Abstract
We have previously described the creation by Tn916 mutagenesis of avirulent transposition mutants from a highly virulent strain of Erysipelothrix rhusiopathiae, the causative agent of swine erysipelas. In this study, we cloned a 2.2-kb DNA fragment which flanked the Tn916 insertion in an avirulent mutant (strain 33H6) and evaluated the possibility that this region could be used for the specific detection of E. rhusiopathiae. According to the sequences of this region, oligonucleotide primers were designed to amplify a 937-bp fragment of the E. rhusiopathiae chromosome by PCR. The specificity of the PCR was investigated by analyzing 64 strains of Erysipelothrix species and 27 strains of other genera different from Erysipelothrix. A 937-bp DNA fragment could be amplified from all E. rhusiopathiae strains tested, and no amplification was observed by using DNAs from the other species tested. To make a rapid and definite diagnosis of swine erysipelas in slaughterhouses, we developed an enrichment broth cultivation-PCR combination assay, which used a commercially available DNA extraction kit, to identify E. rhusiopathiae in the specimens from swine with arthritis. After samples were enriched in selective broth culture, detection of E. rhusiopathiae was tested by either conventional methods or the PCR. Of 102 samples tested, 15 samples were positive by conventional methods and 12 of the 15 samples were positive by the PCR. The detection limit of the PCR was 103 CFU per reaction mixture for the PCR-positive samples. These results indicate that this PCR technique could be used as a first-line screening technique for the specific detection of E. rhusiopathiae in specimens.
The gram-positive bacterium Erysipelothrix rhusiopathiae is widely distributed in nature and causes erysipelas in a variety of animals, including birds, and erysipeloid in humans (15). Erysipelas in swine, a severe disease causing great economic losses in the swine industry, may occur as an acute septicemia or chronic polyarthritis and endocarditis (16).
The genus Erysipelothrix has long been thought to be represented by the single species E. rhusiopathiae. Thus far, serovars 1 through 23 and type N have been described among isolates of E. rhusiopathiae. However, DNA-DNA hybridization by Takahashi et al. (11) showed that the genus Erysipelothrix comprises at least two distinct species, E. rhusiopathiae comprising serovars 1a, 1b, 2, 4, 5, 6, 8, 9, 11, 12, 15, 16, 17, 19, and 21 and type N and Erysipelothrix tonsillarum comprising serovars 3, 7, 10, 14, 20, 22, and 23. Moreover, serovars 13 and 18 are considered to be members of two separate and new species. Although these species are phenotypically very similar to each other (11), E. rhusiopathiae is the only species which causes disease in swine (9, 10–12) and chickens (13).
In Japan, the diagnosis of swine erysipelas in slaughterhouses is currently carried out by traditional methods, namely, the cultivation and subsequent identification of E. rhusiopathiae on the basis of growth and biochemical characteristics. However, these methods are time-consuming and laborious. Therefore, the development of a rapid and simple method for the diagnosis of the disease has been desired. Recently, Makino et al. (5) developed a PCR method based on the DNA sequence encoding 16S rRNA for the diagnosis of swine erysipelas. However, this method could not differentiate between E. rhusiopathiae and E. tonsillarum. E. tonsillarum strains are sometimes isolated from healthy pigs (9, 10, 12); therefore, it is very important to distinguish E. rhusiopathiae from E. tonsillarum.
Using transposition mutagenesis with Tn916, we isolated avirulent transposition mutants from a highly virulent E. rhusiopathiae Fujisawa strain (8). The avirulent mutant strain, designated 33H6, reverted to virulence when the transposon was excised from the chromosome, suggesting that the genetic region interrupted by the Tn916 insertion may be closely related to virulence. In this study, we cloned and sequenced the genetic region flanking the Tn916 insertion in 33H6 to design PCR primers and examined its potential use for the specific detection of E. rhusiopathiae. Furthermore, we evaluated the use of PCR for the detection of E. rhusiopathiae in specimens from swine as a rapid and reliable method for routine use for the diagnosis of swine erysipelas.
