Abstract
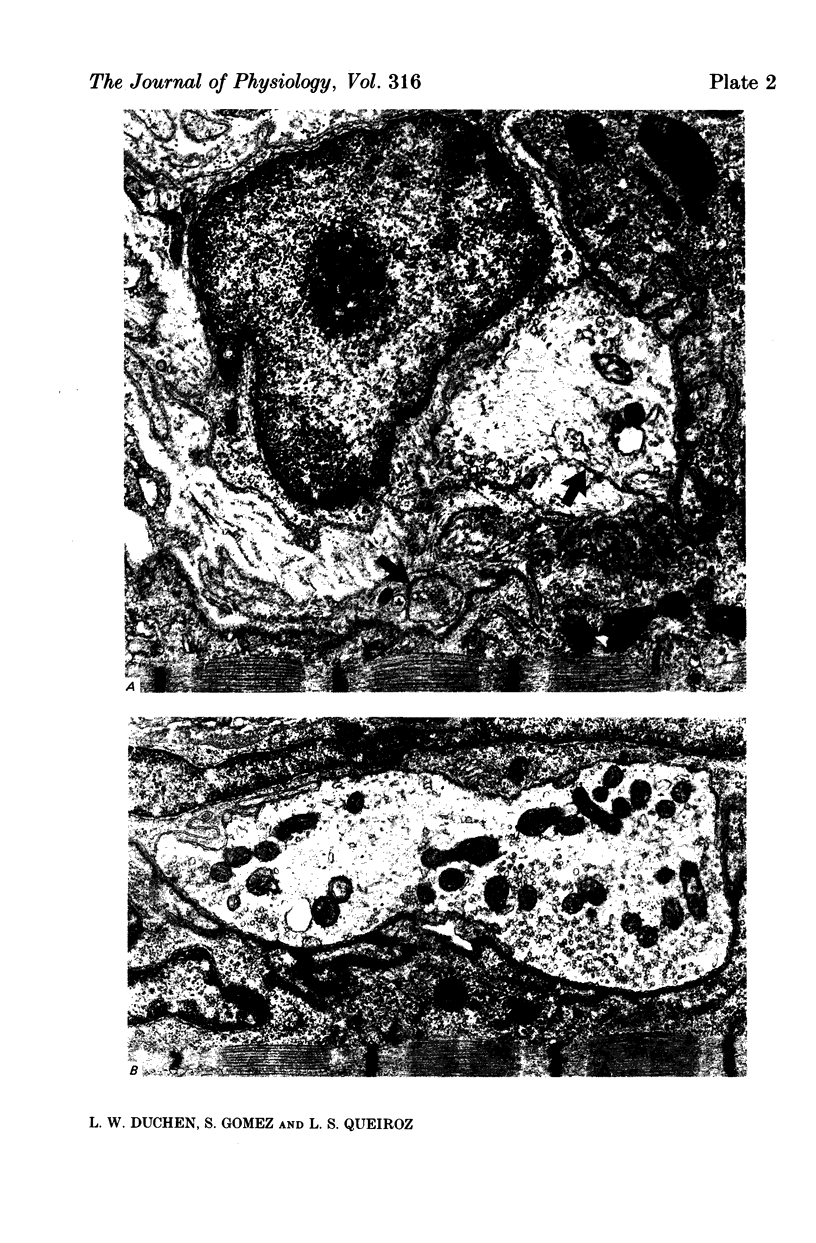
1. A sublethal quantity of black widow spider venom was injected into the calf muscles of mice. After 30 min to 6 weeks soleus muscles were examined by light and electron microscopy and by electrophysiological techniques. 2. Within 30 min motor nerve terminals were swollen and depleted for synaptic vesicles and by 6 h were disrupted and engulfed by Schwann cells. By 24 h every end-plate examined was denervated. Some preterminal myelinated axons also showed degenerative changes. 3. Re-innervation was first seen at 2 days. By 3 days axon terminals were present at most end-plates and by 8 days their morphology was nearly normal. The normal pattern of innervation of the muscle was re-established in that axons re-innervated their original end-plates and very few ultraterminal axonal sprouts were found. 4. Physiological study showed complete failure of transmission and absence of miniature end-plate potentials (m.e.p.p.s) and end-plate potentials (e.p.p.s) until day 3, when muscles responded weakly to indirect stimulation and m.e.p.p.s were recorded at 30% and e.p.p.s at 40% of fibres. The mean quantal content of e.p.p.s was low and there was rapid fatigue on repetitive stimulation. Extrajunctional sensitivity to acetylcholine developed within 1 day, was maximal at 3 days and declined to normal at 12-14 days. 5. The proportion of fibres at which m.e.p.p.s and e.p.p.s were recorded returned to normal by day 6 and mean quantal content was normal by day 9. 6. These findings show that the re-innervation of original end-plates is of importance in facilitating the rapid return of transmission to normal levels and limiting the extent of axonal growth.
