Abstract
We report an outbreak of epidemic Staphylococcus aureus strains characterized by an unusual heterogeneous resistance to methicillin and resistance to tobramycin but susceptibility to gentamicin (gentamicin-susceptible methicillin-resistant S. aureus [GS-MRSA]), contrasting with gentamicin-resistant homogeneous MRSA (GR-MRSA) that have been endemic in our hospital since the 1970s. A total of 97 GS-MRSA strains, which were shown by DNA hybridization to carry the mecA and ant(4′)-Ia genes, were studied. The 40 GS-MRSA strains isolated at the beginning of the outbreak (January 1992 to June 1993) were typed by using resistance patterns, phage typing, serotyping, and pulsed-field gel electrophoresis and were compared with GR-MRSA and methicillin-susceptible S. aureus (MSSA) strains isolated during the same period. Two dominant clones, A::1 and B::3, and one minor clone, C::5, were identified among the 40 GS-MRSA strains, according to pulsotypes (A to C) and their resistance patterns (1, 3, and 5), which were distinguishable from those of GR-MRSA and MSSA strains. A selection of 57 GS-MRSA strains, isolated from 1994 to 1996, were clustered in the same three clones. However, their distribution had changed in comparison with that in the 1992 to 1993 period: clone A::1 remained dominant (47 versus 42.5%), whereas clone B::3 progressively declined (5 versus 35%) and clone C::5, the most susceptible to antibiotics, spread (44 versus 2.5%). Epidemiological investigations revealed that some clones had been introduced via patients transferred from other hospitals and that cross-infection occurred within and between wards. Major changes in the use of antibiotics, especially aminoglycosides, cyclines, and macrolides, likely played a role in the emergence and spread of GS-MRSA strains.
Since its first identification in the early 1960s, methicillin-resistant Staphylococcus aureus (MRSA) has become a major pathogen involved in hospital-acquired infections in many parts of the world (1). In France, the proportion of MRSA strains among total S. aureus strains ranges from 30 to 40%, one of the highest in Europe (21). Until recently, MRSA isolated in France was characterized by a resistance to a wide variety of antibiotics (21).
In our hospital, the proportion of MRSA strains among total S. aureus strains increased between 1975 and 1991 from 18 to 41%. During this period, almost all MRSA strains exhibited a homogeneous resistance to methicillin. In addition, resistance to multiple aminoglycosides, including gentamicin (gentamicin-resistant MRSA [GR-MRSA]), has regularly increased among MRSA strains, from 27% in 1975 to 99% in 1991 (3). Measures for controlling MRSA, based on screening of MRSA carriers, barrier precautions, and isolation and cohorting of patients (14), have been progressively implemented since 1993, especially in intensive care units (ICUs), and have resulted in a decrease in the proportion of MRSA strains in our hospital, from 41% in 1991 to 29% in 1996.
Strikingly, new epidemic MRSA strains more susceptible to antibiotics than GR-MRSA strains have emerged since 1991 to 1992, i.e., just before the implementation of prevention measures, and have tended to replace GR-MRSA strains. This switch was unexpected because MRSA strains are particularly characterized by their ability to develop resistance to antibiotics in current use. These new MRSA strains exhibited a heterogeneous resistance to methicillin and a resistance to tobramycin contrasting with a susceptibility to gentamicin (gentamicin-susceptible MRSA [GS-MRSA]). GS-MRSA strains displayed various resistance patterns, in contrast to endemic GR-MRSA strains, which shared a uniform resistance pattern. In this study, we characterized the GS-MRSA strains by using several typing methods and assessed the correlation among the results yielded by these methods. We also described the spread of GS-MRSA strains in our hospital and investigated the possible reasons for their spread.
MATERIALS AND METHODS
Setting.
Pitié-Salpêtrière Hospital is a university-affiliated public hospital located in Paris, with 2,032-bed acute care facilities comprising five ICUs, 24 general medicine and 12 surgery wards, and a 240-bed long-term care facility. All these wards are located within the same area but are distributed in separate buildings. An average of 80,000 patients are admitted per year.
Bacterial strains.
