Abstract
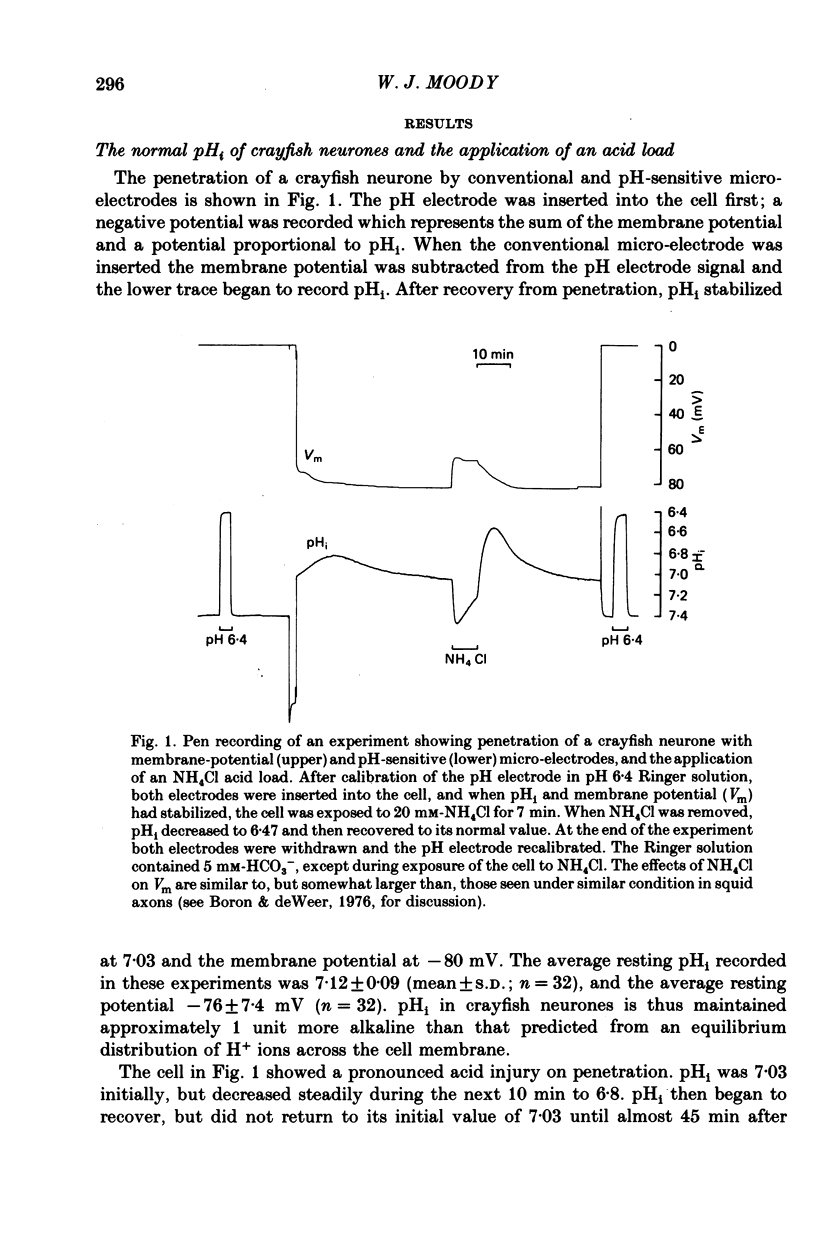
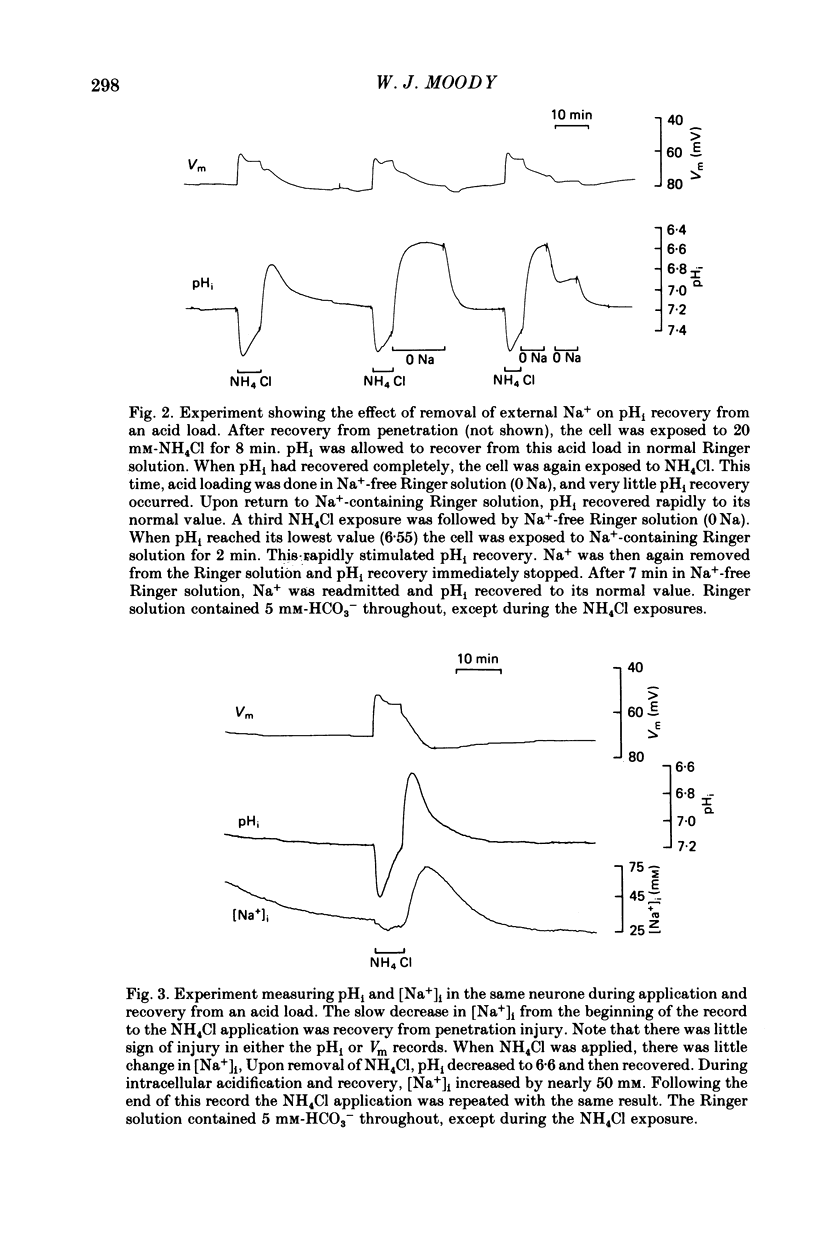
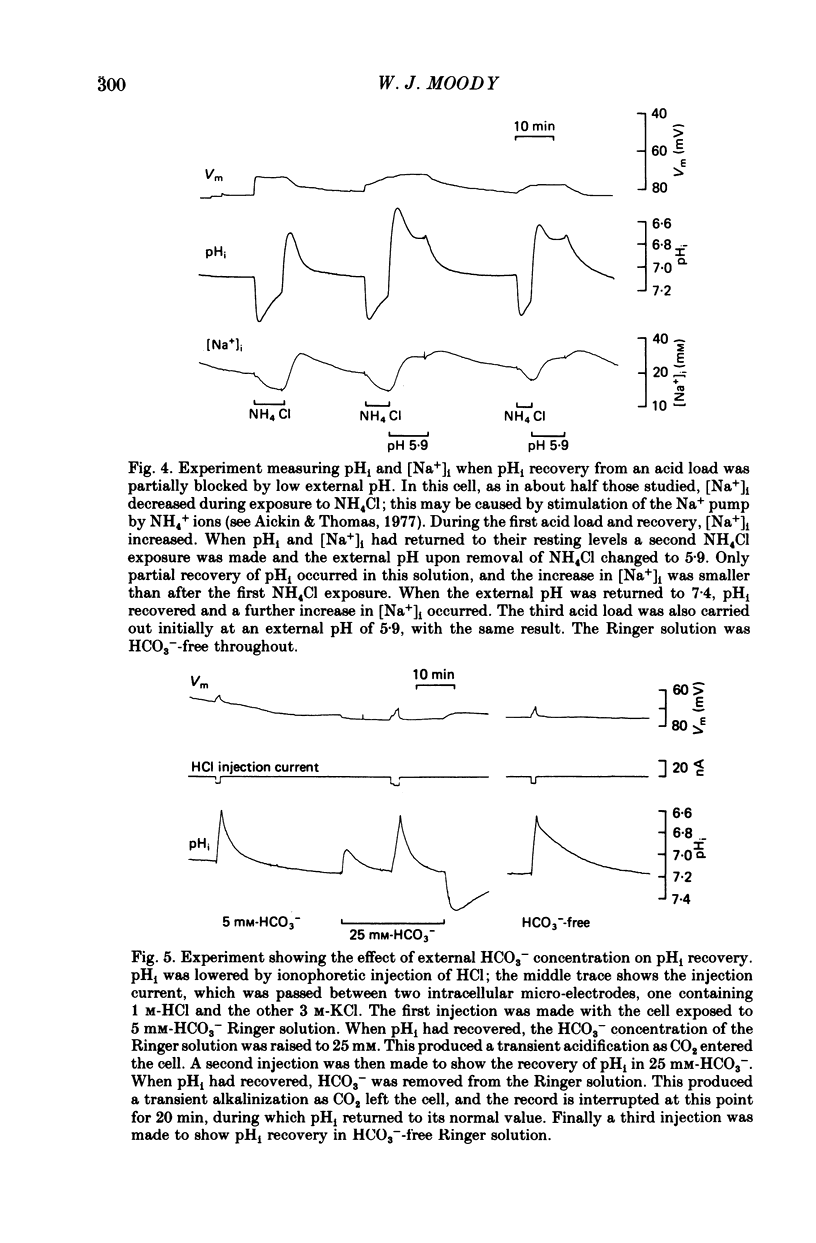
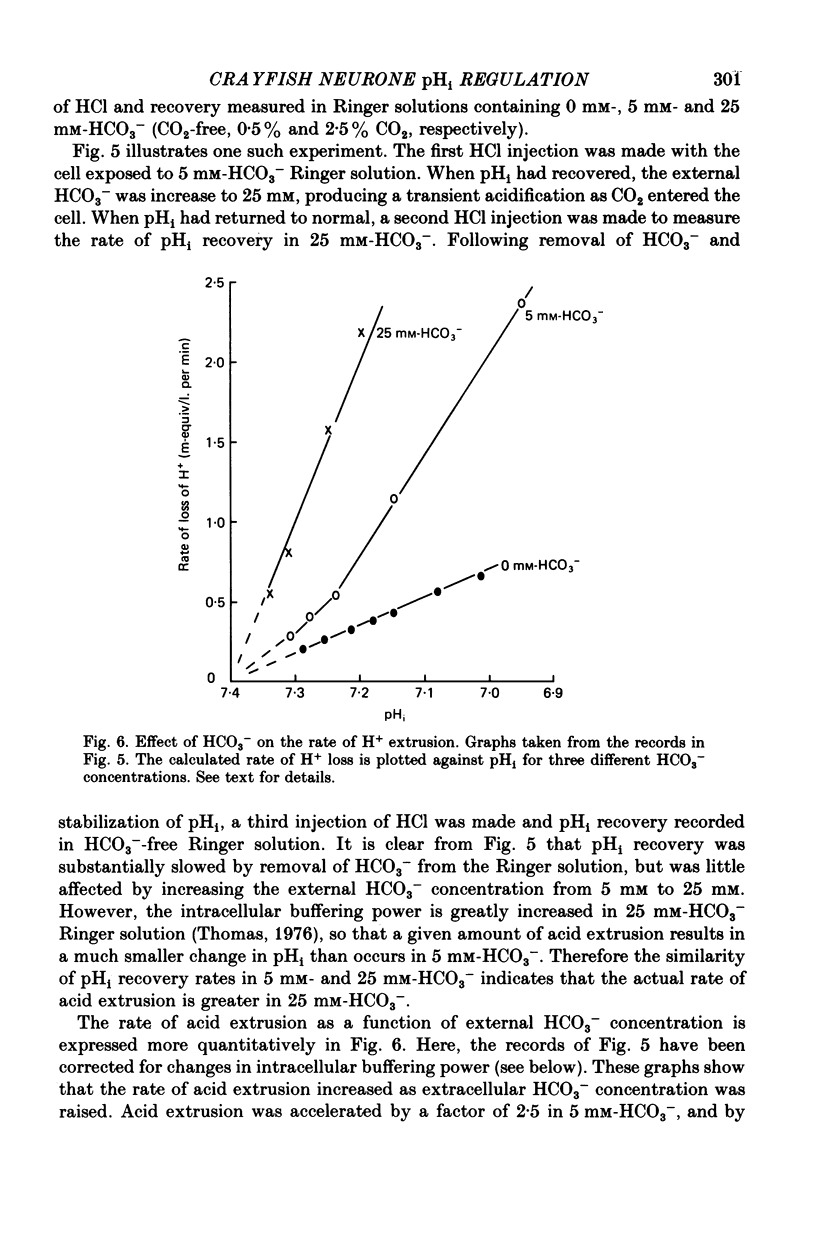
1. Intracellular pH (pHi) regulation in crayfish neurones was studied using pH-, Na+-, and Cl- sensitive micro-electrodes. Neuronal pH regulation has previously been studied only in molluscs. 2. The average resting pHi of crayfish neurones was 7.12 +/- 0.09, which is 1 pH unit more alkaline than that predicted were H+ ions distributed in equilibrium with the membrane potential. 3. When the cytoplasm was acidified (by NH4Cl loading, CO2 application, or HCl injection), pHi recovered towards its resting value. 4. Removal of Na+ from the external solution inhibited pHi recovery from an acid load by more than 90%. pHi recovery resumed immediately when external Na+ was reintroduced. 