MATERIALS AND METHODS
Bacterial strains.
The Erysipelothrix strains and other microorganisms used in this study are listed in Tables 1 and 2, respectively. An avirulent transposition mutant strain, strain 33H6 (8), was used to clone the region flanking the Tn916 insertion site.
TABLE 1.
Erysipelothrix strains used in this study
| Strain | Serovar |
|---|---|
| E. rhusiopathiae | |
| Fujisawa | 1a |
| 422/1E1 | 1b |
| ATCC 19414T | 2 |
| SE-9 | 2 |
| Doggerscharbe | 4 |
| Pécs 67 | 5 |
| Tuzok | 6 |
| Goda | 8 |
| Kaparek | 9 |
| 14B | 9 |
| IV 12/8 | 11 |
| Pécs 9 | 12 |
| Pécs 3597 | 15 |
| Tanzania | 16 |
| 545 | 17 |
| 2017 | 19 |
| Bãno 36 | 21 |
| MEW 22 | N |
| Field isolates (19)a | 1a |
| Field isolates (5) | 2 |
| Field isolates (6) | 6 |
| E. tonsillarum | |
| Wittling | 3 |
| ATCC 43339T | 7 |
| Lengyel-P | 10 |
| 2179 | 10 |
| Iszap-4 | 14 |
| 2553 | 20 |
| Bãno 107 | 22 |
| KS20A | 23 |
| Field isolates (6) | 7 |
| Erysipelothrix spp. | |
| Pécs 56 | 13 |
| 715 | 18 |
The numbers in parentheses indicate the number of strains tested.
TABLE 2.
Non-Erysipelothrix strains used in this study
| Organism group and strain |
|---|
| Gram-positive bacteria |
| Bacillus anthracis Pasteur II |
| Enterococcus faecalis NCTC 775 |
| Listeria monocytogenes EGD |
| Rhodococcus equi ATCC 33701 |
| Staphylococcus aureus ATCC 12600 |
| Streptococcus agalactiae NCTC 11360 |
| Streptococcus dysgalactiae NCDO 2023 |
| Streptococcus pneumoniae NCTC 7465 |
| Streptococcus porcinus NCTC 10228 |
| Streptococcus pyogenes ATCC 12344 |
| Streptococcus suis NCTC 10234 |
| Gram-negative bacteria |
| Actinobacillus pleuropneumoniae 4074 |
| Actinobacillus suis CCM 5586 |
| Actinomyces pyogenes ATCC 19411 |
| Bordetella bronchiseptica A19 |
| Escherichia coli K-12 |
| Haemophilus influenzae ATCC 9795 |
| Haemophilus parasuis ATCC 19417 |
| Klebsiella pneumoniae GN407 rif |
| Pasteurella multocida ATCC 43017 |
| Pseudomonas aeruginosa PAO 9502 |
| Salmonella choleraesuis AHI-5190 |
| Salmonella typhimurium LT2 |
| Yersinia enterocolitica 7-3 rif |
| Mycoplasmas |
| Mycoplasma hyopneumoniae J |
| Mycoplasma hyorhinis BTS7 |
| Mycoplasma hyosynoviae S16 |
DNA preparation.
Total DNAs from the Erysipelothrix strains listed in Table 1 were prepared by the method of Gálan and Timoney (2). Total DNAs from the microorganisms listed in Table 2 were extracted as described by Graves and Swaminathan (4).
Cloning of the DNA region flanking the Tn916 inserted in 33H6.