A total of 115 S. aureus strains isolated from 115 patients hospitalized between 1992 and 1996 were selected for typing. We included all 42 consecutive available GS-MRSA strains isolated at the beginning of the outbreak (from January 1992 to June 1993) and selected 57 GS-MRSA strains throughout the 1994 to 1996 period: 16 in 1994, 19 in 1995, and 22 in 1996. We also included for comparison eight GR-MRSA and eight MSSA strains isolated during the 1992 to 1993 period. Finally, S. aureus ATCC 6583P, CIP 6525 (Collection of the Institut Pasteur), and BM 3002 (6) were used as controls in the dot blotting method.
Antimicrobial susceptibility testing.
Antimicrobial susceptibility was determined by the disk diffusion method on Mueller-Hinton agar (disks and agar were obtained from Diagnostic Pasteur, Marne la Coquette, France) as recommended by the Antibiogram Committee of the French Society for Microbiology (7). Heterogeneous resistance to methicillin was differentiated from homogeneous resistance by incubation of the plates with a 5-μg oxacillin disk at 30 and 37°C. Strains showing a clear zone of inhibition around the oxacillin disk at 37°C and growing in the near vicinity of the disk at 30°C, were considered to be heterogeneous. Strains showing no detectable inhibition of growth around the disk at 30 and 37°C were considered to be homogeneous.
Phage typing.
Bacteriophage typing was performed by the National Reference Laboratory for Staphylococci (Lyon, France) with a standard test dilution (5). The set of phages included the lytic groups I (29, 52, 52A, 79, and 80), II (3A, 3C, 55, and 71), III (6, 42E, 47, 53, 54, 75, 77, 83A, 84, and 85), and V (94 and 96) and the nonallocated phages 81 and 95.
Serotyping.
Serotyping was carried out by the National Reference Laboratory for Staphylococci as previously described (8). Briefly, strains were grown overnight at 37°C in broth containing meat extract and peptone enriched with sodium chloride (2.5%) and glucose (0.2%). Cells were harvested by centrifugation and resuspended in 1 ml of 0.5% formalinized broth. One drop of rabbit serum and 1 drop of the bacterial suspension were placed on a glass plate and mixed by gentle rocking for 20 min at room temperature. Agglutination was determined visually. The immunosera used were a4, a5, b1, c1, e, h1, h2, k1, k1-k2, i1, i2, m, n, 263-I, 263-II, l, o, p, and 18.
PFGE analysis.
Genomic DNA was prepared in low-melting-point agarose plugs and digested with the SmaI restriction enzyme, as previously described by Prevost et al. (17). Electrophoresis was performed with a contour-clamped homogeneous electric field (CHEF DR II; Bio-Rad, Ivry sur Seine, France). Run conditions were 190 V with switching from 8 to 14 s for 9.5 h and from 19 to 28 s for 9.5 h at 14°C.
Pulsed-field gel electrophoresis (PFGE) patterns were analyzed and compared with Gel Compar (Applied Maths, Kortrijk, Belgium) by the unweighted-pair group method of arithmetic average clustering method with the Dice coefficient. PFGE types and subtypes were identified with capital letters and numbers, respectively (e.g., A1). Strains with indistinguishable PFGE patterns were assigned to the same type and subtype. Strains differing by less than three bands were clustered within different subtypes. Strains differing by more than three bands were considered different types (20).
Dot blotting of DNA with the mecA and the ant(4′)-Ia DNA probes.
The probes used were internal fragments of reported sequences of the mecA (679 bp, nucleotides 1050 to 1729) and ant(4′)-Ia (632 bp, nucleotides 2256 to 2888) genes, encoding the penicillin-binding protein PBP2a, responsible for methicillin resistance, and the aminoglycoside nucleotidyl transferase ANT(4′), responsible for tobramycin resistance, respectively (11, 18). These probes were generated by PCR with the primers Mec-1 (5′-CGATAATAGCAATACAATCGC-3′) and Mec-2 (5′-GATTGAAAGGATCTGTACTGG-3′) for the mecA gene and Ant-1 (5′-GGATGATGTTAAGGCTATTGG-3′) and Ant-2 (5′-GCACAGATGGTCATAACCTG-3′) for the ant(4′)-Ia gene. Total DNA from S. aureus strains was extracted as previously described (16) and was dotted onto a nylon membrane and successively hybridized with the mecA and ant(4′)-Ia probes. Probe labelling and hybridization were carried out by using the direct ECL nonradioactive labelling kit (Amersham, Les Ulis, France). The ATCC 6583P strain was used as a negative control for mecA and ant(4′)-Ia genes. CIP 6525 and BM 3002 strains were used as positive controls for mecA and ant(4′)-Ia genes, respectively.
