speed for 10 min and the supernatant was discarded. The retrieved pellet was diluted in 80 μl of lysis buffer (10 mM Tris hydrochloride [pH 8.3], 0.45% Nonidet P-40, 0.45% Tween 20, 100 μg of proteinase K per ml), and the mixture was incubated in a 56°C water bath for 3 h. The proteinase K was subsequently inactivated by incubating the samples at 95°C for 15 min.
Amplification of DNA.
DNA amplification was performed in two rounds as described previously (8) with a PTC-100 (MJ Research) thermocycler. For the first round, 5 μl of DNA template was added to 95 μl of a reaction mixture containing 10 μl of Mg2+-free buffer (Promega), 6 μl of 25 μM MgCl2, 2 μl of 10 mM (each) deoxynucleoside triphosphate, 1 μl of bovine serum albumin (10 mg/ml), 65.6 μl of sterile water, 0.4 μl of Taq DNA polymerase (Promega), and 5 μl of each primer (5 μM [each] of primers ECC [5′-AGAACGAACGCTGGCGGCAAGCC-3′] and ECB [5′-CGTATTACCGCGGCTGCTGGCA-3′]). The thermocycle profile consisted of 40 cycles of 94°C for 1 min, 45°C for 2 min, and 72°C for 1 min and 30 s. Beginning with the third round of amplification, each cycle at 72°C was extended by 1 s. Nested PCR was performed by using 5 μl of the product from the outside amplification (first round) with primers HE3 (5′-TATAGGTACCGTCATTATCTTCCCTAT-3′) and “canis” (5′-CAATTATTTATAGCCTCTGGCTATAGGA-3′). The second thermocycle profile consisted of 40 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 15 s. Beginning with the third round of amplification, each cycle at 72°C was extended by 1 s. The PCR products were visualized on a 1.5% agarose gel by using ethidium bromide and UV light. Positive and negative controls were used; positive controls were retrieved from E. canis-infected DH82 cells, and negative controls were retrieved from noninfected DH82 cells.
RESULTS
Clinical signs.
All dogs showed typical clinical signs consistent with acute CME by day 15 postinoculation. They were not treated medically, and by days 30 to 40 all dogs were clinically healthy. They remained clinically healthy throughout the remainder of the study period (total, 34 months).
PCR results.
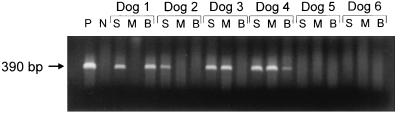
At least one of the three different samples (spleen, bone marrow, and blood) from four of the dogs (dogs 1 to 4) were PCR positive, while two dogs (dogs 5 and 6) were PCR negative. Splenic samples from all four PCR-positive dogs were positive, while bone marrow aspirates and blood samples from two of the four positive dogs were positive (Fig. 1).
FIG. 1.
PCR results for six beagle dogs 34 months postinoculation with E. canis. S, splenic aspirate; M, bone marrow aspirate; B, blood sample; P, positive control retrieved from E. canis-infected DH82 cells; N, negative control retrieved from noninfected DH82 cells.
Serological test results.
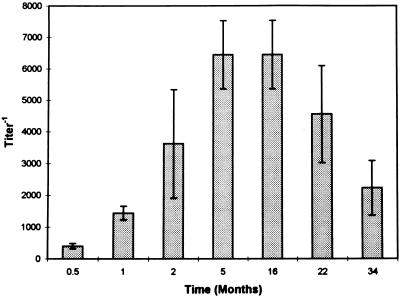
All dogs seroconverted by day 15 postinoculation. Geometric means of the IFA test titers during the different stages of the disease are presented in Fig. 2. A progressive increase in the IFA test titers was documented during the first 5 months postinfection. Titers remained high for an additional period of more than 11 months and declined thereafter. Five of the six dogs (dogs 1 to 5) remained seropositive (anti-E. canis antibody IFA test titers greater than 1:40) at 34 months postinoculation, while dog 6 was found to be seronegative (Table 1).
FIG. 2.