Full text
PDF




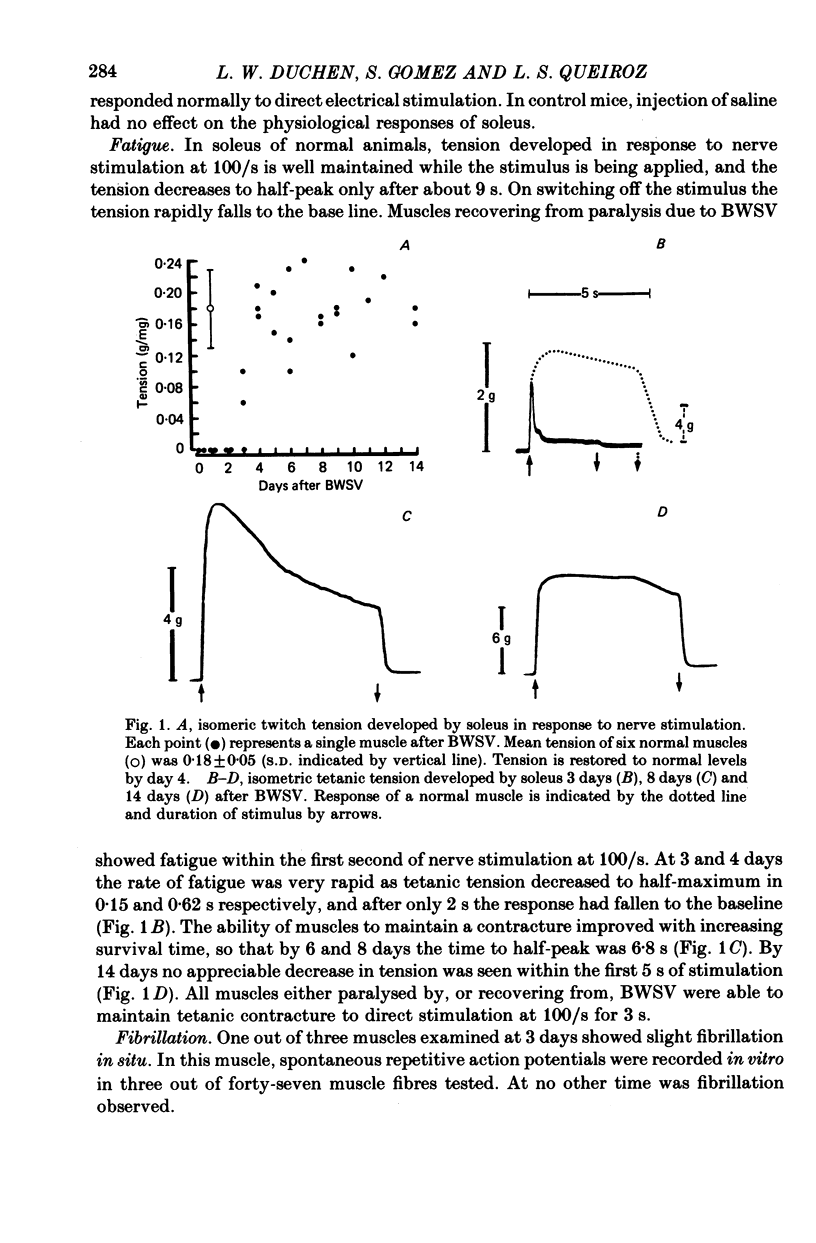
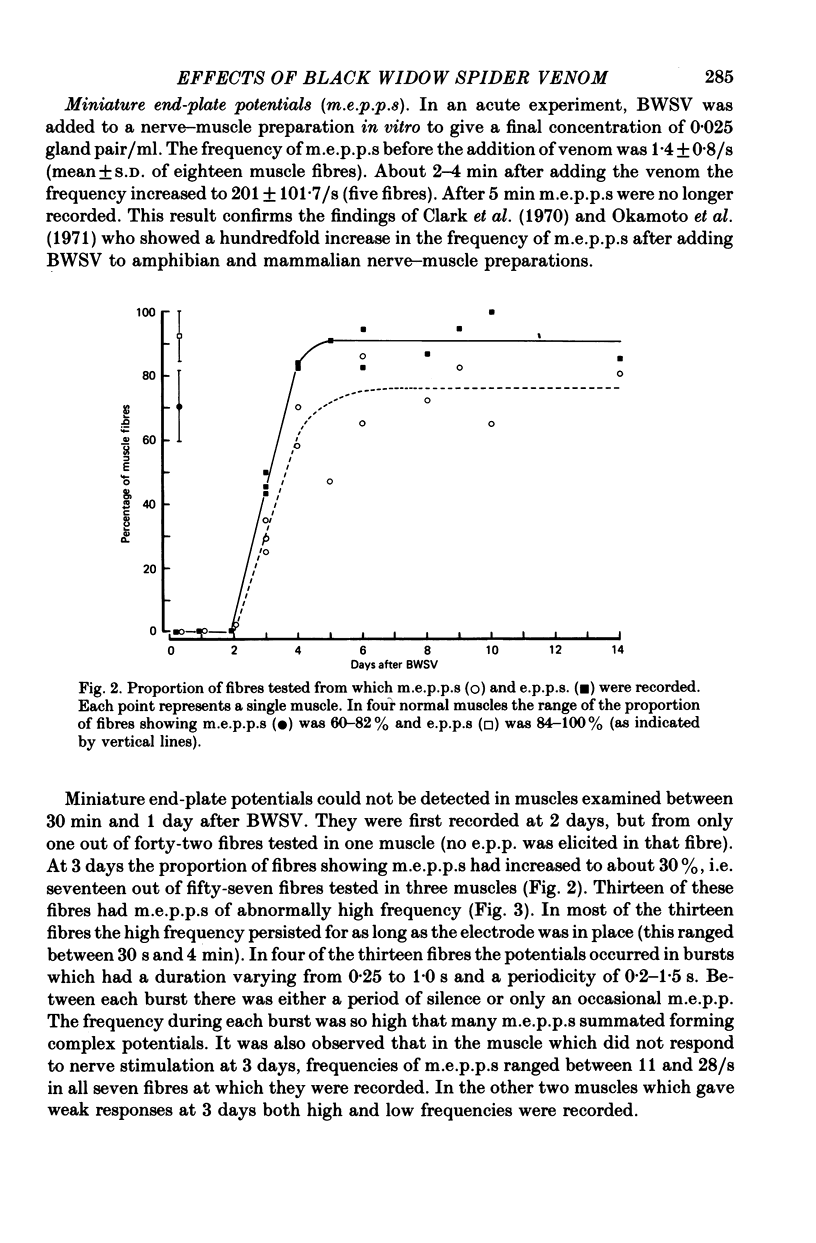
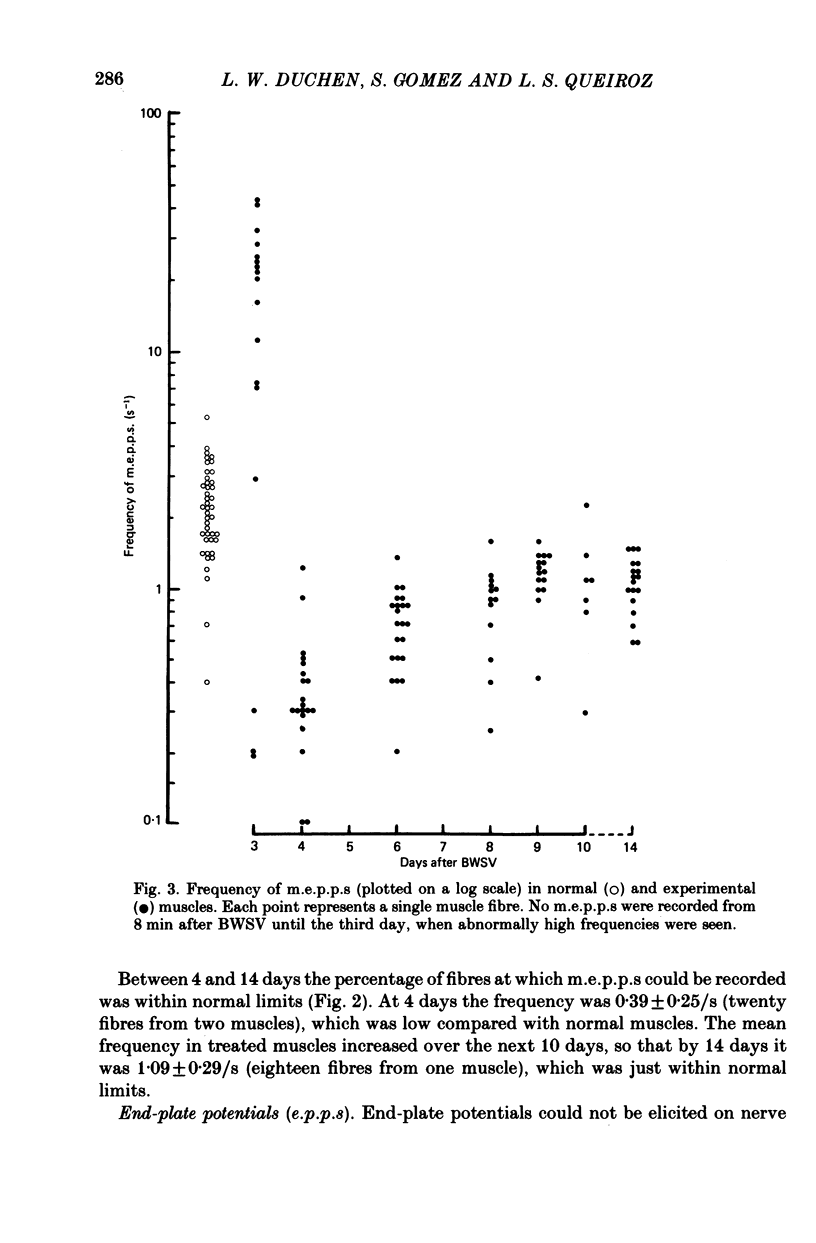
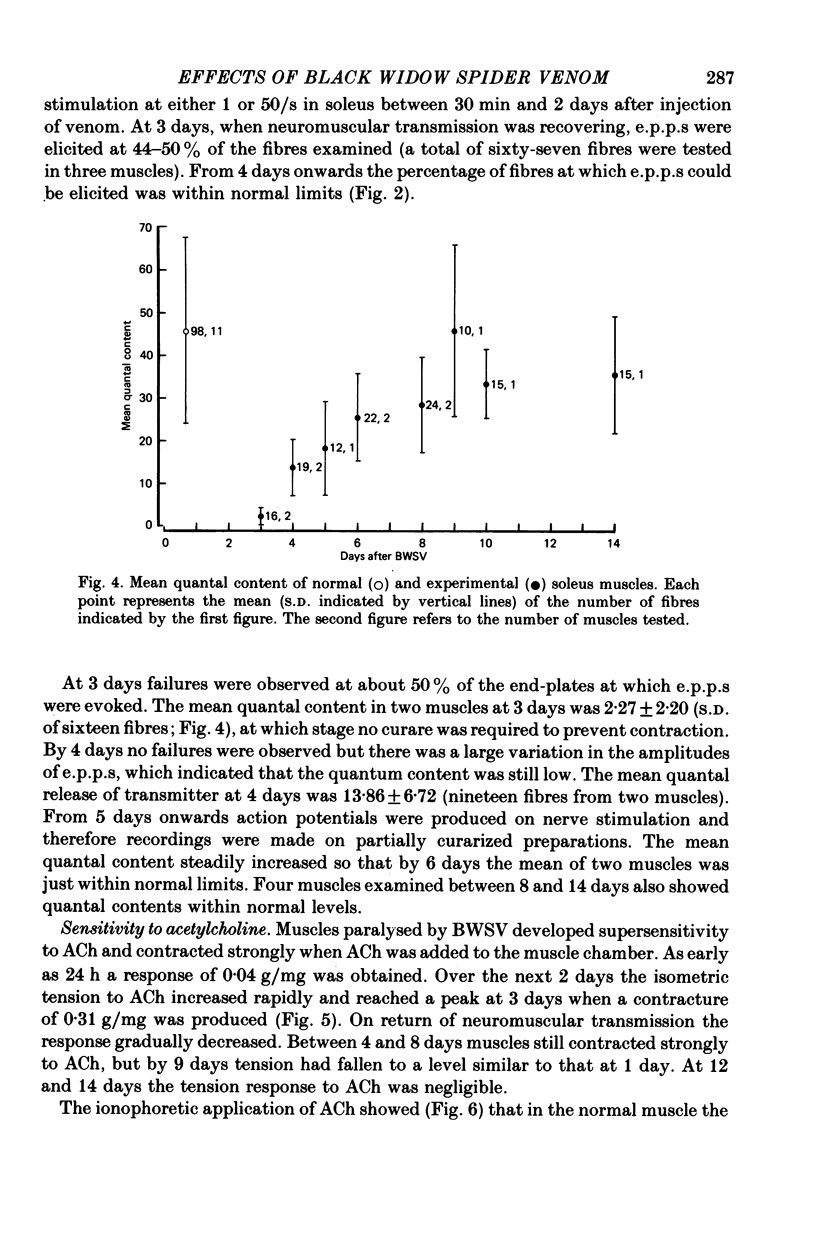
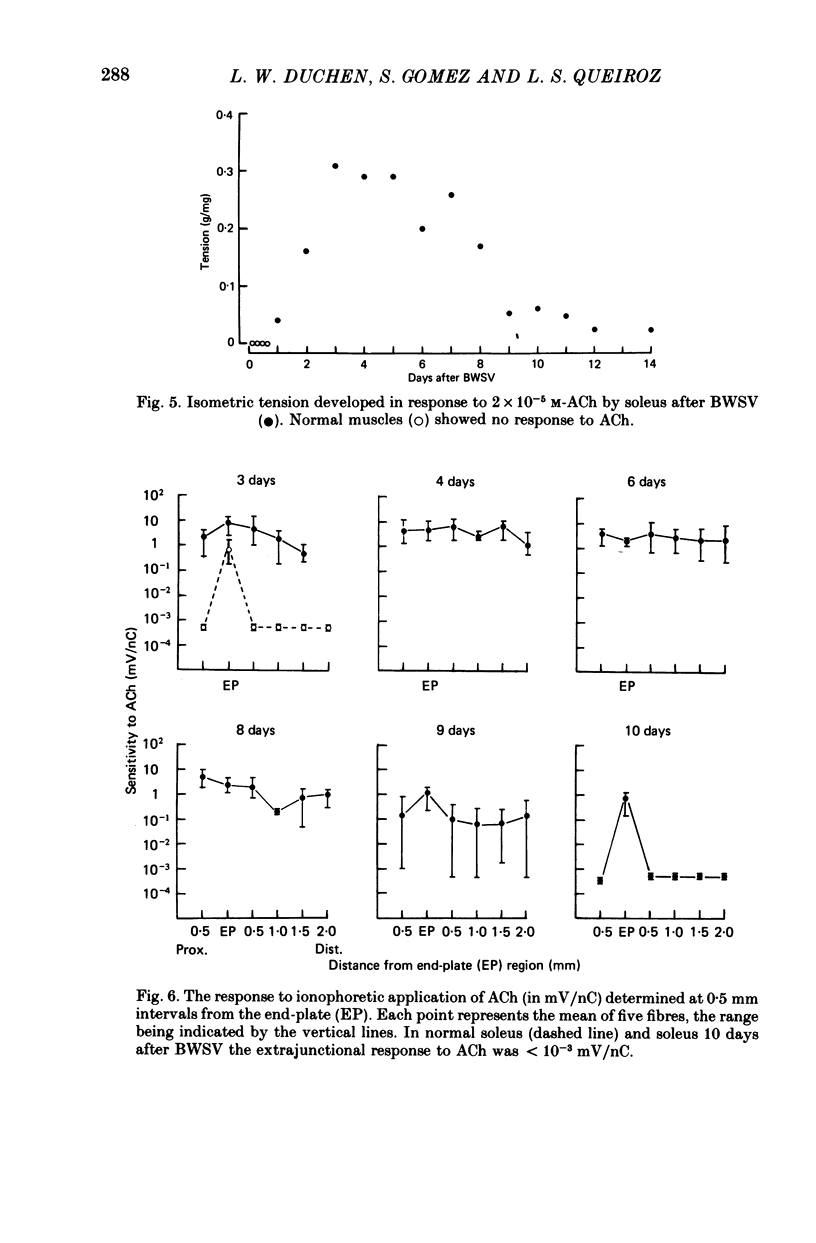


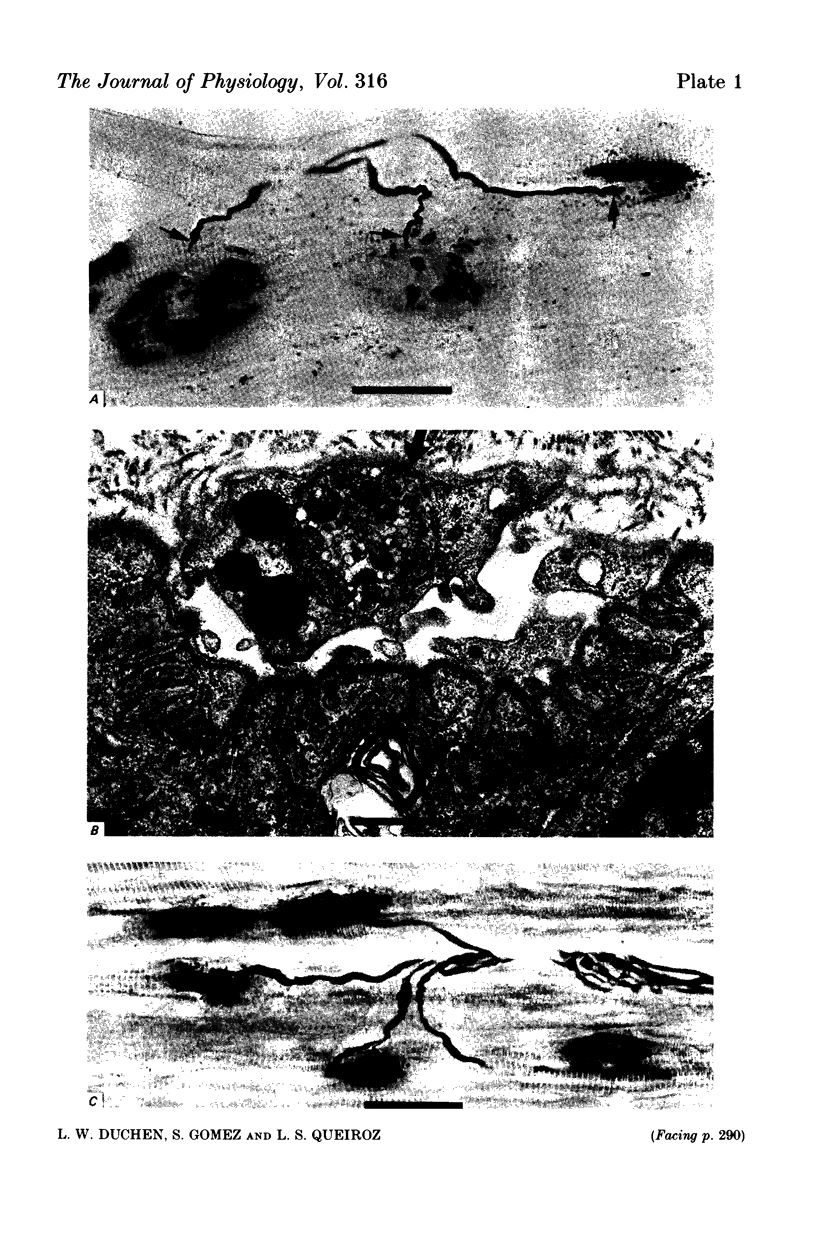

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Limbrick A. R., Miledi R. Acute muscle denervation induced by beta-bungarotoxin. Proc R Soc Lond B Biol Sci. 1976 Nov 12;194(1117):545–553. doi: 10.1098/rspb.1976.0093. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M., Taylor R. S. The formation of synapses in reinnervated mammalian striated muscle. J Physiol. 1973 Sep;233(3):481–500. doi: 10.1113/jphysiol.1973.sp010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Grohovaz F., Hurlbut W. P. Freeze-fracture studies of frog neuromuscular junctions during intense release