5. The resting intracellular Na+ concentration ([Na+]i) of crayfish neurones was 15-25 mM. During pHi recovery from an acid load, [Na+]i increased by 10-50 mM. 6. Reducing the external HCO3(-) concentration from 5 mM to 0 mM slowed pHi recovery by an average of about 45%. This slowing was appreciable even in cells in which Na+ removal almost totally blocked pHi recovery. 7. The resting intracellular Cl- concentration ([Cl-]i) was 30-40 mM, indicating that these cells actively accumulate Cl-. During pHi recovery from an acid load, [Cl-]i decreased by 3-5 mM. 8. In the presence of the anion exchange inhibitor SITS (4-acetamide-4'-isothiocyanostilbene-2,2'-disulphonic acid), pHi recovery was slowed to the rate which was normally seen in HCO3(-)-free Ringer solution. SITS abolished the dependence of pHi recovery on the external HCO3(-) concentration. 9. It is concluded that pHi regulation in crayfish neurones involves two separate mechanisms: a Na+-dependent, HCO3(-)-independent acid extrusion process, and a Cl---HCO3(-) exchange which is probably also Na+-dependent.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Thomas R. C. An investigation of the ionic mechanism of intracellular pH regulation in mouse soleus muscle fibres. J Physiol. 1977 Dec;273(1):295–316. doi: 10.1113/jphysiol.1977.sp012095. [DOI] [PMC free article] [PubMed] [Google Scholar]
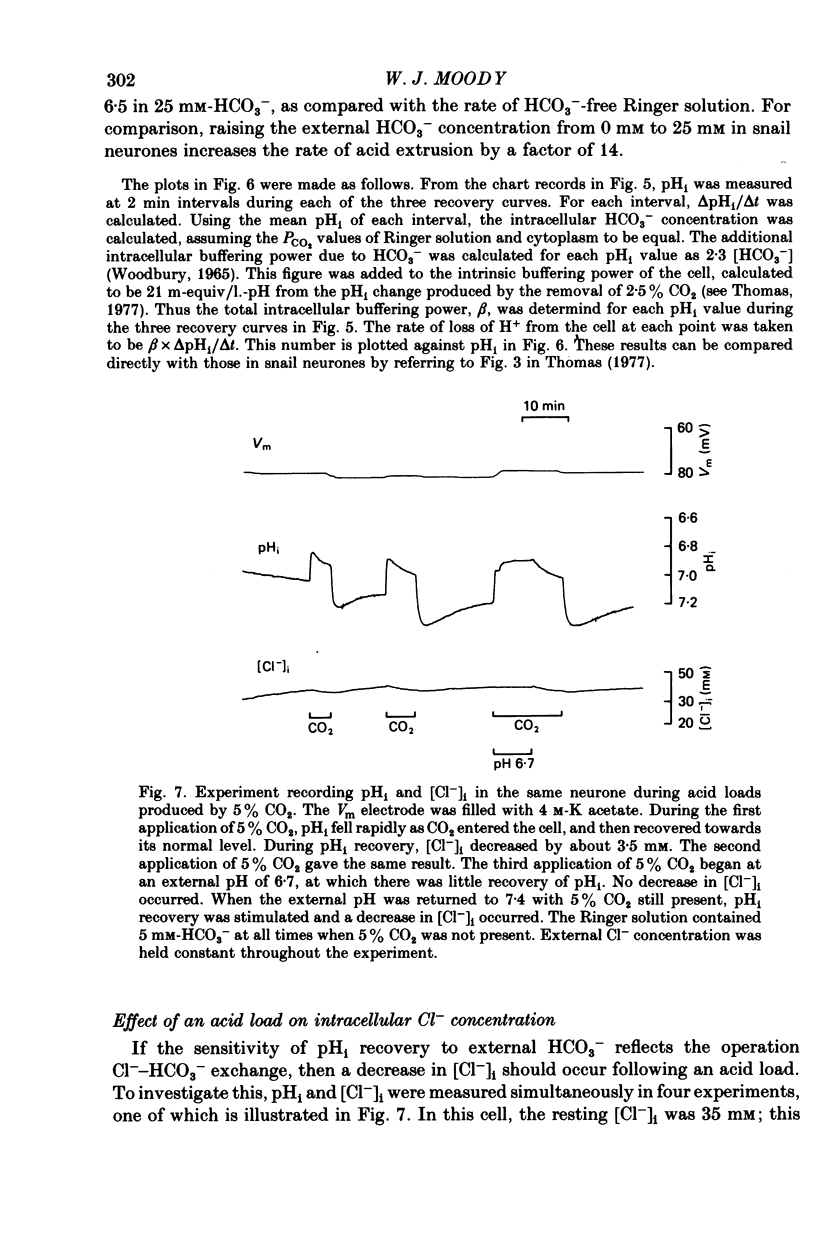
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
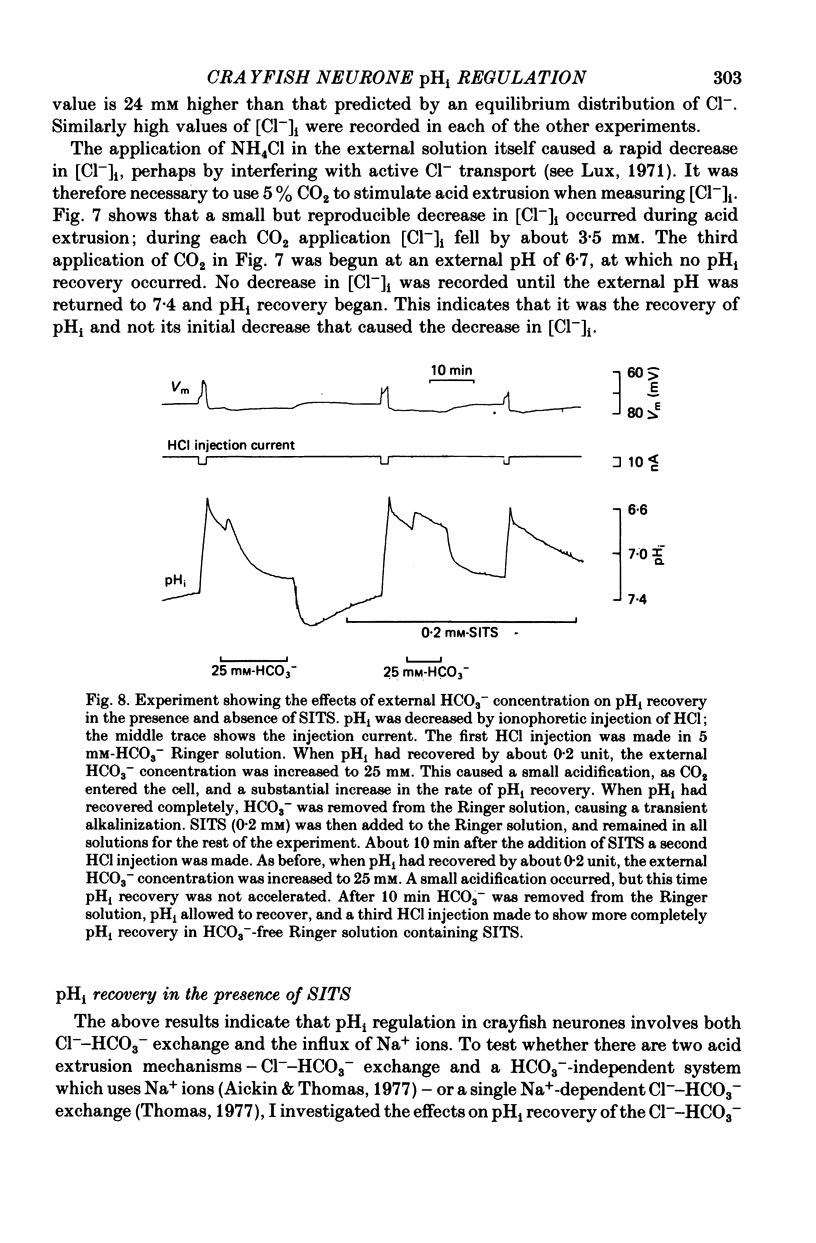
- Boron W. F., De Weer P. Active proton transport stimulated by CO2/HCO3-, blocked by cyanide. Nature. 1976 Jan 22;259(5540):240–241. doi: 10.1038/259240a0. [DOI] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F. Intracellular pH transients in giant barnacle muscle fibers. Am J Physiol. 1977 Sep;233(3):C61–C73. doi: 10.1152/ajpcell.1977.233.3.C61. [DOI] [PubMed] [Google Scholar]
