Abstract
Fatty acid composition is crucial for determining meat quality. Pigeon meat, renowned for its tender texture, high protein content, and abundant polyunsaturated fatty acid (PUFAs), yet the developmental dynamics and molecular mechanisms of intramuscular fatty acid deposition remain unclear. Previous studies suggest that lipid metabolism is often governed by coordinated gene expression programs. Therefore, we hypothesized that fatty acid profiles in pigeon muscle are regulated by gene co-expression modules identifiable via weighted gene co-expression network analysis (WGCNA). To test this, we analyzed pectoral muscles from pigeons at five developmental stages (28 days to 48 months) using gas chromatography–mass spectrometry (GC–MS) and transcriptomic sequencing. A total of 39 fatty acids were identified, with key PUFAs such as DHA increasing and EPA decreasing over time, while overall MUFAs declined and PUFAs peaked at 6 months, revealing distinct stage-dependent patterns in fatty acid composition. WGCNA revealed that three gene modules (green, yellow, turquoise) were significantly associated with fatty acid traits. GO Enrichment analysis indicated their involvement in ribosome activity, mitochondrial pathways, and unsaturated fatty acid biosynthesis, while KEGG pathway highlighted oxidative phosphorylation and phosphatidylinositol signaling. Protein-protein interaction (PPI) analysis pinpointed hub genes, including RPS16, NDUFS6, RHOJ, and NUDT12 as key regulators of fatty acid metabolism. This study provides the first co-expression network linking fatty acid composition with transcriptional regulation in pigeons, broadening WGCNA application in avian lipid metabolism. The findings offer new insights into gene networks underlying lipid deposition and suggest targets for improving meat quality through molecular breeding.
Keywords: Columba livia, RNA-seq, WGCNA, PPI network, Regulatory genes
Highlights
-
•
GC–MS identified 39 fatty acids; PUFA peaked at 6 months with age-dependent shifts.
-
•
WGCNA revealed three gene modules linked to fatty acid composition.
-
•
Hub genes RPS16 and NDUFS6 connected ribosomal and mitochondrial roles in lipid metabolism.
1. Introduction
In livestock and poultry products, fatty acid composition and content are crucial determinants of meat quality (Wood et al., 2004). These components not only influence meat sensory attributes (e.g., flavor, tenderness, and aroma) but are also exert profound effects on human health. Among fatty acids, polyunsaturated fatty acids (PUFAs), particularly omega-3 and omega-6 fatty acids, have garnered significant attention due to their well-documented benefits, including anti-inflammatory effects (Xiangrong et al., 2017), cardiovascular protection(Santos, May, & Buenoz, 2023), and immune system regulation (Gabriela et al., 2022). The fatty acid profiles in animal muscles vary substantially among species, influenced by dietary composition, developmental stage, and genetic background (Sebastià et al., 2024). Comparative studies show that poultry meats (e.g., chicken) tend to have higher PUFA and lower saturated fatty acid (SFA) concentrations than porcine meats, which are typically richer in SFAs (Zhao, Xing, Lu, & Wang, 2018).
Meat pigeons (Columba livia) are a poultry species whose muscle generally contains relatively high protein and low total fat, with detectable levels of polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Chang et al., 2017). Compared with chicken, pigeon meat has been reported to exhibit different PUFA/SFA ratios and atherogenic indices (Cordero, Alonso-Calleja, García-Fernández, & Capita, 2019). In China, pigeon production has reached an annual output of over 600 million birds (Yang et al., 2025), providing a substantial basis for nutritional and genetic studies. Despite this growing nutritional and economic importance, the fatty acid composition of pigeon muscle and the regulatory mechanisms underlying lipid metabolism remain poorly understood. Most existing studies on pigeons have focused on growth performance and general nutrient content, whereas the transcriptomic and gene regulatory basis of lipid accumulation is largely unexplored.
Previous studies in chickens (Kong et al., 2014; Urszula et al., 2022)、cattle (Mingyue et al., 2021)、and sheep (Kong et al., 2024) have demonstrate that fatty acid deposition is a polygenic trait regulated by multiple genes, quantitative trait loci (QTLs), and SNPs involved in lipid metabolism (Li et al., 2024). These findings reflect a growing research focus on the genetic mechanisms underlying fatty acid composition in animal science. In particular, genetic studies have further unveiled species-specific inheritance patterns. For instance, Keiichi et al. reported demonstrating strong genetic correlations between genomic loci and fatty acid composition, particularly for monounsaturated fatty acids (MUFA; genetic correlations: 0.77 for MUFA content, 0.79 for specific MUFA isomers) (Keiichi, Noriaki, Takeshi, & Kenji, 2017). Similarly, in ovine models, Karamichou et al. documented high heritability estimates for muscle fatty acids (SFA: 0.90; MUFA: 0.73; PUFA: 0.40), underscoring the substantial genetic potential for improving fatty acid traits through selective breeding (Karamichou, Richardson, Nute, McLean, & Bishop, 2006).
With the advancement of high-throughput sequencing, transcriptome-based methods such as RNA-seq, when combined with systems biology tools like weighted gene co-expression network analysis (WGCNA), offer powerful means to investigate the transcriptional architecture of complex traits. These tools enable identification of co-expression modules and hub genes associated with fatty acid synthesis, transport, and degradation (Peter & Steve, 2008; Wang et al., 2022; Xiong & Chen, 2024). In livestock, WGCNA has been widely used to explore lipid metabolism. For example, in pigs, has been used to link muscle gene expression with intramuscular fatty acid composition, identifying co-expression modules and hub genes associated with specific fatty acid classes and metabolic pathways (Sebastià et al., 2024). In ruminants such as buffalo, WGCNA has been used to link growth, fat deposition, and intramuscular amino acid traits with key gene modules, identifying candidate genes enriched in metabolic and fatty acid pathways (Shuzhe et al., 2022). In poultry, previous work has primarily focused on adipose tissue development or general metabolic traits in chickens (Luo et al., 2022), but studies targeting intramuscular fatty acid regulation remain scarce. WGCNA has been successfully applied to fat deposition studies in pigs and ruminants, but is still rarely used in avian species such as pigeons.
Therefore, the lack of data on gene regulatory networks governing fatty acid traits in meat pigeons represents a clear knowledge gap. To address this, we employed an integrative approach combining RNA-seq and WGCNA to analyze the transcriptome of pigeon pectoral muscle across five developmental stages, alongside detailed fatty acid profiling using GC–MS/MS. We hypothesize that age-dependent variation in intramuscular fatty acid composition is driven by stage-specific gene co-expression modules, and that identification of key hub genes will provide novel insights into lipid regulation and lay a foundation for molecular breeding aimed at improving meat quality traits.
2. Materials and methods
2.1. Ethics statement
All experimental procedures were performed in strict accordance with the Guidelines for the Care and Use of Laboratory Animals issued by the Ministry of Science and Technology of the People's Republic of China. The study protocol was reviewed and approved by the Animal Welfare Committee of Shanghai Academy of Agricultural Sciences (Approval No.: SAASPZ0522056).
2.2. Meat pigeon rearing and sample collection
All meat pigeons used in this study belonged to the commercial strain Shenwang No. 1, provided by Shanghai Jinhuang Pigeon Industry Co., Ltd., (Shanghai, China). Experimental pigeons were reared under uniform housing and feeding conditions. A total of 60 pigeons were selected from the flock, using visual assessment of feather condition, activity level, and body size as criteria. Pigeons were allocated into five age-based groups (28 days, 3 months, 6 months, 12 months, and 48 months), each consisting of 12 pigeons with a balanced sex ratio (6 males and 6 females). All 12 individuals per group were used for targeted fatty acid analysis, while 6 individuals per group were selected for transcriptome sequencing, serving as biological replicates for each respective analysis. Prior to slaughter, pigeons were fasted for 12 h with water and feed withheld. Slaughter was conducted via cervical dislocation followed by immediate exsanguination, in compliance with institutional animal welfare protocols and ethical guidelines (Approval No.: SAASPZ0522056). From each pigeon, two independent samples (∼200 mg each) were collected from the left pectoral muscle without scalding or plucking, and each was placed into a separate RNase-free cryogenic tube. One sample was used for transcriptome sequencing, and the other for targeted fatty acid analysis. For 5 age groups (n = 12 pigeons each), a total of 120 tubes (two per pigeon) were obtained for downstream analyses. All samples were immediately snap-frozen in liquid nitrogen and stored at −80 °C until analysis. For fatty acid profiling, each sample was processed in technical triplicates; for transcriptome sequencing, samples were processed once without technical replication, following standard RNA-seq protocols.
2.3. Fatty acids metabolite extraction
Samples were thawed at 4 °C, and 50 mg of each sample was mixed with 5 mL of cold dichloromethane-methanol (2:1 v/v; dichloromethane: Sigma-Aldrich, St. Louis, MO, USA; methanol, Fisher Chemical, USA). The mixture was vortexed thoroughly, sonicated at a low temperature for 30 min, and washed with 2 mL Milli-Q water. The lower organic phase was collected and dried with a stream of nitrogen. The residue was reconstituted with an internal standard solution of methyl nonadecanoate (C19:0 ≥ 98 %, Sigma-Aldrich, USA, 1 μg/mL) and 2 mL n-hexane (Sigma-Aldrich, St. Louis, MO, USA) for fatty acid methyl esterification, and incubated for 30 min. Following esterification, 2 mL of Milli-Q water was added, and the mixture was vortexed. The supernatant (1000 μL) was dried with nitrogen, redissolved in n-hexane, and transferred to GC–MS vials (Agilent Technologies, Santa Clara, CA, USA) for analysis.
Fatty acid quantification was performed using the isotope internal standard method, and calibration curves were generated by serially diluting mixed standard solutions. Each calibration point was processed following the same protocol as test samples. Peak area ratios between analytes and internal standards were used to calculate compound concentrations.