Standard recombinant DNA procedures were performed as described elsewhere (6). Chromosomal DNA from transposition mutant 33H6 was digested with the restriction enzyme EcoRI, which does not cut within the Tn916 sequence, ligated to EcoRI-digested pUC19 (Takara, Tokyo, Japan), and then transformed into Escherichia coli JM109 (Takara). Plasmid preparations from colonies that grew on Luria-Bertani (LB; Difco Laboratories, Detroit, Mich.) agar supplemented with tetracycline (10 μg/ml) and ampicillin (50 μg/ml) were confirmed to possess Tn916 by Southern hybridization with pAM120 (3) as a probe. A recombinant plasmid (pYS186) was obtained and was used for further experiments.
One unique property of Tn916 is its ability to be excised from the recombinant plasmid when it is cloned in E. coli in the absence of tetracycline, leaving the vector and the sequences flanking Tn916. This property was used for cloning of a region interrupted by the Tn916 insertion (3). The E. coli cells harboring pYS186 were grown in LB medium containing ampicillin but not tetracycline. EcoRI digestion of the plasmid DNA from the resultant culture showed a 2.7-kb fragment, corresponding to the pUC19 vector, and a 2.2-kb EcoRI fragment, corresponding to the DNA sequences that flanked Tn916, indicating that the 2.2-kb EcoRI fragment of the plasmid arose after Tn916 was excised from pYS186. The plasmid was designated pYS22, and the DNA sequence of its insert was determined.
Southern hybridization.
Southern hybridization was performed as described previously (8) with the DIG DNA Labeling and Detection kit (Boehringer Mannheim-Yamanouchi, Tokyo, Japan). The amplified 937-bp DNA fragment from E. rhusiopathiae Fujisawa was cloned into pCR II (Original TA Cloning Kit; Invitrogen). The insert of pCR II was labeled with digoxigenin as described previously (8) and was used as a probe.
Nucleotide sequence and data analyses.
Nucleotide sequencing was performed by the dideoxy-sequencing technique of Sanger et al. (7) by using a model 377 DNA sequencer (Applied Biosystems, Inc., Foster City, Calif.). Oligonucleotide primers consisting of regions approximately every 100 to 300 bases along both strands of the templates in plasmid pYS22 were synthesized. DNA data were analyzed by the GENETYX system, version 7.3 (SDC, Tokyo, Japan).
PCR primers.
Oligonucleotide primer sequences were designed from the nucleotide sequence of the insert of pYS22 and were synthesized on a model 391 DNA synthesizer (Applied Biosystems). The primers used were ER1 (5′-CGATTATATTCTTAGCACGCAACG-3′) and ER2 (5′-TGCTTGTGTTGTGATTTCTTGACG-3′).
DNA amplification.
Amplification reaction mixtures were prepared in a volume of 50 μl containing 10 mM Tris-HCl (pH 8.3); 50 mM KCl; 1.0 mM MgCl2; bovine serum albumin (10 μg/ml); 0.1% Triton X-100; 200 μM (each) dATP, dCTP, dGTP, and dTTP (Perkin-Elmer); 1.0 μM (each) primer; 2.5 U of Taq DNA polymerase (Amplitaq; Perkin-Elmer); and 50 ng of template DNA. PCR consisting of denaturation at 94°C for 1 min, annealing at 63°C for 30 s, and extension at 72°C for 1 min was performed for 30 cycles on a DNA thermocycler (Perkin-Elmer). The amplified products were separated on 0.8% agarose gels and were stained with ethidium bromide.
Detection of E. rhusiopathiae in specimens.
Synovial fluid (0.5 to 1.5 ml) or swabs of arthritis lesions from swine with arthritis were cultured in 10 ml of tryptic soy broth (pH 7.6) (Difco) supplemented with 0.1% Tween 80, 0.3% Tris(hydroxymethyl)aminomethane, crystal violet (5 μg/ml), and 0.03% sodium azide for selective enrichment. The broths were incubated at 37°C for 24 h, plated onto tryptic soy agar (Difco) supplemented with 5% horse blood, and then incubated at 37°C for a further 24 h. After incubation, suspected colonies were isolated and their H2S production in sulfide-indole-motility (SIM) agar (Nissui, Tokyo, Japan) supplemented with 0.1% Tween 80 was confirmed. In parallel, the enriched broth cultures were subjected to the PCR assay.