Antibiotics consumption.
Antibiotics consumption was measured as defined daily doses per 1,000 patient days (15). The fold change in antibiotics consumption was used to examine trends.
RESULTS
Description of outbreak.
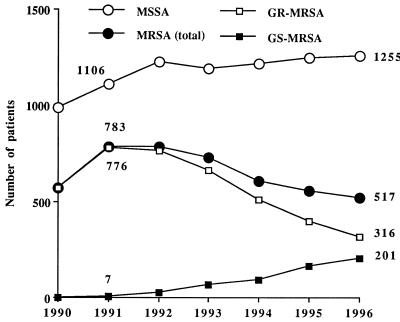
The total number of patients in the hospital with clinical specimens positive for MRSA decreased from 783 in 1991 to 517 in 1996 (Fig. 1). The corresponding ratio of patients with clinical specimens positive for MRSA decreased from 14 to 10 per 1,000 admissions. In contrast, the number of patients with clinical specimens positive for MSSA remained unchanged at around 1,200 (ratio, 22 per 1,000 admissions) each year. The first strains (n = 7) of GS-MRSA were isolated in 1991 from patients hospitalized in five different wards. After that, the number of patients with clinical specimens positive for GS-MRSA steadily increased to 201 in 1996 (ratio, 4 per 1,000 admissions) whereas the number with clinical specimens positive for GR-MRSA decreased from 776 in 1991 to 316 in 1996 (ratio, 14 and 6 per 1,000 admissions, respectively). During the 1991 to 1993 period, most of the GS-MRSA strains were isolated from patients in long-term care facilities and neurosurgery wards. During the 1994 to 1996 period, GS-MRSA spread to almost all wards, but not to the same extent. In 1996, the proportion of GS-MRSA strains among total MRSA strains reached 90% in long-term care facilities whereas it was 47, 43, and 20% in the general medicine and surgery wards and ICUs, respectively.
FIG. 1.
Number of patients with clinical specimens positive for S. aureus, Pitié-Salpêtrière Hospital, 1990 to 1996.
Hybridization with the mecA and the ant(4′)-Ia probes.
CIP 6525, the 8 GR-MRSA strains, 40 of the 42 GS-MRSA strains isolated from January 1992 to June 1993, and all 57 GS-MRSA strains isolated from 1994 to 1996 harbored the mecA gene. The two strains of GS-MRSA which did not hybridize to the mecA probe were subsequently excluded from the study. BM 3002 and all the GS-MRSA and GR-MRSA strains harbored the ant(4′)-Ia gene. As expected, ATCC 6583P and all of the MSSA strains failed to hybridize with mecA or ant(4′)-Ia (data not shown).
Phenotypic and genotypic characterization of GS-MRSA strains isolated during the 1992 to 1993 period. (i) Resistance pattern.
The 40 GS-MRSA strains displayed six distinct resistance patterns (Table 1). All of the strains were resistant to kanamycin, tobramycin, amikacin, and neomycin and exhibited a heterogeneous resistance to methicillin. Seventeen (42.5%) of these 40 strains had resistance pattern 1, characterized by resistance to macrolides-lincosamides-streptogramin B (MLSB), pefloxaxin, and spectinomycin. Six strains (15%) had resistance pattern 2 (2a to 2c), differing from resistance pattern 1 by additional resistance to streptomycin, rifampin, and cyclines. Additional resistance to chloramphenicol and fosfomycin defined the subtypes 2b and 2c, respectively. Fourteen strains (35%) had resistance pattern 3 (3a and 3b), differing from resistance pattern 1 by susceptibility to spectinomycin. Additional resistance to cyclines and rifampin defined the subtypes 3a and 3b, respectively. The remaining three strains had unique resistance patterns (4 to 6) but were all susceptible to MLSB, spectinomycin, and streptomycin. Finally, the eight GR-MRSA strains were grouped in resistance pattern 7, which differed from resistance pattern 2 by additional resistance to gentamicin and homogeneous resistance to methicillin.
TABLE 1.