2.4. GC–MS/MS analysis
Samples were separated using an Agilent DB-23 GC column (60 m × 250 μm × 0. 15 μm; Agilent Technologies, Santa Clara, CA, USA) mounted on a 7890B GC System. The temperature program was set as follows: initial temperature of 80 °C held for 1 min, ramped to 180 °C at 20 °C/min, held for 5 min, then ramped to 220 °C at 5 °C/min and held for 8 min, followed by a final ramp to 280 °C at 5 °C/min and held for 3 min. Helium was used as the carrier gas as a constant flow rate of 1.0 mL/min. Quality control (QC) samples were included to assess system stability and repeatability.
Mass spectrometry analysis was performed using a 5977B MSD mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) with following parameters: inlet temperature 280 °C; ion source temperature 230 °C; transfer line temperature 250 °C. The electron impact ionization energy was set to 70 eV. The chromatographic peak areas and retention times were extracted using the MSD ChemStation software. A standard calibration curve was constructed to quantify the content of medium- and long-chain fatty acids in the samples.
2.5. RNA extraction and transcriptome sequencing
Total RNA from meat pigeon was isolated using Trizol Reagent (Invitrogen Life Technologies, Carlsbad, CA, USA). RNA concentration and purity was assessed by NanoDrop 2000 (Thermo Scientific, Waltham, MA, USA), and integrity was confirmed using 1 % agarose gel electrophoresis or Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA; RNA 6000 Nano Kit). Samples with RIN ≥ 7.0 were used for library construction. For library preparation 3 μg RNA was used purify polyadenylated mRNA via poly-T magnetic beads, which was fragmented and subjected to the first-strand cDNA synthesis with random hexamers and Super Script II, followed by second-strand synthesis using DNA Polymerase I and RNase H. After end repair, 3′ adenylation, and Illumina PE adapter ligation, fragments (400–500 bp) were size-selected by AMPure XP and amplified via 15-cycle PCR. Purified libraries were quantified on a Bioanalyzer 2100 system and sequenced on a NovaSeq 6000 platform (Illumina, San Diego, CA, USA) at Shanghai Personal Biotechnology Cp. Ltd. (Shanghai, China) using paired-end 150 bp reads. On average, each sample generated ∼6.5 Gb of clean reads.
2.6. Quality control and differentially expressed gene analysis
Sequencing generated image files were processed by the platform's software to produce raw FASTQ Data. To ensure data quality, sequencing reads were filtered using fastp (v0.22.0) to remove adapters, low-quality sequences (Q < 20), and contaminants, yielding high quality clean data. Quality statistics including Q20, Q30, GC content, and total/clean reads per sample are summarized in Supplementary Table 1. Reference genome and annotation files of Columba livia were downloaded from the NCBI RefSeq database (assembly accession: GCF_036013475.1_bColLiv1.pat.W.v2; genome size: 1.33 Gb), and used for read alignment and gene annotation. Filtered reads were mapped to the reference genome using HISAT2 (v2.1.0). Gene expression was quantified by counting reads mapped to each gene with HTSEq. (v0.9.1), and normalized using FPKM/ TPM. FPKM and TPM normalization methods were selected because they account for both sequencing depth and gene length, enabling accurate comparison of gene expression levels across different samples and conditions. Raw read counts were used for statistical analyses. Difference gene expression analysis was performed with DESeq2 (v1.38.3) under criteria of |log2Fold Change| > 1, and adjusted P-value <0. 05.
2.7. Weighted gene co-expression network analysis (WGCNA)
Based on targeted fatty acid profiling of meat pigeon pectoral muscle, the most abundant fatty acids and their contents were selected as phenotypic data for trait-module association analysis. Fatty acid metabolism traits were integrated with transcriptome sequencing data for weighted gene co-expression network analysis (WGCNA). The WGCNA package was utilized to construct the gene co-expression network. First, hierarchical clustering of all samples was performed based on gene expression profiles. The TOM (Topological Overlap Matrix) similarity measure was applied to calculate gene co-expression similarity coefficients, and the pickSoftThreshold function was used to determine the optimal soft-thresholding power (β), ensuring the network exhibited scale-free topology. A weighted co-expression network was then established using these parameters, enabling classification of genes into distinct modules. Co-expression relationships within the network were visualized via heatmaps.
2.8. Module eigengene analysis
The module eigengene (ME), defined as the first principal component of each module, was calculated. By integrating biological traits with module hub genes profiles, Pearson correlation coefficients and corresponding p-values were used to evaluate the correlations between module MEs and fatty acid content phenotypes. Modules significantly correlated with biological traits were identified based on a significance threshold of |correlation coefficient| ≥ 0.6 and p < 0.05. The relationships among modules and traits were visualized using hierarchical clustering dendrograms and module-trait heatmaps, offering a comprehensive overview of the trait-module association analysis.
2.9. Functional enrichment analysis
In WGCNA, hub genes within modules are typically identified as those with high gene significance (GS) and module membership (MM). For modules significantly associated with fatty acid composition traits, GS and MM values were calculated, and candidate hub genes were selected based on criteria of MM > 0. 8 and GS > 0. 2. Gene Ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were performed using the R package clusterProfiler (v4.6.0). Genes significantly enriched in lipid/fatty acid metabolism-related biological processes and pathways were further prioritized as intramodular hub genes. Enrichment results were visualized using ggplot2 (v3.4.4) and ComplexHeatmap (v2.16.0).
2.10. PPI network analysis
Protein-protein interaction (PPI) network analysis was carried out to explore gene-gene interactions and identify hub genes. The PPI network was constructed using the STRING database (https://string-db. org/) and visualized with Cytoscape (version 3. 9. 1). Genes with a degree value >10 were selected for maximal clique centrality (MCC) analysis. The top 10 genes ranked by MCC scores were used to generate PPI subnetworks within the module, and the genes with the highest scores were designated as module-specific hub genes.
2.11. RT-qPCR analysis
To validate the expression of hub genes identified from trait-specific modules, total RNA was reverse transcribed into cDNA using the PrimeScript™ RT Reagent Kit (Takara, Dalian, China). Quantitative real-time PCR (RT-qPCR) was conducted on a LightCycler480 II PCR system (Roche, Rotkreuz, Switzerland) with 2× SGExcel FastSYBR Mixture (B532955 Sangon). The amplification program involved an initial 3 min denaturation at 95 °C, followed by 45 cycles of 15 s denaturation at 95 °C and 45 s annealing/extension at 60 °C. Gene expression levels were normalized to the internal reference gene, β-actin. All primers were designed using Primer Premier 3 (https://primer3.ut.ee/), and their sequences are provided in Table 1. Relative gene expression levels were calculated using the 2 -(∆∆Ct) method.
Table 1.
The primers used RT-qPCR analysis.
| Gene | Primer | Primer sequence |
|---|---|---|
| β-actin | Left Primer | AGTACCCCATTGAACACGGT |
| Right Primer | ATACATGGCTGGGGTGTTGA | |
| RPS16 | Left Primer | GGCGGAAGAAAACAGCAACT |
| Right Primer | CCACCCTTTACACGGACTCT | |
| NDUFS6 | Left Primer | GGACGACAAAAGGAGGTGAA |
| Right Primer | GTAGCCACATGTTCCGGTCT | |
| RHOJ | Left Primer | CTGACGGGAGCAGCAATAAC |
| Right Primer | GGGTCTCAGCTGGTTGTAGT | |
| HAT1 | Left Primer | CCTGAGGATTTGGAGGATGA |
| Right Primer | GCAAAAACCAGGAGGAATGA | |
| NUDT12 | Left Primer | TCCCAGTCTCCAAGGTGTTC |
| Right Primer | ACTTTGACTCCAGCCTCCTC |
2.12. Statistical analysis
All data were analyzed using SPSS Statistics 27.0.1 (IBM Corp., Armonk, NY, USA). Results are expressed as mean ± standard deviation (SD). For comparisons among multiple groups, one-way analysis of variance (ANOVA) was used, followed by Tukey's multiple comparison test to assess pairwise differences. A p-value <0.05 was considered statistically significant. Graphs were generated using Origin 2021 software (Origin Lab Corporation, Northampton, MA, USA).
3. Results and analysis
3.1. Fatty acid metabolism profile in pigeon pectoral muscle
Targeted fatty acid metabolomics analysis using gas chromatography-tandem mass spectrometry (GC–MS/MS) was performed on the pectoral muscle of meat pigeons at five developmental ages. A total of 39 fatty acids were identified, including 16 saturated fatty acids (SFAs), 9 monounsaturated fatty acids (MUFAs), and 14 polyunsaturated fatty acids (PUFAs). The total fatty acid content in the pectoral muscle of pigeons at 28 days, 3 months, 6 months, 12 months, and 4 years of age was 1331. 84 μg/g, 1279. 42 μg/g, 1270. 16 μg/g, 938. 04 μg/g, and 873. 49 μg/g, respectively. As depicted in Fig. 1, SFA levels remained stable from 28 days to 6 months but decreased significantly at 12 months and 48 months. MUFA content declined gradually with age, while PUFA content increased initially, peaking at 6 months (402.705 μg/g), before decreasing. Notably, MUFA content exceeded both PUFA and SFA levels throughout the developmental period in pigeon pectoral muscle.
Fig. 1.
Box plot of SFA, MUFA, and PUFA in meat pigeons across various age groups.
Eight major fatty acids were identified in pigeon pectoral muscle, comprising two SFAs and six PUFAs, which collectively accounting for over 85 % of the total fatty acid content. Among SFAs, palmitic acid (C16:0) was the most abundant, followed by stearic acid (C18:0), with their combined levers dominated the total fatty acids profile (Table 2). Oleic acid (C18:1N9C) was the primary monounsaturated fatty acid (MUFA), showing a slight age-dependent decrease (Table 3). As shown in Table 4, Linoleic acid (C18:2N6C) and arachidonic acid (C20:4 N6) were the predominant PUFA, both peaking at 6 months. Notably, eicosapentaenoic acid (EPA, C20:5 N3) and docosahexaenoic acid (DHA, C22:6 N3)-PUFAs with significant human health benefits – displayed contrasting age-related trends. EPA content declined from 29.40 μg/g at 28 days to 8. 56 μg/g at 48 months, whereas DHA content increased significantly, rising from 44. 49 μg/g to 92. 15 μg/g over the same period. Additionally, the PUFA/SFA ratio ranged from 1.0 to 1.3, and peaking at 12 months.
Table 2.