PCR with enriched samples.
To avoid cross-contamination of samples, sample handling was minimized. Samples (1.0 ml of enriched broth cultures) were introduced into microcentrifuge tubes and were pelleted by centrifugation. The bacterial cells were resuspended in 50 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]) containing lysozyme (10 mg/ml) and N-acetylmuramidase SG (50 μg/ml; Seikagaku Kogyo Co., Tokyo, Japan) and were then incubated at 37°C for 1 h. After incubation, 100 μl of proteinase K (1 mg/ml in TE buffer) was added, and the mixture was incubated for a further 30 min. The samples were pelleted by centrifugation, and this was followed by the DNA extraction procedures with the InstaGene Matrix (Bio-Rad Laboratories Hercules, Calif.) according to the manufacturer’s instructions. Briefly, the bacterial pellet was resuspended in 200 μl of InstaGene matrix, and the mixture was incubated at 56°C for 30 min. The tube was vortexed for 10 s and placed in boiling water for 8 min. Next, the tube was vortexed for 10 s and centrifuged, and 10 μl of the resulting supernatant was used in the PCR mixture.
Nucleotide sequence accession number.
The sequence data were submitted to the DDBJ/EMBL/GenBank database, and the sequence was assigned accession no. D64177.
RESULTS
Cloning and sequencing of the DNA region flanking the Tn916 insertion in 33H6.
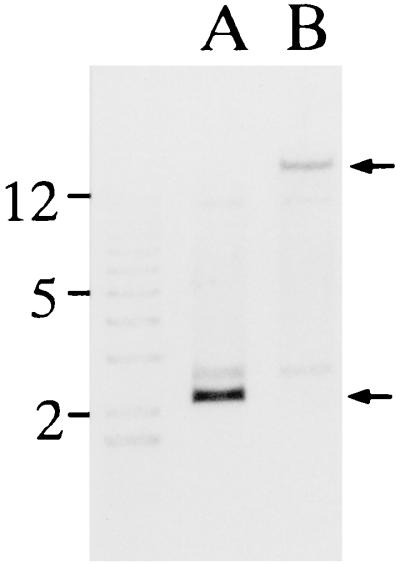
Southern hybridization analysis of chromosomal DNA from transposition mutant strain 33H6 indicated that a single Tn916 insertion occurred within a 18.6-kb EcoRI fragment of the 33H6 chromosome (8). The 18.6-kb EcoRI fragment of 33H6 chromosomal DNA including the entire Tn916 transposon was cloned into pUC19 to generate pYS186. By culturing the E. coli cells harboring pYS186 in medium containing ampicillin but not tetracycline, Tn916 was excised from pYS186 to generate pYS22. When the genomic DNA either from the parent strain (strain Fujisawa) or from 33H6 was digested with EcoRI and examined by Southern hybridization by using the digoxigenin-labeled 2.2-kb EcoRI fragment of plasmid pYS22 as a probe, the probe hybridized only to a single EcoRI fragment of 2.2 kb in Fujisawa and to a fragment of 18.6 kb in 33H6, demonstrating that the 2.2-kb EcoRI fragment of pYS22 arose after Tn916 was excised from pYS186 (Fig. 1). The insert region of pYS22 was subsequently sequenced, and the primer sets ER1 and ER2, which are expected to amplify a 937-bp fragment of the E. rhusiopathiae chromosome, were chosen for PCR amplification.
FIG. 1.
Southern hybridization analysis of EcoRI-digested genomic DNAs from strain Fujisawa (lane A) and 33H6 (lane B) probed with the digoxigenin-labeled 2.2-kb EcoRI fragment of plasmid pYS22. The arrows indicate the 18.6-kb (top) and 2.2-kb (bottom) EcoRI fragments. Molecular size markers (1-kb ladder; GIBCO-BRL) are indicated on the left (in kilobases).