Geometric mean and standard error IFA test titers for six beagles at 15 and 30 days and 2, 5, 16, 22, and 34 months postinoculation with E. canis. All dogs were seronegative preinfection.
TABLE 1.
Platelet counts and IFA test titers for six clinically healthy beagles 34 months postinfection with E. canis with no medical treatment
| Dog no. | Platelet count (103) | IFA test titer |
|---|---|---|
| 1 | 260 | 1:5,120 |
| 2 | 157 | 1:1,280 |
| 3 | 184 | 1:2,560 |
| 4 | 140 | 1:5,120 |
| 5 | 317 | 1:640 |
| 6 | 470 | Negative |
Hematological test results.
At 34 months postinoculation complete blood counts and differential leukocyte counts were within the normal ranges; however, thrombocyte counts were not. Three of the four PCR-positive dogs were found to be thrombocytopenic (platelet counts, <200,000), while the two PCR-negative dogs (dogs 5 and 6) were found to have normal platelet counts (Table 1). No E. canis morulae were observed upon microscopic examination of any of the blood, bone marrow, or splenic smears.
DISCUSSION
The data obtained in this study for the first time prove experimentally that clinically healthy dogs in the subclinical phase of CME are carriers of the rickettsia, that they can harbor E. canis for years without developing the chronic clinical disease, and that dogs (probably immunocompetent dogs) can eliminate the parasite and recover from CME without medical treatment (as occurred in two of the six dogs).
Mild thrombocytopenia was recorded in three of the four PCR-positive dogs, while the two PCR-negative dogs had normal platelet counts. These findings substantiate the findings of Codner and Farris-Smith (7) and Waner et al. (20), who documented mild thrombocytopenia as an important and consistent hematological finding in the subclinical phase of CME.
This study also improves our understanding of the significance of serological testing in the subclinical phase of CME. Even though all dogs were clinically healthy 30 to 40 days postinfection with E. canis, a progressive increase in the IFA test titers was documented during the first 5 months postinfection, suggesting a continuous antigenic stimulation. Titers remained high for an additional period of more than 11 months and declined thereafter. The decrease in mean IFA test titers and the fact that two dogs were PCR negative 34 months postinfection suggests that this decrease in IFA test titers may represent the recovery phase of the disease in these dogs. Our results also suggest that negative serological results represent elimination of the parasite and recovery from the disease, as occurred in dog 6, which was found to be seronegative and PCR negative. On the other hand, positive IFA test results were shown not to be a reliable indicator of the carrier state, because dogs having anti-E. canis antibodies may not carry the parasite, as demonstrated in dog 5. It is probable that those antibodies remained from the antigenic stimulation that occurred during the period when the dog was a carrier of the rickettsia. Data for this dog (dog 5) suggest that PCR is a suitable method for determining whether a seropositive dog is a carrier of E. canis.
Ehrlichial DNA was retrieved from the spleens of all four PCR-positive dogs. These findings reveal the importance of the spleen in the pathogenesis and establishment of the disease and correlate with the fact that splenectomized dogs experimentally infected with E. canis suffered the acute disease in a milder manner, probably due to removal of a major organ in which colonization of the parasite takes place (12). Our findings also suggest that of the spleen, bone marrow, and blood, the spleen is probably the last organ to harbor E. canis parasites during recovery. However, the possibility that the parasite is “hiding” in splenic macrophages, avoiding the immune reaction, cannot be ruled out. Our results show that DNA extracted from splenic aspirates is the best source for a sample for PCR in order to diagnose the E. canis carrier state during subclinical ehrlichiosis and that the amplification of DNA extracted from blood or bone marrow samples would not give accurate results and may even provide misleading results. Our findings also show that microscopic evaluation of Giemsa-stained smears prepared from blood, bone marrow, and splenic aspirates is an insensitive technique for the diagnosis of subclinical CME. It is probable that the number of parasites in a subclinically infected animal is too small to be observed by microscopic examination of blood, bone marrow, or splenic smears; however, by amplifying the DNA of the rickettsia by PCR, positive results could have been obtained.