Composition and Content of SFA in meat pigeons across various age groups.
| Fatty Acid (ug/g) |
28 Days | 3 Months | 6 Months | 12 Months | 48 Months |
|---|---|---|---|---|---|
| C6:0 | 0.07 ± 0.01 | 0.28 ± 0.40 | 0.14 ± 0.05 | 0.29 ± 0.40 | 0.15 ± 0.17 |
| C8:0 | 0.29 ± 0.03a | 1.3 ± 1.0b | 1.20 ± 0.87ab | 1.24 ± 0.66ab | 0.82 ± 0.78ab |
| C10:0 | 0.31 ± 0.12a | 0.35 ± 0.12a | 0.65 ± 0.23b | 0.29 ± 0.08a | 0.25 ± 0.07a |
| C11:0 | 0.07 ± 0.01ab | 0.07 ± 0.01ab | 0.08 ± 0.01b | 0.06 ± 0.01a | 0.06 ± 0.01a |
| C12:0 | 2.30 ± 0.81ab | 2.07 ± 0.94ab | 2.90 ± 0.90b | 1.71 ± 0.80a | 1.94 ± 0.97ab |
| C13:0 | 0.1 ± 0.04 | 0.10 ± 0.02 | 0.14 ± 0.06 | 0.08 ± 0.05 | 0.09 ± 0.04 |
| C14:0 | 47.2 ± 9.6ab | 43.2 ± 17.6ab | 52.8 ± 17.7b | 28.9 ± 17.4a | 31.2 ± 14.0a |
| C15:0 | 8.3 ± 1.7 | 7.0 ± 1.9 | 7.5 ± 2.5 | 5.6 ± 3.4 | 5.3 ± 1.7 |
| C16:0 | 3276 ± 396b | 3115 ± 713 | 2994 ± 647b | 2138 ± 786a | 2002 ± 431a |
| C17:0 | 25.9 ± 6.2 | 24.8 ± 7.6 | 24.3 ± 8.1 | 20.5 ± 10.9 | 18.9 ± 5.0 |
| C18:0 | 2051 ± 235abc | 2226 ± 399bc | 2358 ± 447c | 1842 ± 562ab | 1700 ± 243a |
| C20:0 | 14.1 ± 2.7b | 13.3 ± 2.8ab | 12.1 ± 3.9ab | 9.2 ± 3.3a | 10.0 ± 3.8ab |
| C21:0 | 3.81 ± 0.76b | 3.75 ± 0.62b | 3.106 ± 0.54b | 2.06 ± 0.45a | 1.94 ± 0.48a |
| C22:0 | 4.86 ± 0.55d | 3.26 ± 0.57c | 2.52 ± 0.70b | 1.64 ± 0.21a | 2.15 ± 0.80ab |
| C23:0 | 0.21 ± 0.04 | 0.23 ± 0.03 | 0.22 ± 0.07 | 0.19 ± 0.10 | 0.17 ± 0.08 |
| C24:0 | 0.67 ± 0.18b | 0.57 ± 0.12ab | 0.52 ± 0.21ab | 0.41 ± 0.16b | 0.44 ± 0.22ab |
| Total SFA | 319.8 ± 37.0b | 320.1 ± 62.8b | 321.2 ± 65.4b | 238.3 ± 79.9a | 222.1 ± 39.5a |
Note: Different letters following the numbers indicate significant differences in fatty acids among different age groups (p < 0.05). The same applies below.
Table 3.
Composition and Content of MUFA in meat pigeons across various age groups.
| Fatty Acid (ug/g) |
28 Days | 3 Months | 6 Months | 12 Months | 48 Months |
|---|---|---|---|---|---|
| C14:1 N-5 | 13.0 ± 8.2b | 12.6 ± 8.4b | 9.1 ± 2.3ab | 4.1 ± 3.0a | 4.2 ± 3.0a |
| C15:1n5 cis | 4.96 ± 0.57 | 4.77 ± 0.64 | 4.74 ± 0.44 | 4.81 ± 0.85 | 5.64 ± 0.94 |
| C16:1 N-7 cis | 1358 ± 460c | 1100 ± 594bc | 853 ± 268b | 335 ± 192a | 374 ± 246a |
| C17:1 N-7 cis | 16.3 ± 5.2 | 12.6 ± 3.6 | 10.8 ± 2.5 | 19.5 ± 16.8 | 12.4 ± 7.2 |
| C18:1 N-9 T | 22.8 ± 4.9c | 19.4 ± 6.5bc | 15.5 ± 5.5abc | 10.7 ± 5.7a | 12.8 ± 7.9b |
| C18:1 N-9 | 4587 ± 893b | 4335 ± 1142ab | 3979 ± 1163ab | 3036 ± 1458a | 286 ± 1195 a |
| C20:1 N-9 cis | 5.5 ± 8.5b | 35.9 ± 7.8ab | 27.7 ± 12.6a | 26.7 ± 13.7a | 28.9 ± 16.9a |
| C22:1 N-9 | 6.74 ± 0.79c | 5.00 ± 0.93b | 2.82 ± 0.93a | 2.17 ± 0.93a | 2.6 ± 1.7a |
| C24:1 N-9 cis | 10.2 ± 2.2 | 12.1 ± 4.0 | 13.8 ± 3.5 | 19.1 ± 11.9 | 19.8 ± 11.5 |
| Total MUFA | 674 ± 135b | 615 ± 182b | 546 ± 158ab | 384 ± 187a | 370 ± 163a |
Table 4.
Composition and Content of PUFA in meat pigeons across various age groups.
| Fatty Acid (ug/g) |
28 Days | 3 Months | 6 Months | 12 Months | 48 Months |
|---|---|---|---|---|---|
| C18:2 N-6,9 all-trans | 2.88 ± 0.72c | 1.82 ± 0.62b | 0.84 ± 0.22a | 0.83 ± 0.52a | 0.67 ± 0.22a |
| C18:2 N-6,9 all-cis | 3048 ± 435ab | 3175 ± 660ab | 3320 ± 667b | 2827 ± 1044ab | 2369 ± 510a |
| C18:2 N-6,9,12 all-cis | 12.4 ± 4.8c | 10.2 ± 3.5bc | 7.9 ± 2.8ab | 4.1 ± 2.1a | 3.9 ± 2.2a |
| C18:3 N-3,6,9 all-cis | 33.6 ± 12.1b | 24.7 ± 10.2ab | 34.0 ± 15.8b | 19.2 ± 13.0ab | 15.5 ± 3.2 |
| C20:2 N-6,9 all-cis | 104.7 ± 16.3d | 65.6 ± 17.4c | 49.0 ± 10.1b | 33.5 ± 11.7ab | 32.5 ± 7.2a |
| C20:3 N-6,9,12 all-cis | 70.8 ± 13.0c | 51.1 ± 10.6b | 39.3 ± 9.4ab | 34.8 ± 15.2a | 29.7 ± 6.7a |
| C20:3 N-3,6,9 all-cis | 6.0 ± 1.2c | 2.76 ± 0.47a | 4.3 ± 1.1b | 3.1 ± 1.1ab | 3.5 ± 1.2ab |
| ARA | 965 ± 178a | 957 ± 130a | 1670 ± 190b | 1050 ± 281a | 1044.1 ± 23.7a |
| EPA | 29.4 ± 10.0b | 13.5 ± 5.4a | 13.3 ± 4.7a | 6.0 ± 2.1a | 8.6 ± 5.8a |
| C22:2 N-6,9 cis | 1.52 ± 0.40b | 0.83 ± 0.23a | 0.97 ± 0.45a | 0.75 ± 0.36a | 0.78 ± 0.35a |
| C22:4 N-6,9,12,15 cis | 160.2 ± 19.6b | 160.1 ± 39.9b | 159.2 ± 38.1b | 130.2 ± 25.7a | 114.4 ± 12.1a |
| DPA | 198.0 ± 62.1ab | 234.3 ± 51.7b | 196.1 ± 49.1ab | 153.6 ± 40.7a | 172.2 ± 48.9ab |
| DPA | 51.2 ± 9.9a | 64.9 ± 12.6ab | 75. ± 9.6b | 63.3 ± 13.4ab | 58.2 ± 16.7a |
| DHA | 44.5 ± 8.3a | 55.425 ± 19.741ab | 67.743 ± 14.057ab | 88.289 ± 53.145ab | 92.152 ± 36.851b |
| Total PUFA | 337.7 ± 43.2 | 344.1 ± 57.4 | 402.7 ± 61.2 | 315 ± 103 | 281.8 ± 42.5 |
3.2. Differentially expressed gene analysis
Transcriptome sequencing was performed on the pectoral muscle tissues of meat pigeons at five developmental stages. After stringent data filtering and quality control, all transcriptomic datasets met the requirement for downstream analysis. Mapping the quality-controlled reads to the pigeon reference genome yielded an overall mapping rate exceeding 94 %, with a unique mapping rate of over 91 % for all samples. A total of 17,095 expressed genes were identified, underscoring the reliability of the sequencing data for subsequent analyses. Principal component analysis (PCA) of gene expression profiles across developmental stages showed that PC1 and PC2 explained 37.5 % and 29.1 % of the total variance, respectively (Fig. 2a), indicating high quality and strong sample reproducibility. Additionally, correlation analysis (Fig. 2b) revealed distinct clustering patterns consistent with developmental stages. Samples within the same age group exhibited high intra-group correlation, reflecting metabolic homogeneity, whereas inter-group correlations differed significantly across developmental stages.
Fig. 2.
Transcriptome Sequencing and Functional Analysis of Differentially Expressed Genes in Pectoral Muscle of Meat Pigeons across various age groups. (a) PCA of the pectoral muscle transcriptome across various age groups. (b) Correlation analysis of transcriptomes in pectoral muscle across various age groups. (c) Differentially expressed genes in the pectoral muscle of Meat Pigeons various age groups.