Specificity of the PCR.
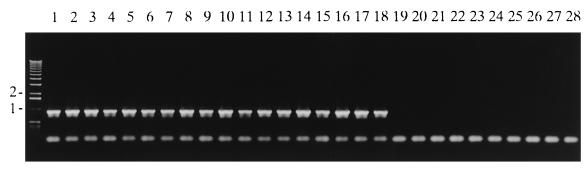
To test the specificity of the PCR detection system, we examined a total of 64 strains including all serovars of the Erysipelothrix species listed in Table 1. The PCR of DNAs from all strains of E. rhusiopathiae tested amplified a single fragment corresponding to 937 bp, but no amplification was observed by using DNAs from strains from the species other than E. rhusiopathiae. Results for 28 of the samples are depicted in Fig. 2. All of the amplified bands hybridized with the probe prepared from E. rhusiopathiae Fujisawa DNA (data not shown). These results demonstrate that the DNA fragments were specifically amplified from the homologous DNA sequences in E. rhusiopathiae and, thus, that the PCR system is specific for E. rhusiopathiae.
FIG. 2.
Specificity of PCR amplification. PCR products were detected by electrophoresis on an agarose gel. The samples were Fujisawa (lane 1), 422/1E1 (lane 2), ATCC 19414T (lane 3), SE-9 (lane 4), Doggerscharbe (lane 5), Pécs 67 (lane 6), Tuzok (lane 7), Goda (lane 8), Kaparek (lane 9), 14B (lane 10), IV 12/8 (lane 11), Pécs 9 (lane 12), Pécs 3597 (lane 13), Tanzania (lane 14), 545 (lane 15), 2017 (lane 16), Bãno 36 (lane 17), MEW 22 (lane 18), Wittling (lane 19), ATCC 43339T (lane 20), Lengyel-P (lane 21), 2179 (lane 22), Iszap-4 (lane 23), 2553 (lane 24), Bãno 107 (lane 25), KS20A (lane 26), Pécs 56 (lane 27), 715 (lane 28). Molecular size markers (1-kb ladder; GIBCO-BRL) are indicated on the left (in kilobases).
We extended the PCR technique to strains of other genera, listed in Table 2, including microorganisms able to cause arthritis and endocarditis in swine. All samples tested were negative (data not shown), confirming the specificity of the PCR system.
Detection of E. rhusiopathiae in specimens by cultivation-PCR combination assay.
Cultivation of the specimens in enrichment broth is usually conducted for the detection of E. rhusiopathiae in specimens collected from swine in slaughterhouses with clinical signs of arthritis or endocarditis. To make a rapid and practical procedure for routine use for diagnostic purposes, we combined a PCR which used a commercially available DNA extraction kit (InstaGene Matrix) with cultivation in enrichment broth to specifically detect the bacteria in the specimens. In preliminary experiments, we found that when the InstaGene Matrix alone was used to prepare DNA from the serial 10-fold dilutions of the enriched samples, the detection limit of the PCR was approximately 105 CFU per reaction tube (data not shown). To increase the efficiency of DNA extraction from the specimens, we used lysozyme, N-acetylmuramidase, and proteinase K prior to the InstaGene Matrix procedures. When these enzymes were used, the detection limit of the PCR was at least 103 CFU per reaction tube (data not shown).
After a total of 102 specimens of synovial fluid or arthritis lesion were enriched in selective medium, they were subjected to either conventional culture techniques or PCR testing for the detection of E. rhusiopathiae. Of 102 samples tested, suspected Erysipelothrix colonies were isolated from 15 samples. These colonies were characterized as Erysipelothrix bacteria by confirming their H2S production in SIM agar, and they were eventually identified as E. rhusiopathiae by PCR testing. Of 102 enriched samples, the PCR detected the bacteria in 12 of the 15 samples which were positive by conventional methods. DNA was amplified from all of the samples in reaction tubes which contained 103 CFU or more of the bacteria (data not shown).