of neurotransmitter. I. Effects of black widow spider venom and Ca2+-free solutions on the structure of the active zone. J Cell Biol. 1979 Apr;81(1):163–177. doi: 10.1083/jcb.81.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. W., Hurlbut W. P., Mauro A. Changes in the fine structure of the neuromuscular junction of the frog caused by black widow spider venom. J Cell Biol. 1972 Jan;52(1):1–14. doi: 10.1083/jcb.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. W., Mauro A., Longenecker H. E., Jr, Hurlbut W. P. Effects of black widow spider venom on the frog neuromuscular junction. Effects on the fine structure of the frog neuromuscular junction. Nature. 1970 Feb 21;225(5234):703–705. doi: 10.1038/225703a0. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontali N., Ceccarelli B., Gorio A., Mauro A., Siekevitz P., Tzeng M. C., Hurlbut W. P. Purification from black widow spider venom of a protein factor causing the depletion of synaptic vesicles at neuromuscular junctions. J Cell Biol. 1976 Mar;68(3):462–479. doi: 10.1083/jcb.68.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOELLE G. B., FRIEDENWALD J. A. A histochemical method for localizing cholinesterase activity. Proc Soc Exp Biol Med. 1949 Apr;70(4):617–622. doi: 10.3181/00379727-70-17013. [DOI] [PubMed] [Google Scholar]
- Kao I., Drachman D. B., Price D. L. Botulinum toxin: mechanism of presynaptic blockade. Science. 1976 Sep 24;193(4259):1256–1258. doi: 10.1126/science.785600. [DOI] [PubMed] [Google Scholar]
- Kawai N., Mauro A., Grundfest H. Effect of black widow spider venom on the lobster neuromuscular junctions. J Gen Physiol. 1972 Dec;60(6):650–664. doi: 10.1085/jgp.60.6.