Resistance patterns of the 40 GS-MRSA strains (patterns 1 to 6) and the 8 GR-MRSA strains (pattern 7) isolated during the 1992 to 1993 period
| Pattern no. | Resistancea | No. of strains (%) |
|---|---|---|
| 1 | MLSB, Pf, Sp | 17 (42.5) |
| 2 | 6 (15) | |
| 2a | MLSB, Pf, Sp, St, Rf, Tc-Mc | 2 |
| 2b | MLSB, Pf, Sp, St, Rf, Tc-Mc, Cl | 2 |
| 2c | MLSB, Pf, Sp, St, Rf, Tc-Mc, Fs | 2 |
| 3 | 14 (35) | |
| 3a | MLSB, Pf, Tc-Mc | 6 |
| 3b | MLSB, Pf, Rf | 8 |
| 4 | Fa | 1 (2.5) |
| 5 | Pf | 1 (2.5) |
| 6 | L, Pf, Cl, SA | 1 (2.5) |
| 7 | 8 (100) | |
| 7a | MLSB, Pf, Sp, St, Rf, Tc-Mc, G | 4 |
| 7b | MLSB, Pf, Sp, St, Rf, Tc-Mc, Fs, G | 4 |
M, macrolides; L, lincosamides; SA, streptogramin A; SB, streptogramin B; Pf, pefloxacin; Sp, spectinomycin; St, streptomycin; Rf, rifampin; Tc, tetracycline; Mc, minocycline; Cl, chloramphenicol; Fs, fosfomycin; G, gentamicin.
(ii) Phage typing.
Sixteen (40%) GS-MRSA strains were untypeable by phage typing. Among the 34 typeable strains, 31 were included in group III and were lysed by phages 47, 53, 54, 77, 84, and 85 or by phage 85 alone. Three GS-MRSA strains displayed phage type 95 in addition to phage types 47, 53, 54, 77, 84, and 85. Most GR-MRSA strains were lysed by phage 77 alone. Among the MSSA strains, only one was typeable and it belonged to group V (phages 94 and 96).
(iii) Serotyping.
Three GS-MRSA strains (7.5%), belonging to resistance patterns 1, 2c, and 3b, were untypeable by serotyping. This method defined three groups among the 37 typeable strains. The first group included 19 strains reacting with serum a5 (Table 2). These were 16 of the 17 strains with resistance pattern 1 and three strains with resistance patterns 2c, 5, and 6, respectively. The second group included four strains reacting with antigens c1/18 and 18 and belonging to resistance patterns 2a and 2b. The third group included 14 strains reacting with serum c1/o. Of these, 13 had resistance pattern 3 and one had resistance pattern 4. Most of the GR-MRSA strains were characterized by antigens a5 and 18, which is displayed by the GS-MRSA strains belonging to resistance patterns 1 and 2. In contrast, antigen expression was more heterogeneous among MSSA strains (data not shown).
TABLE 2.
Typing of GS-MRSA strains isolated during the 1992 to 1993 period
| Pulsotype | Serotype | Resistance patterna | No. of strains (%) | Clone |
|---|---|---|---|---|
| A | 23 (57.5) | |||
| A1 | a5 | 1 | 11b | A::a5::1 |
| A5 | a5 | 1 | 5 | A::a5::1 |
| A6 | a5 | 1 | 1 | A::a5::1 |
| A3 | a5 | 2c | 2b | A::a5::2 |
| A2 | c1/18 | 2a | 2 | A::18::2 |
| A4 | 18 | 2b | 2 | A::18::2 |
| B | 15 (37.5) | |||
| B1 | c1/o | 3a | 5 | B::c1/o::3 |
| B1 | c1/o | 3b | 2b | B::c1/o::3 |
| B2 | c1/o | 3b | 7 | B::c1/o::3 |
| B3 | c1/o | 4 | 1 | B::c1/o::4 |
| C | 2 (5) | |||
| C1 | a5 | 5 | 1 | C::a5::5 |
| C2 | a5 | 6 | 1 | C::a5::6 |
For definitions, see Table 1.
One of these strains was untypeable by serotyping.
(iv) PFGE analysis.