Larvae and nymphs are reported to become infected with E. canis while feeding only on acutely ill dogs (14, 19). The presence of ehrlichial DNA in blood samples, as occurred in two dogs (dogs 1 and 4) 34 months postinfection with E. canis, implies that the blood of dogs in the subclinical phase of CME may also be infective. Because there is no intermediate host in the pathogenesis of CME, the rickettsia may be transmitted by transfusions of infected blood. This problem is of importance in regions where E. canis is endemic and where a high percentage of the dog population is seropositive (2, 4, 16) and may carry the parasite. We therefore recommend that clinicians should screen dogs serologically for CME and not use the blood of clinically healthy seropositive dogs for blood transfusions. Seropositive dogs should undergo repeated serological testing until they are seronegative or their splenic samples are PCR negative.
Some dogs suffering from the subclinical stage of CME can develop the severe, life-threatening, chronic stage of the disease. The conditions that lead to the development of the chronic stage are not fully understood; however, it may be related to the breed, the immune status of the animal, stress conditions, coinfections with other parasites, the strain of the parasite, or geographical location (11, 17). Therefore, the risk of developing the chronic, severe form of the disease should be considered for dogs with subclinical cases of infection and should not be overlooked; thus, we recommend that these dogs with subclinical cases of infection be treated. To date, the treatment regimen for subclinical ehrlichiosis is empirical and requires further investigation.
In conclusion, this study documents new information regarding the pathogenesis and the diagnosis of subclinical ehrlichiosis. It also highlights the significance of serological testing during this stage of the disease. Interpretation of this information can prove to be useful to clinicians in providing a more accurate diagnosis and therefore can enable better treatment and prognosis. In an era of new emerging ehrlichial zoonoses (such as human granulocytic ehrlichiosis and human monocytic ehrlichiosis) (1, 6, 15) and recognition of the persistence of such infections in human patients (as documented with Ehrlichia chaffeensis, an organism closely related to E. canis) (10), our findings may prove valuable and may allow for a better understanding of the persistence of human and canine ehrlichial infections.
ACKNOWLEDGMENT
We thank Niels C. Pedersen from the Center for Companion Animal Health at the University of California, Davis, for professional assistance.
REFERENCES
- 1.Anderson B E, Dawson J E, Jones D C, Wilson K H. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baneth G, Waner T, Koplah A, Weinstein S, Keysary A. Survey of Ehrlichia canis antibodies among dogs in Israel. Vet Rec. 1996;138:257–259. doi: 10.1136/vr.138.11.257. [DOI] [PubMed] [Google Scholar]
- 3.Barlough J E, Madigan J E, DeRock E, Bigornia L. Nested polymerase chain reaction for detection of Ehrlichia equi genomic DNA in horses and ticks (Ixodes pacificus) Vet Parasitol. 1996;63:319–329. doi: 10.1016/0304-4017(95)00904-3. [DOI] [PubMed] [Google Scholar]
- 4.Botros B A, Elmolla M M S, Salib A W, Calamaio C A, Dasch G A, Arthur R R. Canine ehrlichiosis in Egypt—seroepidemiologic survey. Onderstepoort J Vet Res. 1995;62:41–43. [PubMed] [Google Scholar]
- 5.Breitschwerdt E B, Hegarty B C, Hancock S I. Doxycycline treatment and challenge infection with two Ehrlichia canis strains. J Vet Int Med. 1997;11:132. . (American College of Veterinary Internal Medicine abstract.) [Google Scholar]