DISCUSSION
Diagnosis of E. rhusiopathiae infection in swine is currently based primarily on cultivation and identification of the bacteria from specimens, such as synovial fluid and lymph nodes from animals. However, identification of E. rhusiopathiae by traditional methods is a laborious, time-consuming procedure that can take up to 3 to 4 days. Therefore, a more rapid and simple method for the identification of E. rhusiopathiae from the specimens is needed. Although a previous study (5) demonstrated that PCR based on the DNA sequence encoding 16S rRNA can be used to detect Erysipelothrix strains, the method could not differentiate E. rhusiopathiae from E. tonsillarum. Because the frequency of isolation of E. tonsillarum from healthy pigs is high (9, 10, 12), discrimination of these closely related species is very important.
In this study, we used primers designed from the sequences of chromosomal loci which are presumably associated with the virulence of E. rhusiopathiae. The primers were specifically selected because their use did not result in nonspecific reactions with DNAs from E. tonsillarum strains, and the specificity of each primer was investigated by using 48 strains of E. rhusiopathiae and 14 strains of E. tonsillarum. PCR assays with the primers could successfully differentiate between E. rhusiopathiae and E. tonsillarum, demonstrating that the PCR system is an improvement over the present methods of diagnosing swine erysipelas. Furthermore, no amplification was observed with strains of serovars 13 and 18, which are proposed to be new distinct species (11). These results suggested that the PCR system may differentiate E. rhusiopathiae from not only E. tonsillarum but also new Erysipelothrix species. However, the very few strains belonging to serovars 13 and 18 were tested. More strains belonging to these serovars must be collected and tested to support this hypothesis.
The specificity of this PCR system was further investigated with a number of microorganisms including pathogens, such as Streptococcus suis, Mycoplasma hyosynoviae, and Mycoplasma hyorhinis, which can cause arthritis or endocarditis in swine. The results obtained with all samples of these microorganisms tested were negative, confirming that this PCR system can be applied to specimens from animals with suspected erysipelas infection.
The PCR assay was evaluated with specimens of synovial fluid from swine with arthritis. Some studies have described PCR procedures that detect pathogenic organisms directly from clinical specimens. However, the application of such methods to swine erysipelas appeared to be difficult because low levels of E. rhusiopathiae bacteria are common in specimens from animals with chronic arthritis and direct detection of E. rhusiopathiae from the specimens is difficult (14). Therefore, we combined the PCR with cultivation in enrichment broth to detect E. rhusiopathiae from the specimens from animals. This method is advantageous because cultivation of specimens from animals in enrichment broth is a routine practice in diagnostic laboratories and the bacteria obtained by the enrichment are sometimes used for other tests such as antibiotic susceptibility testing and serovar determination. For the rapid and easy preparation of DNA from the enriched samples, we used lysozyme, N-acetylmuramidase, and proteinase K treatment prior to the InstaGene Matrix procedures. The detection limit of the PCR with clinical specimens was 103 CFU per reaction mixture. Although the sensitivity of the PCR appeared to be low, it could be further improved by using a larger sample volume or extending the cultivation period.
In conclusion, we developed a PCR based on the sequence of the genetic region presumably related to the virulence of E. rhusiopathiae for the rapid and accurate identification of E. rhusiopathiae. In addition, we combined the PCR with cultivation in enrichment broth to detect E. rhusiopathiae in clinical specimens. The method developed in this study requires minimal sample manipulation for DNA extraction. Nevertheless, it is simple to perform and can be used as a first-line screening technique for the specific detection of E. rhusiopathiae in a large number of specimens, which sometimes must be processed in a relatively short period of time.
ACKNOWLEDGMENTS
We thank Y. Ike for the kind gift of pAM120. We also thank T. Takahashi, S. Iwamatsu, H. Ishikawa, I. Uchida, M. Osaki, and H. Kobayashi for kind donations of strains.