Differentially expressed genes (DEGs) across five developmental stages of meat pigeons were identified, as depicted in fig. 2c. Comparative analysis revealed 955 DEGs between 28-day-old (D28) and 3-month-old (M3) pigeons, with 312 upregulated and 643 downregulated genes. In the M3 vs.M6 comparison, 886 DEGs were detected, including with 428 upregulated and 468 downregulated genes. The M6 vs. M12 comparison identified 1431 DEGs, with 378 upregulated and 1053 downregulated genes. Finally, the M12 vs. M48 comparison showed 281 DEGs, of which 202 were upregulated and 79 were downregulated. Analyzing these stage-specific DEG profiles in pectoral muscle may elucidated the molecular mechanisms governing fatty acid biosynthesis.
3.3. WGCNA analysis
Fourteen fatty acids with relatively high concentrations, namely Myristic acid (C14:0), Palmitic acid (C16:0), Palmitoleic acid (C16:1 n-7), Stearic acid (C18:0), Oleic acid (C18:1 n-9), Linoleic acid (C18:2 n-6,9), arachidonic acid (ARA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), docosahexaenoic acid (DHA), Myristoleic acid (C14:1 n-5), γ-Linoleic acid (C18:2 n-6,9,12), 11Z-Eicosenoic acid (C20:1 n-9) and 11Z,14Z-Eicosadienoic acid (C20:2 n-6,9), were selected for weighted gene co-expression network analysis (WGCNA) in combination with transcriptomic data. The optimal soft threshold power (β) was determined to be 4 as indicated by the stabilization of correlation coefficients (Fig. 3a). Hierarchical clustering analysis identified six distinct gene co-expression modules, labeled as blue, brown, red, royal blue, Green, and Yellow (Fig. 3b).
Fig. 3.
Weighted gene co-expression network analysis. (a) Soft threshold distribution plot. (b) Identification of gene co-expression modules. (c) Gene co-expression modules with varying gene counts. (d) Correlation analysis between co-expression modules and the phenotypic values of 14 fatty acid contents.
This study identified six gene co-expression modules with varying gene counts (Fig.3c). The turquoise module contained 3084 genes, was the largest, while the remaining modules had between 125 and 635 genes. The green and yellow modules clustered together in the dendrogram, indicating similar eigengene expression patterns, where each eigengene (ME) represents the first principal component summarizing the expression pattern of all genes in a module. Correlation analysis between the six modules and 14 breast muscle fatty acids (Fig.3d) revealed that five modules significantly correlated with 10 fatty acids (p < 0.05), confirming the applicability of the module eigengene (ME)-based analysis described in the methodology. The turquoise module, with the most genes, showed significant positive correlations with six fatty acids (C16:0, C16:1 n-7, EPA, C18:2 n-6,9,12, C20:1 n-9, and C20:2 n-6,9; p < 0.05), with EPA and C20:2 n-6,9 exhibiting highly significant correlations (p < 0.01) implying a key role in polyunsaturated fatty acids (PUFAs) synthesis and accumulation. Conversely, the brown module negatively correlated with C16:1 n-7, EPA, C18:2 n-6,9,12, and C20:2 n-6,9 (p < 0.05), while the red module showed negative correlations with C18:1 n-9 and DHA (p < 0.05). Both the yellow and green modules were highly significantly positively correlated with arachidonic acid (ARA) (p < 0.01). These finding suggest that genes within these five modules contribute to regulating fatty acid composition in pigeon pectoral muscle. Together, these results derived from the WGCNA pipeline provide a robust framework for identifying gene networks involved in the regulation of intramuscular fatty acid composition.
3.4. Functional enrichment analysis
WGCNA was employed to explore the regulatory relationship between gene co-expression modules and fatty acid composition. Results showed that the turquoise, green, and yellow modules were significantly positively correlated (p < 0.05) with eight fatty acids, while the Brown and Red modules exhibited significant negative correlations (p < 0.05) with six fatty acids, indicating that these five modules likely contain key regulators of fatty acid composition. Using gene significance (GS) > 0.2 and module membership (MM) > 0.8 as criteria, 1484 hub genes were identified from the positively correlated modules, and 662 from the negatively correlated ones. To elucidate their biological functions and associated signaling pathways, GO functional annotation and KEGG pathway enrichment analyses were conducted for these modules. The top 20 enriched terms were visualized via GO bar plots and KEGG bubble plots based on enrichment scores. In the positively correlated, GO analysis revealed that hub genes were primarily associated with ribosome-related processes, mitochondrial metabolism, and peptide biosynthesis, suggesting roles in protein translation and mitochondrial function (Fig. 4a). KEGG pathway analysis further showed significant enrichment in unsaturated fatty acid biosynthesis, protein export, phosphatidylinositol signaling, and inositol phosphate metabolism, indicating involvement in lipid biosynthesis and regulation (Fig. 4b). Conversely, for the negatively correlated Brown and Red modules, GO enrichment indicated that the hub genes were mainly involved in protein degradation and ribosome assembly (Fig. 4c). KEGG pathways analysis highlighted enrichment in the proteasome, eukaryotic ribosome biogenesis, and RNA transport and degradation pathways, suggesting roles in protein turnover and RNA metabolism (Fig. 4d). These processes may negative regulate fatty acid synthesis and accumulation, providing insights into the molecular mechanisms governing fatty acid composition in muscle tissue.
Fig. 4.
Trait-associated gene enrichment in specific modules. (a) GO enrichment analysis of positively correlated module. (b) KEGG pathway enrichment analysis of positively correlated module. (c) GO enrichment analysis of negatively correlated module. (d) KEGG pathway enrichment analysis of negatively correlated module.
3.5. PPI network construction
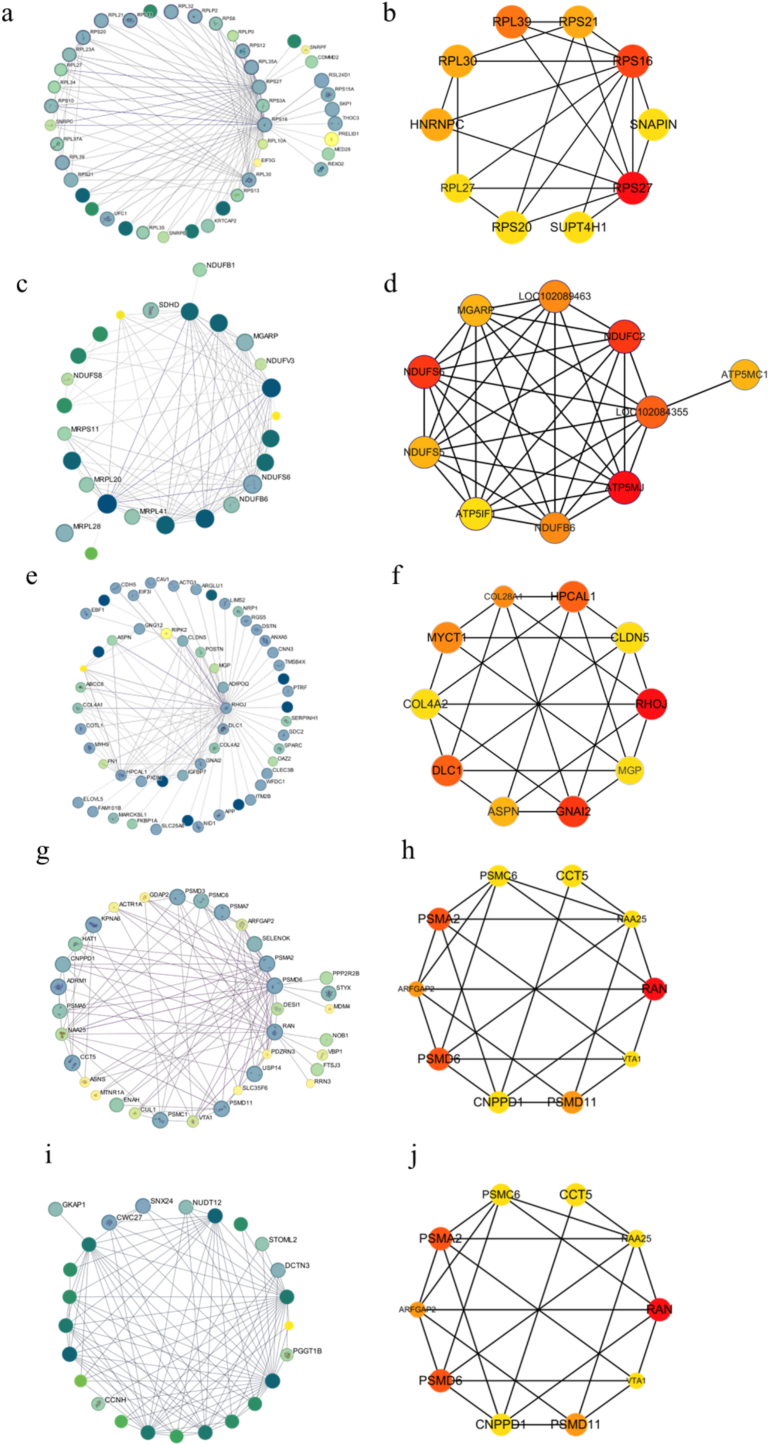
To identify key regulatory genes associated with fatty acid traits, the top 100 genes with the strongest correlations from each of the five WGCNA modules significantly linked to fatty acid profiles were selected. Protein-protein interaction (PPI) networks were a constructed using Cytoscape, and hub genes within each module's PPI network were identified via the MCC algorithm in the CytoHubba plugin. Corresponding PPI subnetworks were generated to visualize core gene interactions. In the green module, the PPI network consisted of 44 nodes and 100 edges (Fig. 5a). The top 10 hub genes by MCC ranking- RPS27, RPS16, RPL39, RPS21, RPL30, HNRNPC, RPL27, RPS20, SNAPIN, and SUPT4H1-formed a subnetwork with 10 nodes and 22 edges (Fig. 5b). Notably, ribosomal genes such as RPS16, RPS27, and RPL39 were repeatedly identified as hub genes, suggesting their critical role in fatty acid metabolism regulation. Ribosomal proteins are known to modulate protein synthesis efficiency, influencing muscle cell growth, development, and lipid metabolism processes, highlighting their biological significance in the context of intramuscular fatty acid deposition. The yellow module's PPI network had 26 nodes and 100 edges (Fig. 5c), with an MCC-derived subnetwork of 10 nodes and 36 edges. Hub genes including ATP5MJ, NDUFS6, NDUFC2, LOC102084355, LOC102089463, NDUFB6, MGARP, NDUFS5, ATP5MC1, and ATP5IF suggested involvement in mitochondrial energy metabolism and fatty acid regulation. For the turquoise module, the PPI network contained 56 nodes and 100 edges (Fig. 5d), and its core subnetwork had 10 nodes and 24 edges. Identified hub genes like RHOJ, GNAI2, DLC1, HPCAL1, COL28A1, MYCT1, ASPN, MGP, COL4A1, and CLDN5, related to vascular function and cell signaling, may influence fatty acid metabolism. The brown module's PPI network comprised 35 nodes and 100 edges (Fig. 5e), with a 10 nodes and 23 edges MCC subnetwork. Core genes such as RAN, HAT1, PSMA2, PSMD6, NAA25, CNPPD1, PSMC1, KPNA6, USP14, and PSMD11 involved in nuclear transport, protein degradation, potentially regulate lipid metabolic pathways. In the red module, the PPI network was composed of 25 nodes and 100 edges (Fig. 5f). The top 10 MCC-ranked hub genes, including several uncharacterized LOC genes and NUDT12, likely contribute to fatty acid metabolism regulation despite their unknown functions.