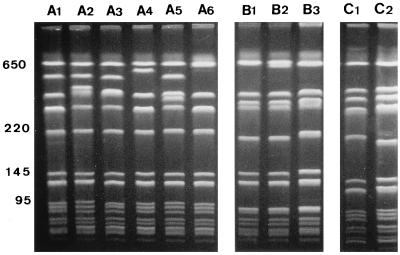
PFGE analysis of the GS-MRSA strains revealed three major pulsotypes (A to C), which exhibited a similarity coefficient of 73%. Pulsotypes A, B, and C were divided into six (A1 to A6), three (B1 to B3), and two (C1 and C2) subtypes, respectively (Fig. 2). Pulsotypes A and B were predominant, including 23 and 15 strains, respectively, while only 2 strains had pulsotype C (Table 2). Most of the strains with pulsotype A were associated either with serotype a5 and resistance pattern 1 or with serotype 18 and resistance pattern 2. All the strains with pulsotype B were associated with serotype c1/o and resistance pattern 3, except one strain exhibiting the unusual resistance pattern 4. The two strains with pulsotype C displayed the serotype a5, as did most strains with pulsotype A, but had resistance pattern 5 or 6.
FIG. 2.
PFGE of SmaI-digested genomic DNA from representative GS-MRSA strains of each pulsotype and subtype. The scale (in kilobases) is at the left.
Pulsotypes of GR-MRSA strains were closely related (the similarity coefficient ranged from 80 to 100%) but were distinct from pulsotypes A, B, and C of the GS-MRSA strains (similarity coefficient, 66%). In contrast, pulsotypes of MSSA strains were more heterogeneous (data not shown).
(v) Spread of GS-MRSA clones.
The GS-MRSA strains were clustered on the basis of the pulsotypes (A to C), serotypes (a5, 18, and c1/o), and resistance patterns (1 to 6) (Table 2). Phage typing results were not taken into account for analysis because of the method’s low discriminatory power.
The clones characterized by pulsotype A included 57.5% of the GS-MRSA strains. The dominant clone (A::a5::1), mainly clustered temporally and spatially in neurology and surgery wards (Table 3), was introduced into our hospital via one patient transferred from another hospital. The clone A::18::2 was isolated from patients hospitalized at the same time in two general medicine wards. The clone A::a5::2 appeared sporadically.
TABLE 3.
Spread of the first GS-MRSA clones in the wards of Pitié-Salpêtrière Hospital between January 1992 and June 1993
| Ward | Clone(s)a isolated during (mo and yr):
|
|||||
|---|---|---|---|---|---|---|
| Jan–Mar 92 | Apr–Jun 92 | Jul–Sep 92 | Oct–Dec 92 | Jan–Mar 93 | Apr–Jun 93 | |
| General medicine (2 wards) | A1, A1 | A1, A1 | ||||
| Neurology (3 wards) | A | A | A | |||
| Renal | B1 | |||||
| Pneumology | Cb | |||||
| Long-term care facility | B | B, B, B | B, B, B, B | A, B, B | ||
| Orthopedic surgery | A | B | B | |||
| Cardiac surgery | A, A, A | |||||
| Neurosurgery (2 wards) | Ab | A, B | A, A, A, A, A | |||
| Other surgery (3 wards) | C1b | A, A2 | A | |||
| Surgical ICU | A2 | B | ||||
A, clone A::a5::1; A1, clone A::18::2; A2, clone A::a5::2; B, clone B::c1/o::3; B1, clone B::c1/o::4; C, clone C::a5::5; C1, clone C::a5::6. The number of entries represents the frequency with which a clone was isolated in a ward for each 3-month period.
Introduced into the hospital via a transferred patient.
The clones characterized by pulsotype B included 37.5% of the GS-MRSA strains. Twelve of the 14 strains included in the dominant clone (B::c1/o::3) were isolated from patients hospitalized at the same time in the long-term care facility (n = 10) and the orthopedic surgery ward (n = 2). Temporal and spatial overlaps were found among the patients hospitalized in these two wards. The clone B::c1/o::4 was isolated from only one patient.
Each of the clones characterized by pulsotype C (C::a5::5 and C::a5::6) included only one strain, both introduced into our hospital via two patients transferred from other hospitals.
Phenotypic and genotypic characterization of GS-MRSA strains isolated during the 1994 to 1996 period.