- Caldwell-Violich M., Requena J. Magnesium content and net fluxes in squid giant axons. J Gen Physiol. 1979 Dec;74(6):739–752. doi: 10.1085/jgp.74.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger J. L., Winkler M. M., Shen S. S., Steinhardt R. A. Intracellular pH controls protein synthesis rate in the sea urchine egg and early embryo. Dev Biol. 1979 Feb;68(2):396–406. doi: 10.1016/0012-1606(79)90213-6. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Epel D. Intracellular pH and activation of sea urchin eggs after fertilisation. Nature. 1976 Aug 19;262(5570):661–664. doi: 10.1038/262661a0. [DOI] [PubMed] [Google Scholar]
- Knauf P. A., Rothstein A. Chemical modification of membranes. I. Effects of sulfhydryl and amino reactive reagents on anion and cation permeability of the human red blood cell. J Gen Physiol. 1971 Aug;58(2):190–210. doi: 10.1085/jgp.58.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody W., Jr Appearance of calcium action potentials in crayfish slow muscle fibres under conditions of low intracellular pH. J Physiol. 1980 May;302:335–346. doi: 10.1113/jphysiol.1980.sp013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L. D., Thomas R. C. A twelve-way rotary tap for changing physiological solutions. J Physiol. 1975 Feb;245(2):22P–23P. [PubMed] [Google Scholar]
- Russell J. M. Chloride and sodium influx: a coupled uptake mechanism in the squid giant axon. J Gen Physiol. 1979 Jun;73(6):801–818. doi: 10.1085/jgp.73.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S. S., Steinhardt R. A. Direct measurement of intracellular pH during metabolic derepression of the sea urchin egg. Nature. 1978 Mar 16;272(5650):253–254. doi: 10.1038/272253a0. [DOI] [PubMed] [Google Scholar]
- TAKEDA K., KENNEDY D. SOMA POTENTIALS AND MODES OF ACTIVATION OF CRAYFISH MOTONEURONS. J Cell Physiol. 1964 Oct;64:165–181. doi: 10.1002/jcp.1030640203. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Comparison of the mechanisms controlling intracellular pH and sodium in snail neurones. Respir Physiol. 1978 Apr;33(1):63–73. doi: 10.1016/0034-5687(78)90085-3. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular sodium activity and the sodium pump in snail neurones. J Physiol. 1972 Jan;220(1):55–71. doi: 10.1113/jphysiol.1972.sp009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976 Mar;255(3):715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turin L., Warner A. E. Intracellular pH in early Xenopus embryos: its effect on current flow between blastomeres. J Physiol. 1980 Mar;300:489–504. doi: 10.1113/jphysiol.1980.sp013174. [DOI] [PMC free article] [PubMed] [Google Scholar]