Fig. 5.
PPI Network Analysis. (a) PPI network constructed for the green module. (b) PPI subnetwork of the green module based on MCC analysis. (c) PPI network constructed for the yellow module. (d) PPI subnetwork of the yellow module based on MCC analysis. (e) PPI network constructed for the turquoise module. (f) PPI subnetwork of the turquoise module based on MCC analysis. (g) PPI network constructed for the brown module. (h) PPI subnetwork of the brown module based on MCC analysis. (i) PPI network constructed for the red module. (j) PPI subnetwork of the red module based on MCC analysis. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In summary, Table 5 lists a set of candidate hub genes across five modules that exhibit strong associations with fatty acid traits. Among them, RPS16, NDUFS6, ATP5MJ, RHOJ, GNAI2, RAN, HAT1, and NUDT12 repeatedly emerged as top-ranked hub genes. These genes are hypothesized to be key regulators of intramuscular fatty acid metabolism in pigeon breast muscle, offering important insights for basic research and potential applications in enhancing meat quality.
Table 5.
Hub Genes.
| Correlation | Module | Trait | Hub Genes |
|---|---|---|---|
| Significantly positively correlated (p < 0. 05) |
Green | ARA | RPS27, RPS16, RPL39, RPS21, RPL30, HNRNPC, RPL27, RPS20, SNAPIN, SUPT4H1 |
| Yellow | C18:0, ARA | ATP5MJ, NDUFS6, NDUFC2, LOC102084355, LOC102089463, NDUFB6, MGARP, NDUFS5, ATP5MC1, ATP5IF1 | |
| Turquoise | C16:0, C16:1, C18:2, C20:1, C20:2, EPA | RHOJ, GNAI2, DLC1, HPCAL1, COL28A1, MYCT1, ASPN, MGP, COL4A1, CLDN5 | |
| Significantly negatively correlated (p < 0. 05) |
Brown | EPA, C18:2 N-6,9,12, C20:2 N-6,9 | RAN, HAT1, PSMA2, PSMD6, NAA25, CNPPD1, PSMC1, KPNA6, USP14, PSMD11 |
| Red | C18:1 N-9, DHA | LOC135577198, LOC135577195, LOC135577141, LOC135577104, LOC135577155, LOC135577087, LOC135577177, LOC135577160, NUDT12, LOC135575258 |
3.6. RT-qPCR analysis
To validate the expression patterns of the core hub genes identified from the five WGCNA-derived co-expression modules, RT-qPCR analysis was performed on five representative genes (RHOJ, HAT1, NUDT12, NDUFS6, and RPS16) across five developmental stages of pigeon pectoral muscle. The relative expression levels (2^-ΔΔCt) obtained by qPCR were statistically compared with the RNA-seq-based FPKM values using Pearson correlation analysis. The results showed that three genes—RHOJ (r = 0.623, p < 0.001), HAT1 (r = 0.572, p < 0.001), and NUDT12 (r = 0.554, p = 0.002)—exhibited significant positive correlations between qPCR and RNA-seq data, indicating strong concordance in expression trends between the two methods (Fig. 6 and Table 6). Although NDUFS6 and RPS16 did not show statistically significant correlations, their overall expression patterns were still comparable between platforms. These findings confirm the reliability of the RNA-seq results and support the selected hub genes' involvement in muscle development. The integration of qualitative and quantitative validation provides a solid foundation for future functional studies of regulatory genes associated with pigeon muscle growth and meat quality.
Fig. 6.
Validation and expression analysis of hub genes using RT-qPCR. The X-axis represents different developmental stages of samples; the left Y-axis indicates the relative expression levels of genes (2−ΔΔCt).
Table 6.
Validation of RNA-seq results by Pearson correlation analysis with RT-qPCR expression levels for five representative hub genes.
| Gene | Pearson correlation (r) | p-value | Sample size (n) | Significance |
|---|---|---|---|---|
| RHOJ | 0.623 | < 0.001 | 30 | ** |
| HAT1 | 0.572 | < 0.001 | 30 | ** |
| NUDT12 | 0.554 | 0.002 | 30 | ** |
| NDUFS6 | −0.083 | 0.662 | 30 | n.s. |
| RPS16 | −0.068 | 0.723 | 30 | n.s. |
Notes: p < 0.01, statistically significant; n.s., not significant.
4. Discussion
Fatty acids, serving as essential energy sources and functional molecules in poultry growth and metabolism, influencing not only the nutritional quality of meat products but also participating in multiple molecular regulatory processes. PUFAs have been shown to modulate muscle fiber composition and immune responses by activating the peroxisome proliferator-activated receptor (PPAR) signaling pathway—a key mechanism governing lipid metabolism and inflammation (Jannas-Vela et al., 2023). This suggests that changes in fatty acid composition during pigeon development may functionally affect meat quality and immune competence through PPAR-mediated transcriptional regulation, thereby directly impacting the nutritional value and physiological functions of meat products. Our study demonstrated significant variations in the contents of saturated (SFA), monounsaturated (MUFA), and polyunsaturated (PUFA) fatty acids across different developmental stages of pigeons. Specifically, MUFA levels declined gradually with age before stabilizing, while PUFA levels increased, suggesting that MUFAs primarily support energy supply and membrane structure during early development, whereas PUFAs become more crucial for signal transduction and cellular function regulation as pigeons mature. These findings align with previous research on poultry fatty acid metabolism (Chen, Zhao, Zhang, & Geng, 2017; Zheng, Pan, & Cao, 2013). Notably, pigeon meat contains higher PUFA levels compared to other livestock and poultry meats (Chang et al., 2017). PUFAs, especially Omega-3 fatty acids like eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are vital for human health, exhibiting anti-inflammatory and regulatory properties (Banaszak et al., 2024). In our study, the levels of EPA and DHA showed distinct age-dependent trends. The higher EPA content in younger pigeons may be linked to its role in anti-inflammatory responses and energy metabolism, while the increased DHA levels in older pigeons likely contribute to nervous system maturation and functional optimization (Wang et al., 2014). Overall, these findings highlight the stage-specific physiological roles of functional fatty acids during pigeon development. Similar age-related patterns of EPA and DHA have also been reported in ducks, where higher EPA levels in early stages contribute to immune regulation and energy supply, and increased DHA levels in later stages support neural development (Wang et al., 2022). Likewise, quail studies have revealed comparable trends (Juodka, Nainienė, Šiukščius, Leikus, & Šarauskas, 2024). The consistency across species suggests that different avian animals may share conserved developmental characteristics in fatty acid metabolism. Furthermore, The PUFA/SFA ratio showed distinct age-dependent variation, reaching its highest value at 6 months (1.25), indicating a more favorable nutritional profile at this developmental stage. Compared with previous reports, pigeon muscle displayed comparable or higher PUFA enrichment levels. For instance, poultry meat typically exhibits a PUFA/SFA ratio around 0.76–0.80) (Zhang et al., 2022), while duck meat shows a ratio of approximately 1.0 (Banaszak, Kuźniacka, Biesek, Maiorano, & Adamski, 2020), highlighting the nutritional advantage of pigeon meat in terms of PUFA content, particularly during mid-development.
Fatty acid profiling identified that C14:0, C16:0, C16:1 n-7, C18:0, C18:1 n-9, C18:2 n-6,9, arachidonic acid (ARA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), docosahexaenoic acid (DHA), C14:1 n-5, C18:2 n-6,9,12, C20:1 n-9, and C20:2 n-6,9 as the predominant fatty acids in squab pectoral muscle. Their relatively high levels, indicated that these fatty acids constitute the major components of intramuscular fat. To elucidate the molecular regulatory mechanisms of fatty acid composition, WGCNA was performed by integrating transcriptomic data with the quantified fatty acid contents. WGCNA has been widely applied in studies of muscle development and lipid deposition in livestock and poultry, enabling the systematic identification of core regulatory factors underlying complex phenotypes. For instance, Xing et al. constructed co-expression modules associated with intramuscular fat content in chickens and identified key regulatory genes such as HAT1 and TNIP1, which play crucial roles in fatty acid synthesis, transport, and meat quality formation (Xing et al., 2020). Similarly, Zhao et al. integrated transcriptomic and metabolomic data in pigs and used WGCNA to identify several candidate regulators, including PPARG and PLIN1, that are closely linked to lipid metabolism and muscle fiber-type transitions (Zhao et al., 2020). In the present study, we systematically constructed, for the first time in pigeons, a co-expression network linking fatty acid content with transcriptional regulation. This not only extends the application of WGCNA to special poultry species but also highlights its unique strength in uncovering novel regulatory mechanisms. The analysis revealed five gene modules significantly associated with fatty acid levels in pigeon pectoral muscle. The turquoise, green, and yellow modules showed significant positive correlations with multiple fatty acids, while the brown and red modules exhibited strong negative correlations, suggesting that key genes within these modules are involved in fatty acid synthesis, catabolism, and transport. GO enrichment and KEGG pathway analyses revealed that hub genes were primarily associated with oxidative phosphorylation, unsaturated fatty acids biosynthesis, and ribosome-related pathways, indicating their potential roles in lipid metabolism regulation. Additionally, PPI network analysis of cor genes in significantly correlated modules provided further insights into the molecular mechanisms underlying fatty acid deposition in pigeon pectoral muscle.