- 6.Chen S M, Dumler J S, Bakken J S, Walker D H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Codner E C, Farris-Smith L. Characterization of the subclinical phase of ehrlichiosis in dogs. J Am Vet Med Assoc. 1986;189:47–50. [PubMed] [Google Scholar]
- 8.Dawson J E, Biggie K L, Warner C K, Cookson K, Jenkins S, Levine J F, Olson J G. Polymerase chain reaction evidence of Ehrlichia chaffeensis, an etiologic agent of human ehrlichiosis, in dogs from southern Virginia. Am J Vet Res. 1996;57:1175–1179. [PubMed] [Google Scholar]
- 9.Donatien A, Lestoquard A. Existance en Algerie d’une rickettsia du chien. Bul Soc Pathol Exot. 1935;28:418–419. [Google Scholar]
- 10.Dumler S J, Sutker W L, Walker D H. Persistent infection with Ehrlichia chaffeensis. Clin Infect Dis. 1993;17:903–905. doi: 10.1093/clinids/17.5.903. [DOI] [PubMed] [Google Scholar]
- 11.Harrus S, Waner T, Bark H. Canine monocytic ehrlichiosis: an update. Compend Contin Educ Pract Vet. 1997;19:1–10. [Google Scholar]
- 12.Harrus, S., T. Waner, A. Keysary, I. Aroch, H. Voet, and H. Bark. Investigation of splenic functions in canine monocytic ehrlichiosis. Vet. Immunol. Immunopathol., in press. [DOI] [PubMed]
- 13.Keysary A, Waner T, Rosner M, Warner C K, Dawson J E, Zass R, Biggie K L, Harrus S. The first isolation, in vitro propagation, and genetic characterization of Ehrlichia canis in Israel. Vet Parasitol. 1996;62:331–340. doi: 10.1016/0304-4017(95)00866-7. [DOI] [PubMed] [Google Scholar]
- 14.Lewis G E, Ristic M, Smith R D, Lincoln T, Stephenson E H. The brown dog tick Rhipicephalus sanguineus and the dog as experimental host of Ehrlichia canis. Am J Vet Res. 1977;38:1953–1955. [PubMed] [Google Scholar]
- 15.Magnarelli L A, Stafford K C, Mather T N, Yeh M T, Horn K D, Dumler J S. Hemocytic rickettsia-like organisms in ticks: serologic reactivity with antisera to ehrlichiae and detection of DNA of agent of human granulocytic ehrlichiosis by PCR. J Clin Microbiol. 1995;33:2710–2714. doi: 10.1128/jcm.33.10.2710-2714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthewman L A, Kelly P J, Mahan S M, Semu D, Tagwira M, Bobade P A, Brouqui P, Mason P R, Raoult D. Western blot and indirect fluorescent antibody testing for antibodies reactive with Ehrlichia canis in sera from apparently healthy dogs in Zimbabwe. J S Afr Vet Assoc. 1996;64:111–115. [PubMed] [Google Scholar]
- 17.Ristic M, Holland C J. Canine ehrlichiosis. In: Woldehiwet Z, Ristic M, editors. Rickettsial and chlamydial diseases of domestic animals. Oxford, United Kingdom: Pergamon Press; 1993. pp. 169–186. [Google Scholar]
- 18.Ristic M, Huxsoll D L, Weisiger R M, Hildebrandt P K, Nyindo M B A. Serological diagnosis of tropical canine pancytopenia by indirect immunofluorescence. Infect Immun. 1972;6:226–231. doi: 10.1128/iai.6.3.226-231.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith R D, Sells D M, Stephenson E H, Ristic M, Huxsoll D L. Development of Ehrlichia canis, causative agent of canine ehrlichiosis, in the tick Rhipicephalus sanguineus and its differentiation from a symbiotic rickettsia. Am J Vet Res. 1996;37:119–126. [PubMed] [Google Scholar]
- 20.Waner T, Harrus S, Bark H. Characterization of the subclinical phase of canine ehrlichiosis in experimentally infected beagle dogs. Vet Parasitol. 1997;69:307–317. doi: 10.1016/s0304-4017(96)01130-2. [DOI] [PubMed] [Google Scholar]
- 21.Wen B, Rikihisa Y, Mott J M, Greene R, Hyung-Yong K, Zhi N, Couto G C, Unver A, Bartsch R. Comparison of nested PCR with immunofluorescent antibody assay for detection of Ehrlichia canis infection in dogs treated with doxycycline. J Clin Microbiol. 1997;35:1852–1855. doi: 10.1128/jcm.35.7.1852-1855.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]