This work was supported in part by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan.
REFERENCES
- 1.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gálan J E, Timoney J F. Cloning and expression in Escherichia coli of a protective antigen of Erysipelothrix rhusiopathiae. Infect Immun. 1990;58:3116–3121. doi: 10.1128/iai.58.9.3116-3121.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gawron-Burke C, Clewell D B. Regeneration of insertionally inactivated streptococcal DNA fragments after excision of transposon Tn916 in Escherichia coli: strategy for targeting and cloning of genes from gram-positive bacteria. J Bacteriol. 1984;159:214–221. doi: 10.1128/jb.159.1.214-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graves L M, Swaminathan B. Universal bacterial DNA isolation procedure. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology: principles and applications. Washington, D.C: American Society for Microbiology; 1993. pp. 617–621. [Google Scholar]
- 5.Makino S, Okada Y, Maruyama T, Ishikawa K, Takahashi T, Nakumura M, Ezaki T, Morita H. Direct and rapid detection of Erysipelothrix rhusiopathiae DNA in animals by PCR. J Clin Microbiol. 1994;32:1526–1531. doi: 10.1128/jcm.32.6.1526-1531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 7.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;86:699–703. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimoji Y, Yokomizo Y, Sekizaki T, Mori Y, Kubo M. Presence of a capsule in Erysipelothrix rhusiopathiae and its relationship to virulence for mice. Infect Immun. 1994;62:2806–2810. doi: 10.1128/iai.62.7.2806-2810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi T, Fujisawa T, Benno Y, Tamura Y, Sawada T, Suzuki S, Muramatsu M, Mitsuoka T. Erysipelothrix tonsillarum sp. nov. isolated from tonsils of apparently healthy pigs. Int J Syst Bacteriol. 1987;37:166–168. [Google Scholar]
- 10.Takahashi T, Sawada T, Muramatsu M, Tamura Y, Fujisawa T, Benno Y, Mitsuoka T. Serotype, antimicrobial susceptibility, and pathogenicity of Erysipelothrix rhusiopathiae isolates from tonsils of apparently healthy slaughter pigs. J Clin Microbiol. 1987;25:536–539. doi: 10.1128/jcm.25.3.536-539.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi T, Fujisawa T, Tamura Y, Suzuki S, Muramatsu M, Sawada T, Benno Y, Mitsuoka T. DNA relatedness among Erysipelothrix rhusiopathiae strains representing all twenty-three serovars and Erysipelothrix tonsillarum. Int J Syst Bacteriol. 1992;42:469–473. doi: 10.1099/00207713-42-3-469. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi T, Nagamine N, Kijima M, Suzuki S, Takagi M, Tamura Y, Nakamura M, Muramatsu M, Sawada T. Serovars of Erysipelothrix strains isolated from pigs affected with erysipelas in Japan. J Vet Med Sci. 1996;58:587–589. doi: 10.1292/jvms.58.587. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi T, Takagi M, Yamaoka R, Ohishi K, Norimatsu M, Tamura Y, Nakamura M. Comparison of the pathogenicity for chickens of Erysipelothrix rhusiopathiae and Erysipelothrix tonsillarum. Avian Pathol. 1994;23:237–245. doi: 10.1080/03079459408418992. [DOI] [PubMed] [Google Scholar]
- 14.Timoney J F, Jr, Berman D T. Erysipelothrix arthritis in swine: bacteriologic and immunopathologic aspects. Am J Vet Res. 1970;31:1411–1421. [PubMed] [Google Scholar]
- 15.Wood R L. Swine erysipelas—a review of prevalence and research. J Am Vet Med Assoc. 1984;184:944–949. [PubMed] [Google Scholar]
- 16.Wood R L. Erysipelas. In: Leman A D, et al., editors. Diseases of swine. Ames: Iowa State University Press; 1992. pp. 475–486. [Google Scholar]