A selection of 57 GS-MRSA strains isolated during the 1994 to 1996 period were analyzed for changes in resistance and PFGE patterns. For this purpose, we selected one to three strains isolated from patients with no known epidemiological links for each of the wards affected by the outbreak.
Results of antimicrobial susceptibility testing and PFGE indicated that the resistance patterns and pulsotypes of these 57 GS-MRSA strains were essentially identical to those found during the 1992 to 1993 period and were similarly associated. However, the distribution of the clones defined by pulsotypes A to C and resistance patterns 1 to 6 changed markedly. The clones A::1 and C::6 remained stable, representing 47 and 2% of GS-MRSA strains, respectively. The clones A::2 and B::4 disappeared, and the clone B::3 markedly decreased, from 35% of GS-MRSA strains in 1992 to 1993 to 5% in 1994 to 1996. The clone C::5, the least resistant to antibiotics, dramatically rose from 2.5% in 1992 to 1993 to 44% in 1994 to 1996. Finally, only one strain (2%) displayed a new association between pulsotype A and resistance limited to pefloxacin (as in pattern 5) and spectinomycin.
Routine susceptibility testing performed in the laboratory on all 545 GS-MRSA strains isolated since the beginning of the outbreak confirmed the large increase in resistance pattern 5, from 0% in 1992 to 37% in 1996, and the progressive decrease in pattern 2, from 20% in 1992 to 0.5% in 1996.
Evolution of antibiotics consumption at Pitié-Salpêtrière hospital.
Between 1985 and 1995, for the entire hospital, the overall use of penicillinase inhibitors, cephalosporins, imipenem, fluoroquinolones, and amikacin, which are inactive against all MRSA strains, whether resistant to gentamicin or not, steadily increased (4.5-fold). The use of gentamicin and of cyclines that are active against most of the GS-MRSA strains but not against GR-MRSA strains progressively decreased (four- and twofold, respectively). The use of macrolides, which are active against GS-MRSA strains with resistance pattern 5 but not against those with resistance patterns 1, 2, and 3, remained stable between 1985 and 1990 but declined afterwards.
In the early 1990s, the level of gentamicin use was twofold lower in the wards most affected by the outbreak of GS-MRSA than in those least affected. Between 1990 and 1995, the use of macrolides and cyclines declined more (by two- and fourfold, respectively) in the wards most affected by the outbreak of GS-MRSA than in those least affected (1.5- and 3-fold, respectively).
DISCUSSION
Since 1991, new epidemic S. aureus strains characterized by heterogeneous resistance to methicillin and resistance to tobramycin but susceptibility to gentamicin (GS-MRSA) have emerged and spread to our hospital. This fact was striking because our hospital has been confronted since the 1970s with endemic homogeneous MRSA strains resistant to gentamicin (GR-MRSA).
On the basis of pulsotypes, resistance patterns, and serotypes, GS-MRSA strains were classified into three major clones. Phage typing was not suitable for the phenotypic characterization of GS-MRSA strains, since it failed to type a large number of the strains, as already reported in other MRSA outbreaks (2, 13). Serotyping classified most GS-MRSA strains isolated in 1992 to 1993 into three major groups but was not as discriminating as PFGE analysis, since GS-MRSA and GR-MRSA strains with distinct PFGE patterns were classified into the same serogroups. For these reasons, phage typing and serotyping were not used to type the GS-MRSA strains isolated from 1994 to 1996.
PFGE analysis is presently considered the most reliable method for studying the epidemiology of MRSA strains (20). In our study, PFGE analysis discriminated the new epidemic GS-MRSA strains from endemic GR-MRSA strains and detected among the GS-MRSA strains three major pulsotypes (A, B, and C). Differences in resistance patterns correlated well with differences in pulsotypes. Indeed, GS-MRSA strains resistant to spectinomycin and MLSB always displayed pulsotype A (clone A::1), whereas those susceptible to spectinomycin but resistant to MLSB displayed pulsotype B (clone B::3) and those susceptible to spectinomycin and to MLSB displayed pulsotype C (clone C::5), except one strain with pulsotype B. The same correlation between pulsotypes and resistance patterns was observed among GS-MRSA strains isolated 3 years after the beginning of the outbreak, thus suggesting that the clones are stable.