In the Green module, core genes such as PRS16 and RPS27 showed significant correlations with arachidonic acid (ARA), a fatty acid crucial for cellular signaling and inflammatory responses. ARA metabolism relies on the coordinated action of multiple key enzymes (Hanna & Hafez, 2018). Based on MCC analysis, RPS16 and RPS27 were identified as top hub genes. Interestingly, although RPS16 was identified as a member of the module positively associated with fatty acid content, its Pearson correlation coefficient showed a negative correlation with fatty acid levels. This discrepancy suggests that while RPS16 may be co-expressed with other functionally relevant genes, it may not itself be a direct regulator of fatty acid metabolism. These genes encode structural proteins of the 40S ribosomal subunit; although not directly involved in enzymatic reactions (Wang et al., 2019), they may indirectly influence fatty acid -metabolizing enzymes by regulating protein biosynthesis. High expression levels of RPS16 and RPS27 could enhance ARA metabolic pathway activity through interactions with lipoxygenases (LOX) and cyclooxygenases (COX), thereby regulating of inflammation and cellular membrane phospholipids remodeling (Jian et al., 2021). Moreover, RPS27 has been reported to interact with proteins in cellular signaling pathways, potentially affecting cellular energy metabolism, which is essential for fatty acid activation, transport, and degradation (Xiong, Zhao, He, & Sun, 2011).Similarly, RPL39, encoding a 60S ribosomal subunit protein, influences cell proliferation via protein synthesis and mitochondrial function (Zou & Qi, 2021). Thus, this may modulate fatty acid transport pathways to facilitate intracellular fatty acid utilization, possibly through the regulation of key transport-associated proteins such as fatty acid-binding proteins (FABPs) and CD36 (Liu, Wang, Liang, & Yang, 2024; Mistry et al., 2021), which mediate the uptake and intracellular trafficking of long-chain fatty acids.
The yellow module showed a significant correlation with the contents of stearic acid (C18:0) and arachidonic acid (ARA). Core genes in this module were mainly associated with mitochondrial respiratory chain complex functions, indicating their potential role in fatty acid oxidative metabolism. Among these, NDUFS6 and NDUFC2, ranked highly by MCC analysis, encode essential subunits of mitochondrial complex I, a key component of the electron transport chain responsible for NADH oxidation. These genes are central to maintaining oxidative phosphorylation efficiency and ATP production (Guerrero-Castillo et al., 2017). Previous research has shown that mitochondrial energy metabolism efficiency directly impacts the rate of fatty acid β-oxidation, influencing the balance between lipid synthesis and degradation (Wang, Mohsen, Mihalik, Goetzman, & Vockley, 2011). Thus, NDUFC2 and NDUFS6 likely regulate fatty acid content and composition by modulating mitochondrial energy status and intracellular energy supply. Notably, Huang et al. (Huang et al., 2019) identified NDUFC2-AS, a long non-coding RNA, during buffalo adipocyte differentiation though RNA sequencing. The positive regulatory role of NDUFC2-AS in adipogenesis suggests it may indirectly affect fatty acid synthesis, accumulation, or oxidation, possibly by regulating NDUFC2 expression or downstream signaling pathways. Moreover, ATP5MJ encodes a protein involved in mitochondrial biogenesis, organelle maintenance and transcriptional activation (Fujikawa, Ohsakaya, Sugawara, & Yoshida, 2014). ATP5MJ may indirectly regulate the energy supply for fatty acid oxidation by modulating mitochondrial ATP synthase activity (Giménez-Escamilla et al., 2024). Since ATP is required for fatty acid activation, its availability may influence the intracellular fatty acid flux and subsequent incorporation into triglycerides or oxidation pathways. It may indirectly regulate the energy supply for fatty acid oxidation by modulating mitochondrial ATP synthase activity, which is crucial for maintaining cellular energy homeostasis and fatty acid metabolism dynamics.
Core genes of the turquoise module showed complex regulatory interactions within the protein–protein interaction (PPI) network and were strongly associated with key fatty acids such as C16:0, EPA, and C18:2. Functional annotation indicated that these hub genes were involved in cell signaling, cytoskeletal organization, and extracellular matrix regulation, suggesting roles in fatty acid accumulation and skeletal muscle development. RHOJ and DLC1, members of the Rho GTPase family, regulate cytoskeletal remodeling, endothelial cell migration, and angiogenesis (Xiaoying, Xiaoli, & DongChi, & JiaoZuoyi., 2020). Sordella et al. (Sordella, Jiang, Chen, Curto, & Settleman, 2003) showed that Rho GTPases modulate insulin-like growth factor 1 (IGF-1) signaling, which influences preadipocytes and muscle satellite cells fate. Hasegawa et al. (Kiko et al., 2023) reported that Rac1, another Rho family GTPase, promotes adipogenic differentiation by regulating adipogenesis-related transcription factors, thereby enhancing fatty acid and triglyceride biosynthesis. These findings highlight the importance of Rho GTPases in adipose tissue development, lipid droplet formation, and fatty acid metabolism. Thus, RHOJ and DLC1 may indirectly regulate intracellular fatty acids trafficking and deposition by mediating signaling cytoskeleton-organelles signaling. Moreover, GNAI2 encodes the alpha subunit of guanine nucleotide-binding proteins, which are crucial for transmembrane signaling. These proteins participate in adenylate cyclase hormonal regulation and cellular division (Cezanne et al., 2022; Polit, Rysiewicz, Mystek, Błasiak, & Dziedzicka-Wasylewska, 2020). GNAI2 likely modulates fatty acid uptake and transport efficiency through its role in cellular signaling pathways.
In the brown module, RAN and HAT1 were significantly negatively correlated with multiple fatty acids, including C16:1 N-7, EPA, C18:2 N-6,9,12, and C20:2 N-6,9, indicating their potential regulatory roles in lipid metabolism. Most core genes in this module were annotated as proteasome-related components, suggesting involvement in protein degradation processes associated with fatty acid metabolism. RAN encodes a small GTPase crucial for nucleocytoplasmic transport, spindle assembly, and cell cycle regulation (Lin et al., 2025; Mohamed et al., 2023). Peroxisome proliferator-activated receptor alpha (PPARα), a key lipid metabolism regulator, requires proper nuclear localization for function. Lin et al. showed that RAN can modulate lipid uptake and fatty acid catabolism by interfering PPARα nuclear export via the CRM1-dependent pathway, altering its subcellular distribution (Lin et al., 2025). Thus, RAN may suppress fatty acid accumulation or synthesis though nucleocytoplasmic trafficking or cell cycle–related mechanisms. HAT1 encodes a histone acetyltransferase that acetylates histone H4 at gene promoters, coordinating histone production and acetylation, while enhancing glucose metabolism and cell proliferation. It plays a central role in chromatin remodeling, DNA replication, and transcriptional regulation (Gruber et al., 2019). (Denu, 2019) demonstrated that HAT1 directly binds to and promotes H4 gene expression under growth factor and glucose-dependent conditions, linking metabolic signaling to gene transcription. (Xu et al., 2023) reported that HAT1 forms a nuclear complex with ATP-citrate lyase (ACL) to catalyze histone H2K5 acetylation, a modification associated with cellular growth and metabolic. Although direct histone of HAT1's role in lipid or fatty acid is lacking, its involvement in histone acetylation and gene regulation, combined with metabolic enzymes roles in lipid biosynthesis, suggests that HAT1 may indirectly regulate fatty acid synthesis and metabolism by modulating relevant gene expression.
The red module exhibited a significantly negatively correlated with the contents of C18:1 N-9 and DHA. Among its core genes, NUDT12 encodes a mitochondrial Nudix hydrolase primarily involved in NAD(P)H metabolism and redox homeostasis regulation (Ewa et al., 2019). A study by Görigk et al. (Sarah et al., 2022) demonstrated that NUDT19, another Nudix family member, is crucial for regulating energy metabolism and mitochondrial function in hepatocytes, underscoring the family's importance in maintaining cellular energy balance. Additionally, Nudix family members such as CoA diphosphatase participate in coenzyme A (CoA) metabolism, essential for fatty acid β-oxidation, energy production, and reactive oxygen species (ROS) (Kang et al., 2003). Thus, high expression of NUDT12 may enhance mitochondrial metabolic activity, promote fatty acid oxidation, or inhabit lipid synthesis, reducing fatty acids accumulation in muscle tissue. Moreover, the red module identified several genes that were significantly correlated with fatty acid content but remain functionally unannotated. Similar observations have been reported in WGCNA studies of chickens and pigs, suggesting that these genes may play previously unrecognized roles in lipid metabolism (Guo et al., 2023; Li et al., 2025). Therefore, the candidate genes identified in this study hold substantial potential for future research and merit further functional validation and mechanistic investigation.
Despite systematically integrating transcriptomic data with fatty acid profiles to identify key regulatory modules and hub genes involved in muscle lipid metabolism, this study has several limitations. First, the functions of the identified hub genes were primarily inferred through bioinformatics analyses, lacking direct experimental validation at the protein or cellular level. In particular, the precise molecular mechanisms by which ribosomal and mitochondrial genes influence fatty acid metabolism remain unclear and require targeted functional assays—such as in vivo or in vitro knockdown, overexpression, or gene editing experiments—for further elucidation. Second, the relatively limited sample size at each developmental stage may reduce the statistical power and generalizability of the findings. Future studies should focus on functional validation of these core genes using molecular biology techniques and metabolic flux analysis. In addition, integrating multi-omics approaches—such as proteomics, epigenomics, and metabolomics—will help refine the regulatory networks underlying lipid metabolism. These efforts will enhance our understanding of intramuscular fat deposition and provide theoretical and technical foundations for improving pigeon breeding and developing functional meat products.