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig J., Pecot-Dechavassine M. Relations entre l'apparition des potentiels miniatures spontanes et l'ultrastructure des plaques motrices en voie de reinnervation et de neoformation chez le rat. Brain Res. 1971 Mar 19;27(1):43–57. doi: 10.1016/0006-8993(71)90371-4. [DOI] [PubMed] [Google Scholar]
- Lüllmann-Rauch R. The regeneration of neuromuscular junctions during spontaneous re-innervation of the rat diaphragm. Z Zellforsch Mikrosk Anat. 1971;121(4):593–603. doi: 10.1007/BF00560162. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. Electrophysiology and electron-microscopy of rat neuromuscular junctions after nerve degeneration. Proc R Soc Lond B Biol Sci. 1968 Feb 27;169(1016):289–306. doi: 10.1098/rspb.1968.0012. [DOI] [PubMed] [Google Scholar]
- Miledi R., Zelená J. Sensitivity to acetylcholine in rat slow muscle. Nature. 1966 May 21;210(5038):855–856. doi: 10.1038/210855a0. [DOI] [PubMed] [Google Scholar]
- Namba T., Nakamura T., Grob D. Staining for nerve fiber and cholinesterase activity in fresh frozen sections. Am J Clin Pathol. 1967 Jan;47(1):74–77. doi: 10.1093/ajcp/47.1.74. [DOI] [PubMed] [Google Scholar]
- Okamoto M., Longenecker H. E., Jr, Riker W. F., Jr, Song S. K. Destruction of mammalian motor nerve terminals by black widow spider venom. Science. 1971 May 14;172(3984):733–736. doi: 10.1126/science.172.3984.733. [DOI] [PubMed] [Google Scholar]
- Pestronk A., Drachman D. B. A new stain for quantitative measurement of sprouting at neuromuscular junctions. Muscle Nerve. 1978 Jan-Feb;1(1):70–74. doi: 10.1002/mus.880010110. [DOI] [PubMed] [Google Scholar]
- Saito A., Zacks S. I. Fine structure observations of denervation and reinnervation of neuromuscular junctions in mouse foot muscle. J Bone Joint Surg Am. 1969 Sep;51(6):1163–1178. [PubMed] [Google Scholar]
- Saito A., Zacks S. I. Fine structure of neuromuscular junctions after nerve section and implantation of nerve in denervated muscle. Exp Mol Pathol. 1969 Jun;10(3):256–273. doi: 10.1016/0014-4800(69)90056-2. [DOI] [PubMed] [Google Scholar]
- Tonge D. A. Chronic effects of botulinum toxin on neuromuscular transmission and sensitivity to acetylcholine in slow and fast skeletal muscle of the mouse. J Physiol. 1974 Aug;241(1):127–139. doi: 10.1113/jphysiol.1974.sp010644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge D. A. Physiological characteristics of re-innervation of skeletal muscle in the mouse. J Physiol. 1974 Aug;241(1):141–153. doi: 10.1113/jphysiol.1974.sp010645. [DOI] [PMC free article] [PubMed] [Google Scholar]