GS-MRSA and GR-MRSA strains belonged predominantly to the same phage group (III) and the same serotypes (a5 and 18), and their pulsotypes exhibited a similarity coefficient of 66%. Moreover, all the GS-MRSA strains and the eight GR-MRSA strains isolated during the same period of time harbored the ant(4′)-Ia gene. Recent work has shown that half of the GR-MRSA strains isolated in France between 1991 and 1993 (12), but only 6% of those isolated in 1988 (4), harbored the ant(4′)-Ia gene in addition to the gene encoding the bifunctional enzyme AAC(6′)-APH(2"). These results support the idea that GS-MRSA strains could derive from GR-MRSA strains. Further studies should be undertaken to assess this hypothesis, since the results reported above may also indicate a spread of the ant(4′)-Ia gene.
Unintentional changes in the use of antibiotics observed during the past 10 years in our hospital probably played an important role in favoring the switch between the GR-MRSA strains, which are the most resistant MRSA strains, and GS-MRSA strains, which are the most susceptible. Changes in the use of antibiotics often lead to parallel changes in resistance patterns, sometimes with a delay of several years (10). The first outbreaks of GR-MRSA were described in 1975 to 1976, while gentamicin has been used since the early 1970s (9, 19). In our hospital, the progressive decrease in the use of gentamicin and cyclines between 1985 and 1990 likely facilitated the emergence of MRSA strains susceptible to these antibiotics in the early 1990s, i.e., the GS-MRSA strains belonging to clones A::1 and B::3, which represented 78% of the GS-MRSA strains at the beginning of the outbreak. The subsequent decrease in the use of macrolides could help the recent spread of the clone C::5, which is also susceptible to these antibiotics. Conversely, continuous or increasing selective pressure imposed by the extensive use of β-lactams, fluoroquinolones, and amikacin in our hospital likely explains the resistance to these antibiotics shared by all clones of GS-MRSA and GR-MRSA.
Finally, introduction of several distinct clones of GS-MRSA via patients transferred from other hospitals as well as by cross-infection, demonstrated by typing methods and traditional epidemiological investigation, also contributed to the emergence and spread of various clones in our hospital.
In conclusion, clonal dissemination of new epidemic GS-MRSA strains more susceptible to antibiotics, detected by phenotypic and genotypic methods, was associated with an unintentional change in the use of antibiotics. However, further studies should be undertaken to determine if these changes led to a loss of resistance genes in GR-MRSA strains that have been endemic for several years in our hospital.
ACKNOWLEDGMENTS
We are grateful to Y. Brun and M. Bes from the National Reference Laboratory for Staphylococci for their help in performing phage typing and serotyping.
This work was supported by the Institut National de la Santé et de la Recherche Médicale (grant CRI 950601).
REFERENCES
- 1.Ayliffe, G. A. J. 1997. The progressive intercontinental spread of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 24(Suppl. 1):S74–S79. [DOI] [PubMed]
- 2.Bannerman T L, Hancock G A, Tenover F C, Miller J M. Pulsed-field gel electrophoresis as a replacement for bacteriophage typing of Staphylococcus aureus. J Clin Microbiol. 1995;33:551–555. doi: 10.1128/jcm.33.3.551-555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bismuth R, Jarlier V, Sinègre M, Nguyen J, Sednaoui P. Relation entre la sensibilité bactérienne et la consommation d’antibiotiques. Presse Med. 1983;12:77–81. [PubMed] [Google Scholar]
- 4.Bismuth R, Vermee F, Drugeon H, Courvalin P. Program and abstracts of the 28th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1988. Influence of aminoglycoside-modifying enzymes on the bactericidal activity of aminoglycosides against staphylococci, abstr. 1331; p. 349. [Google Scholar]
- 5.Blair J E, Williams R E O. Phage typing of staphylococci. Bull W H O. 1961;24:771–784. [PMC free article] [PubMed] [Google Scholar]
- 6.Bouanchaud D H, Fouace J M, Bieth G. Physical studies of a Staphylococcus aureus plasmid mediating resistance to streptogramins, lincosamins and aminoglycosides. Ann Microbiol (Paris) 1977;128B:431–437. [PubMed] [Google Scholar]
- 7.Comité de l’Antibiogramme de la Société Française de Microbiologie. 1996. Zone sizes and MIC breakpoints for non-fastidious organisms. Clin. Microbiol. Infect. 2(Suppl. 1):S46–S49. [PubMed]
- 8.Fleurette, J., and A. Modjadedy. 1976. Attempts to combine and simplify two methods for serotyping of Staphylococcus aureus. Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. Abt. 1 Orig. 5(Suppl.):71–80.