5. Conclusion
This study identified 39 fatty acids in the pectoral muscle tissues of pigeons across various age groups (28 days, 3 months, 6 months, 12 months, and 48 months). Predominant fatty acids included C14:0, C16:0, C16:1 N-7, C18:0, C18:1 N-9, C18:2 N-6,9, ARA, EPA, DPA, DHA, C14:1 N-5, C18:2 N-6,9,12, C20:1 N-9, and C20:2 N-6,9. By Integrating transcriptomic data with weighted gene co-expression network analysis (WGCNA), three co-expression modules(green, yellow, and turquoise), were found to have significant positively correlated with pectoral muscle fatty acid composition, whereas the brown and red modules showed significant negative correlations. PPI network analysis of differentially expressed genes identified several hub genes, such as RPS16, RPS27, NDUFS6, ATP5MJ, RHOJ, GNAI2, RAN, HAT1, LOC135577198, and NUDT12, with high Maximal Clique Centrality (MCC) scores. These genes are potential core regulators of fatty acid composition in pigeon pectoral muscle. Collectively, these findings elucidate the molecular regulatory mechanisms of fatty acid composition in pigeon muscle and provide candidate genes for future molecular breeding strategies aimed at improving meat quality traits.
CRediT authorship contribution statement
Suwei Zheng: Writing – original draft, Validation, Methodology, Formal analysis, Data curation. Haobin Hou: Investigation, Conceptualization. Xin Li: Investigation. Xiaoliang Wang: Writing – review & editing. Qiang Meng: Investigation. Zihan Qiao: Investigation. Yingying Tu: Investigation. Yunzhou Yang: Investigation. Daqian He: Investigation. Xiaohui Shen: Project administration. Junfeng Yao: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This study was funded by Shanghai Agricultural Science and Technology Innovation Program (T2023212); Shanghai Meat Pigeon Industry Technology System (2022-2025(012)); Shanghai Academy of Agricultural Sciences Program for Excellent Research Team (2022021).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochms.2025.100299.
Appendix A. Supplementary data
Supplementary material: Supplementary Table 1. Sequencing raw data quality statistics for pigeon muscle samples, including raw reads, raw bases, Q20/Q30 scores, GC content, and N percentage.
Data availability
Data will be made available on request.
References
- Banaszak M., Dobrzyńska M., Kawka A., Górna I., Woźniak D., Przysławski J., Czyż S.D. Role of Omega-3 fatty acids eicosapentaenoic (EPA) and docosahexaenoic (DHA) as modulatory and anti-inflammatory agents in noncommunicable diet-related diseases – Reports from the last 10 years. Clinical Nutrition ESPEN. 2024;63:240–258. doi: 10.1016/j.clnesp.2024.06.053. [DOI] [PubMed] [Google Scholar]
- Banaszak M., Kuźniacka J., Biesek J., Maiorano G., Adamski M. Meat quality traits and fatty acid composition of breast muscles from ducks fed with yellow lupin. Animal. 2020;14(9):1969–1975. doi: 10.1017/s1751731120000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cezanne M., Solis G.P., Koval A., Brückner M., Katanaev V.L., Behrens J., Bernkopf D.B. Gαi2-induced conductin/axin2 condensates inhibit Wnt/β-catenin signaling and suppress cancer growth. Nature Communications. 2022;13(1):674. doi: 10.1038/s41467-022-28286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Tang Q., Wang Q., Fu S., Mu C., Zhang R., Bu Z. Comparative analysis of meat quality and main nutritional components between European meat pigeons and other livestock and poultry. Journal of Food Safety and Quality. 2017;8(06):2035–2040. [Google Scholar]
- Chen X., Zhao N., Zhang Y., Geng Z. Study on fatty acid composition of muscle and expression patterns of hepatic PPARα, FADS2 and ME1 genes during the fattening period in Wanxi white geese. Acta Veterinaria et Zootechnica Sinica. 2017;48(10):1912–1919. [Google Scholar]
- Cordero J., Alonso-Calleja C., García-Fernández C., Capita R. Microbial load and antibiotic resistance patterns of Escherichia coli and enterococcus faecalis isolates from the meat of wild and domestic pigeons. Foods. 2019;8(11):536. doi: 10.3390/foods8110536. https://www.mdpi.com/2304-8158/8/11/536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denu J.M. Histone acetyltransferase 1 links metabolism and transcription to cell-cycle progression. Molecular Cell. 2019;75(4):664–665. doi: 10.1016/j.molcel.2019.08.004. [DOI] [PubMed] [Google Scholar]
- Ewa G.-N., Yixuan W., Xinfu J., Huijuan C., Mateyak M.K., Hart R.P.…Kiledjian M. Structural and mechanistic basis of mammalian Nudt12 RNA deNADding. Nature Chemical Biology. 2019;15(6):575–582. doi: 10.1038/s41589-019-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa M., Ohsakaya S., Sugawara K., Yoshida M. Population of ATP synthase molecules in mitochondria is limited by available 6.8-kDa proteolipid protein (MLQ) Genes to Cells. 2014;19(2):153–160. doi: 10.1111/gtc.12121. [DOI] [PubMed] [Google Scholar]
- Gabriela Á., Susanna D.M., Joel F., Alessandro A., Marcello C., Cristina L., Fabrizio C. Immunomodulatory effects of long-chain n-3 polyunsaturated fatty acids (n-3 PUFA) on porcine monocytes (CD14 +) immune response in vitro. Veterinary Immunology and Immunopathology. 2022;254:110523. doi: 10.1016/j.vetimm.2022.110523. [DOI] [PubMed] [Google Scholar]
- Giménez-Escamilla I., Benedicto C., Pérez-Carrillo L., Delgado-Arija M., González-Torrent I., Vilchez R.…Roselló-Lletí E. Alterations in mitochondrial oxidative phosphorylation system: Relationship of complex V and cardiac dysfunction in human heart failure. Antioxidants. 2024;13(3):285. doi: 10.3390/antiox13030285. https://www.mdpi.com/2076-3921/13/3/285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J.J., Geller B., Lipchik A.M., Chen J., Salahudeen A.A., Ram A.N.…Snyder M.P. HAT1 coordinates histone production and acetylation via H4 promoter binding. Molecular Cell. 2019;75(4):711–724.e715. doi: 10.1016/j.molcel.2019.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Castillo S., Baertling F., Kownatzki D., Wessels H.J., Arnold S., Brandt U., Nijtmans L. The assembly pathway of mitochondrial respiratory chain complex I. Cell Metabolism. 2017;25(1):128–139. doi: 10.1016/j.cmet.2016.09.002. [DOI] [PubMed] [Google Scholar]
- Guo X., Zhang H., Wang H., He X.-X., Wang J.-X., Wei W.…Jiang R.-S. Identification of key modules and hub genes involved in regulating the color of chicken breast meat using WGCNA. Animals. 2023;13 doi: 10.3390/ani13142356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna V.S., Hafez E.A.A. Synopsis of arachidonic acid metabolism: A review. Journal of Advanced Research. 2018;11:23–32. doi: 10.1016/j.jare.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zheng Q., Wang S., Wei X., Li F., Ma Y. High-throughput RNA sequencing reveals NDUFC2-AS lncRNA promotes Adipogenic differentiation in Chinese Buffalo (Bubalus bubalis L.) Genes. 2019;10(9) doi: 10.3390/genes10090689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannas-Vela S., Espinosa A., Candia A.A., Flores-Opazo M., Peñailillo L., Valenzuela R. The role of Omega-3 polyunsaturated fatty acids and their lipid mediators on skeletal muscle regeneration: A narrative review. Nutrients. 2023;15 doi: 10.3390/nu15040871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian K., Natalie B., Chan K.T., Jiachen X., Pearson R.B., Elaine S. Ribosomal proteins and human diseases: Molecular mechanisms and targeted therapy. Signal Transduction and Targeted Therapy. 2021;6(1):323. doi: 10.1038/s41392-021-00728-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juodka R., Nainienė R., Šiukščius A., Leikus R., Šarauskas G. Effects of dietary hempseed or Camelina cakes on fatty acid composition of quail meat. Life. 2024;14(1):53. doi: 10.3390/life14010053. https://www.mdpi.com/2075-1729/14/1/53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L.-W., Gabelli S.B., Bianchet M.A., Xu W.L., Bessman M.J., Mario A.L. Structure of a coenzyme A pyrophosphatase from Deinococcus radiodurans: A member of the Nudix family. Journal of Bacteriology. 2003;185(14):4110–4118. doi: 10.1128/JB.185.14.4110-4118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamichou E., Richardson R.I., Nute G.R., McLean K.A., Bishop S.C. Genetic analyses and quantitative trait loci detection, using a partial genome scan, for intramuscular fatty acid composition in Scottish blackface sheep. Journal of Animal Science. 2006;84(12):3228–3238. doi: 10.2527/jas.2006-204. [DOI] [PubMed] [Google Scholar]
- Keiichi I., Noriaki S., Takeshi H., Kenji O. Genetic relationships between meat quality traits and fatty acid composition in Japanese black cattle. Animal science journal = Nihon chikusan Gakkaiho. 2017;88(1):11–18. doi: 10.1111/asj.12613. [DOI] [PubMed] [Google Scholar]
- Kiko H., Nobuyuki T., Maaya Y., Yoshiki S., Atsu A., Takaya S. Regulation of De novo lipid synthesis by the small GTPase Rac1 in the Adipogenic differentiation of progenitor cells from mouse white adipose tissue. International Journal of Molecular Sciences. 2023;24(5):4608. doi: 10.3390/ijms24054608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y., Li F., Yue X., Xu Y., Bai J., Fu W. SNPS within the SLC27A6 gene are highly associated with Hu sheep fatty acid content. Gene. 2024;927 doi: 10.1016/j.gene.2024.148716. [DOI] [PubMed] [Google Scholar]
- Kong Z.S., Deng T.Y., Sun Z., Xu C.Q., Yang W.Q., Li H.R., Tang K.X. Adjacent SNPs in the transcriptional regulatory region of the FADS2 gene associated with fatty acid and growth traits in chickens. Genetics and molecular research : GMR. 2014;13(2):3329–3336. doi: 10.4238/2014.April.29.11. [DOI] [PubMed] [Google Scholar]