- 9.McGowan J E, Terry P M, Huang T R S, Linstrom Houk C, Davies J. Nosocomial infections with gentamicin-resistant Staphylococcus aureus: plasmid analysis as an epidemiologic tool. J Infect Dis. 1979;140:864–872. doi: 10.1093/infdis/140.6.864. [DOI] [PubMed] [Google Scholar]
- 10.McGowan J E. Antimicrobial resistance in hospital organisms and its relation to antibiotic use. Rev Infect Dis. 1983;5:1033–1048. doi: 10.1093/clinids/5.6.1033. [DOI] [PubMed] [Google Scholar]
- 11.McKenzie T, Hoshino T, Tanaka T, Sueoka N. The nucleotide sequence of pUB110: some salient features in relation to replication and its regulation. Plasmid. 1986;15:93–103. doi: 10.1016/0147-619x(86)90046-6. [DOI] [PubMed] [Google Scholar]
- 12.Miller, G. H., F. J. Sabatelli, L. Naples, R. S. Hare, K. J. Shaw, and the Aminoglycoside Resistance Study Group. 1995. The most frequently occurring aminoglycoside resistance mechanisms—combined results of surveys in eight regions of the world. J. Chemother. 7(Suppl. 2):17–30. [PubMed]
- 13.Mlynarczyk G, Rosdahl V T, Skov R, Mlynarczyk A. Epidemiology of methicillin-resistant Staphylococcus aureus in a Warsaw hospital. J Hosp Infect. 1996;34:151–160. doi: 10.1016/s0195-6701(96)90141-3. [DOI] [PubMed] [Google Scholar]
- 14.Mulligan M E, Murray-Leisure K A, Ribner B S, Standiford H C, John J F, Korvick J A, Kauffman C A, Yu V L. Methicillin-resistant Staphylococcus aureus: a consensus review of the microbiology, pathogenesis, and epidemiology with implications for prevention and management. Am J Med. 1993;94:313–328. doi: 10.1016/0002-9343(93)90063-u. [DOI] [PubMed] [Google Scholar]
- 15.Norris S, Nightingale C H, Mandell G L. Tables of antimicrobial agent pharmacology. In: Mandel G L, Douglas R G, Bennett J E, editors. Principles and practice of infectious diseases. 3rd ed. New York, N.Y: Churchill Livingstone; 1990. pp. 434–460. [Google Scholar]
- 16.Ounissi H, Derlot E, Carlier C, Courvalin P. Gene homogeneity for aminoglycoside-modifying enzymes in gram-positive cocci. Antimicrob Agents Chemother. 1990;34:2164–2168. doi: 10.1128/aac.34.11.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prevost G, Jaulhac B, Piemont Y. DNA fingerprinting by pulsed-field gel electrophoresis is more effective than ribotyping in distinguishing among methicillin-resistant Staphylococcus aureus isolates. J Clin Microbiol. 1992;30:967–973. doi: 10.1128/jcm.30.4.967-973.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryffel C, Tesch W, Birch-Machin I, Reynolds P E, Barberis-Maino L, Kayser F H, Berger-Bächi B. Sequence comparison of mecA genes isolated from methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. Gene. 1990;94:137–138. doi: 10.1016/0378-1119(90)90481-6. [DOI] [PubMed] [Google Scholar]
- 19.Shanson D C, Kensit J G, Duke R. Outbreak of hospital infection with a strain of Staphylococcus aureus resistant to gentamicin and methicillin. Lancet. 1976;i:1347–1348. doi: 10.1016/s0140-6736(76)91986-3. [DOI] [PubMed] [Google Scholar]
- 20.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voss A, Milatovic D, Wallrauch-Schwarz C, Rosdahl V T, Braveny I. Methicillin-resistant Staphylococcus aureus in Europe. Eur J Clin Microbiol Infect Dis. 1994;13:50–55. doi: 10.1007/BF02026127. [DOI] [PubMed] [Google Scholar]