- Li H., Li X., Li S., Zhang H., Gu J., Yuan P.…Li G. Identification of Lipidome characteristics and key candidate genes in breast muscle of Gushi chicken and AA broiler. China Poultry. 2024;46(07):1–11. doi: 10.16372/j.issn.1004-6364.2024.07.001. [DOI] [Google Scholar]
- Li W., Yang S., Liu H., Cao Z., Xu F., Ning C.…Tang H. Identification of key LncRNAs and mRNAs associated with intramuscular fat in pig via WGCNA. BMC Genomics. 2025;26(1):233. doi: 10.1186/s12864-025-11427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Liang Z., Weng Z., Liu X., Zhang F., Chong Y. CRSP8-driven fatty acid metabolism reprogramming enhances hepatocellular carcinoma progression by inhibiting RAN-mediated PPARα nucleus-cytoplasm shuttling. Journal of Experimental & Clinical Cancer Research. 2025;44(1):93. doi: 10.1186/s13046-025-03329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Wang Z., Liang M., Yang L. Rice protein reduces triglyceride levels through modulating CD36, MTP, FATP, and FABP expression in growing and adult rats. Foods. 2024;13(17):2704. doi: 10.3390/foods13172704. https://www.mdpi.com/2304-8158/13/17/2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo N., Shu J., Yuan X., Jin Y., Cui H., Zhao G., Wen J. Differential regulation of intramuscular fat and abdominal fat deposition in chickens. BMC Genomics. 2022;23(1):308. doi: 10.1186/s12864-022-08538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingyue C., Lijun S., Peng P., Bo H., Lin L., Xiaoqing L.…Dongxiao S. Determination of genetic effects and functional SNPs of bovine HTR1B gene on milk fatty acid traits. BMC Genomics. 2021;22(1):575. doi: 10.1186/s12864-021-07893-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry J.J., Hellmich C., Moore J.A., Jibril A., Macaulay I., Moreno-Gonzalez M.…Rushworth S.A. Free fatty-acid transport via CD36 drives β-oxidation-mediated hematopoietic stem cell response to infection. Nature Communications. 2021;12(1):7130. doi: 10.1038/s41467-021-27460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed E., Hamdi N., Vijay M., Yachana M.A., Aljabali A.A., Serrano-Aroca Á.…Tambuwala M.M. Ran GTPase and its importance in cellular signaling and malignant phenotype. International Journal of Molecular Sciences. 2023;24(4):3065. doi: 10.3390/ijms24043065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter L., Steve H. WGCNA: An R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9(1):559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polit A., Rysiewicz B., Mystek P., Błasiak E., Dziedzicka-Wasylewska M. The Gαi protein subclass selectivity to the dopamine D(2) receptor is also decided by their location at the cell membrane. Cell Communication and Signaling: CCS. 2020;18(1):189. doi: 10.1186/s12964-020-00685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos H.O., May J.C., Buenoz A.A. Eating more sardines instead of fish oil supplementation: Beyond omega-3 polyunsaturated fatty acids, a matrix of nutrients with cardiovascular benefits. Frontiers in Nutrition. 2023;10:1107475. doi: 10.3389/fnut.2023.1107475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarah G., Margriet O.D., Tanja K., Delsi A., Christian B., Mareike D.…Hadi A.-H. Nudix hydrolase NUDT19 regulates mitochondrial function and ATP production in murine hepatocytes. BBA - Molecular and Cell Biology of Lipids. 2022;1867(6):159153. doi: 10.1016/j.bbalip.2022.159153. [DOI] [PubMed] [Google Scholar]
- Sebastià C., Gallopin M., Caldas Y.R., Estellé J., Hernández J.V., Castelló A.…Folch J.M. Gene co-expression network analysis for porcine intramuscular fatty acid composition. Animal : An International Journal of Animal Bioscience. 2024;18(9) doi: 10.1016/j.animal.2024.101259. [DOI] [PubMed] [Google Scholar]
- Shuzhe W., Chaoyun Y., Cuili P., Xue F., Zhaoxiong L., Jieping H., Xuefeng W., Fen L., Yun M. Identification of key genes and functional enrichment pathways involved in fat deposition in Xinyang buffalo by WGCNA. Gene. 2022 doi: 10.1016/j.gene.2022.146225. (prepublish) [DOI] [PubMed] [Google Scholar]
- Sordella R., Jiang W., Chen G.-C., Curto M., Settleman J. Modulation of rho GTPase signaling regulates a switch between Adipogenesis and Myogenesis. Cell. 2003;113(2):147–158. doi: 10.1016/s0092-8674(03)00271-x. [DOI] [PubMed] [Google Scholar]
- Urszula K., Sebastian S., Joanna N., Julia G., Jakub J., Dorota W., Katarzyna P. The g.4290 CG polymorphism in the FADS2 gene modifies the fatty acid profile of the pectoralis Superficialis muscle of Ross 308 broiler chickens. Animals : An Open Access Journal from MDPI. 2022;12(15):1882. doi: 10.3390/ani12151882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Dong B., Yang T., Zhang A., Hu X., Wang Z.…Chen G. Dietary linseed oil affects the polyunsaturated fatty acid and transcriptome profiles in the livers and breast muscles of ducks. Frontiers in Nutrition. 2022;9 doi: 10.3389/fnut.2022.1030712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Yang C., Pan C., Feng X., Lei Z., Huang J., Wei X., Li F., Ma Y. Identification of key genes and functional enrichment pathways involved in fat deposition in Xinyang buffalo by WGCNA. Gene. 2022;818(prepublish) doi: 10.1016/j.gene.2022.146225. [DOI] [PubMed] [Google Scholar]
- Wang T., Fu X., Chen Q., Patra J.K., Wang D., Wang Z., Gai Z. Arachidonic acid metabolism and kidney inflammation. International Journal of Molecular Sciences. 2019;20(15):3683. doi: 10.3390/ijms20153683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Mohsen A.-W., Mihalik S., Goetzman E., Vockley J. Evidence for the physical association of mitochondrial fatty acid oxidation and oxidative phosphorylation complexes. Mitochondrion. 2011;11(4):644. doi: 10.1074/jbc.M110.139493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Yu C., Lu S., Xiao M., Zhou Q., Li X. The important role of DHA/EPA in the prevention and treatment of cardiovascular diseases. China Food Additives. 2014;09:164–170. [Google Scholar]
- Wood J.D., Richardson R.I., Nute G.R., Fisher A.V., Campo M.M., Kasapidou E.…Enser M. Effects of fatty acids on meat quality: A review. Meat Science. 2004;66(1):21–32. doi: 10.1016/S0309-1740(03)00022-6. [DOI] [PubMed] [Google Scholar]
- Xiangrong C., Shukai W., Chunnuan C., Baoyuan X., Zhongning F., Weipeng H.…Hefan H. Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway following experimental traumatic brain injury. Journal of Neuroinflammation. 2017;14(1):143. doi: 10.1186/s12974-017-0917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaoying G., Xiaoli G., DongChi, & JiaoZuoyi. Rho GTPases and related signaling complexes in cell migration and invasion. Experimental Cell Research. 2020;388(1) doi: 10.1016/j.yexcr.2020.111824. [DOI] [PubMed] [Google Scholar]
- Xing S., Liu R., Zhao G., Liu L., Groenen M.A.M., Madsen O.…Wen J. RNA-Seq analysis reveals hub genes involved in chicken intramuscular fat and abdominal fat deposition during development. Frontiers in Genetics. 2020;11 doi: 10.3389/fgene.2020.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., Chen Q. Identification of key genes and pathways involved in subcutaneous fat deposition in pigs based on WGCNA method. Modern Animal Husbandry Science & Technology. 2024;09:39–43. doi: 10.19369/j.cnki.2095-9737.2024.09.011. [DOI] [Google Scholar]
- Xiong X., Zhao Y., He H., Sun Y. Ribosomal protein S27-like and S27 interplay with p53-MDM2 axis as a target, a substrate and a regulator. Oncogene. 2011;30(15):1798–1811. doi: 10.1038/onc.2010.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Yue Y., Liu B., Chen Z., Ma X., Wang J.…Zhou D.X. ACL and HAT1 form a nuclear module to acetylate histone H4K5 and promote cell proliferation. Nature Communications. 2023;14(1):3265. doi: 10.1038/s41467-023-39101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Song X., Wu W., Zhang L., Han Z., Wang X., Wang R., Yang M., Zhang Z. Nutritional profiling of breast muscle: A comparative study between Yuzhong pigeons and European meat pigeons. Food Chemistry: X. 2025;25:102157. doi: 10.1016/j.fochx.2025.102157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zhuang H., Cao J., Geng A., Wang H., Chu Q.…Liu H. Breast meat fatty acid profiling and proteomic analysis of Beijing-you chicken during the laying period. Frontiers in Veterinary Science. 2022;9 doi: 10.3389/fvets.2022.908862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Xing Q., Lu Y., Wang Z. Fatty acid composition in different types of meat products. Journal of Hygiene Research. 2018;47(02):254–259. doi: 10.19813/j.cnki.weishengyanjiu.2018.02.016. [DOI] [PubMed] [Google Scholar]
- Zhao X., Hu H., Lin H., Wang C., Wang Y., Wang J. Muscle transcriptome analysis reveals potential candidate genes and pathways affecting intramuscular fat content in pigs. Frontiers in Genetics. 2020;11 doi: 10.3389/fgene.2020.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Pan D., Cao J. Analysis of amino acid and fatty acid composition and content in Zhedong white geese at different ages. Food Science. 2013;34(12):140–142. https://link.cnki.net/urlid/11.2206.TS.20130104.1441.038 [Google Scholar]
- Zou Q., Qi H. Deletion of ribosomal paralogs Rpl39 and Rpl39l compromises cell proliferation via protein synthesis and mitochondrial activity. The International Journal of Biochemistry & Cell Biology. 2021;139:106070. doi: 10.1016/j.biocel.2021.106070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material: Supplementary Table 1. Sequencing raw data quality statistics for pigeon muscle samples, including raw reads, raw bases, Q20/Q30 scores, GC content, and N percentage.
Data Availability Statement
Data will be made available on request.