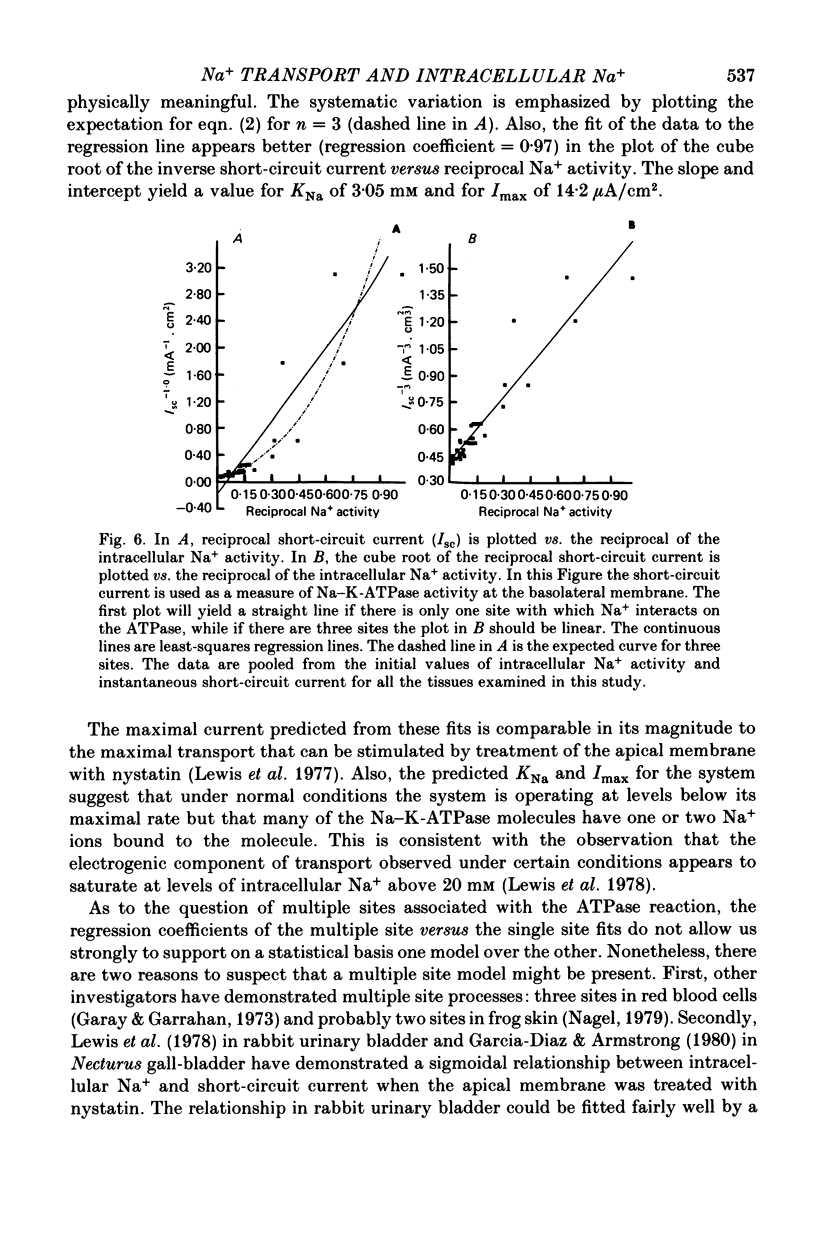
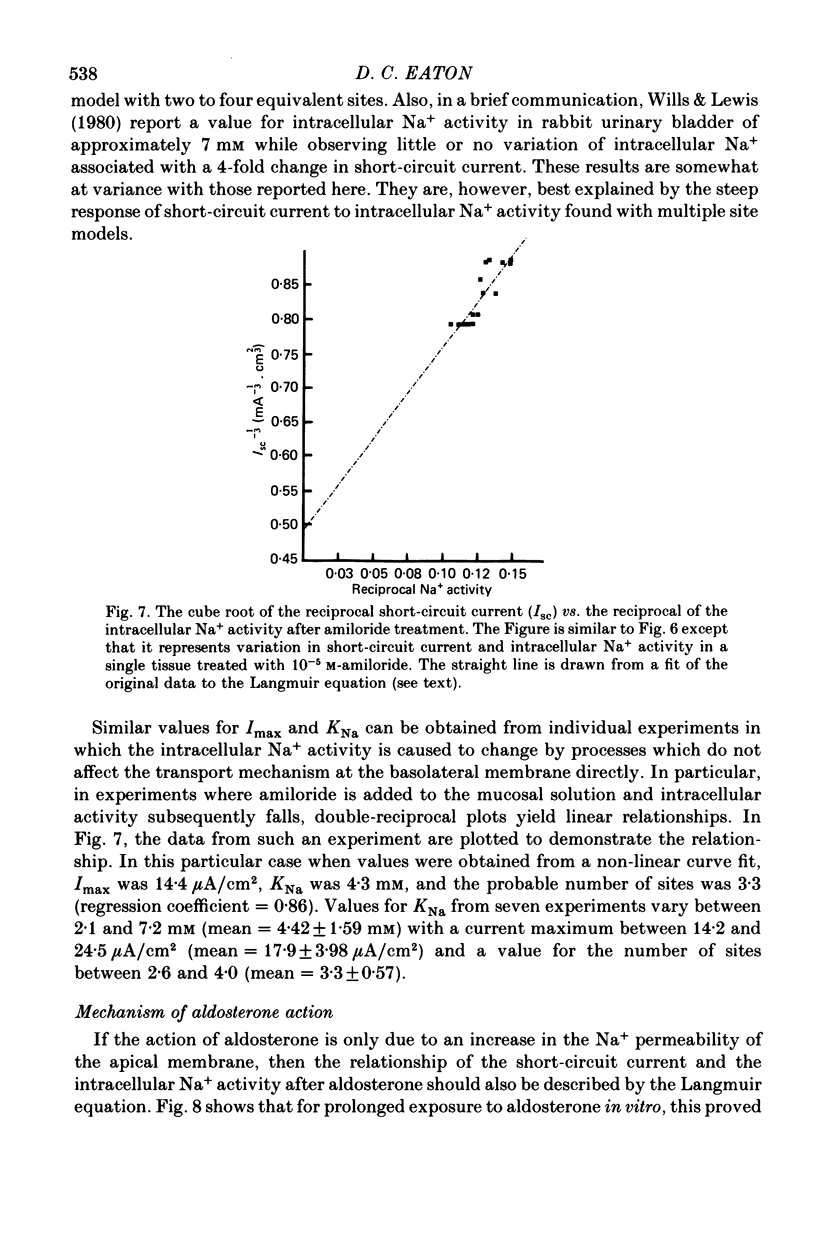
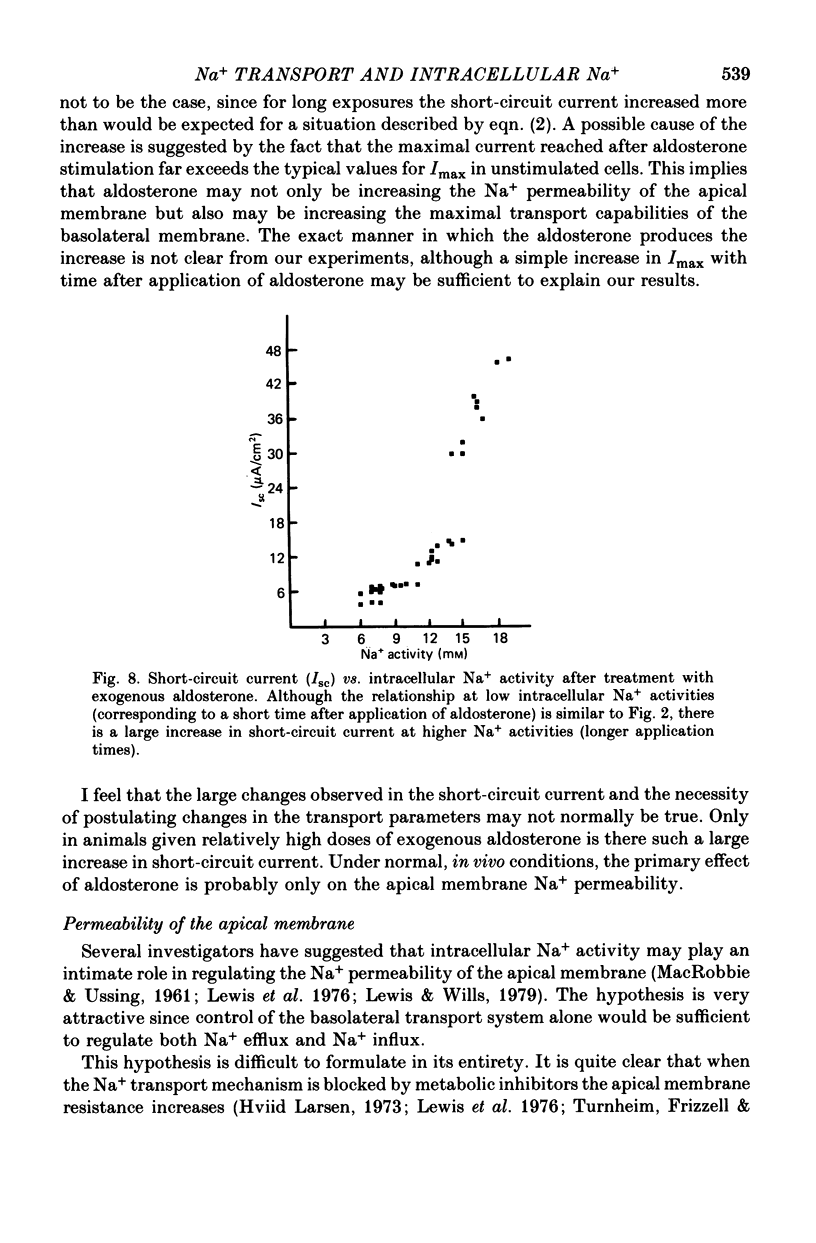
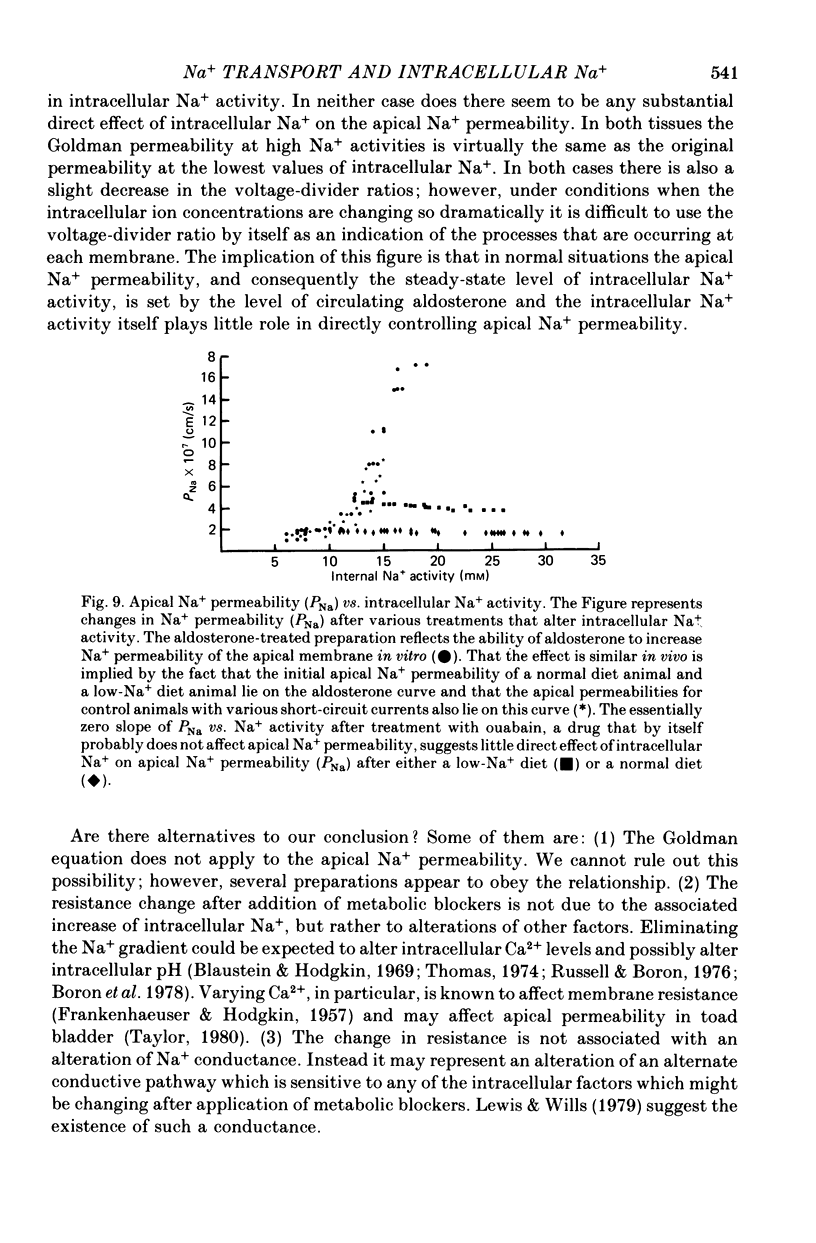
Abstract
1. Intracellular potentials and the intracellular activities of Na+ and K+ were examined using conventional and ion-selective micro-electrodes. 2. In animals on a normal diet, the intracellular Na+ activity was 8.6 +/- 2.9 mM (mean +/- S.D.) with a mean short-circuit current of 2.8 +/- 0.9 microA/cm2. 3. In animals on a low-Na+ diet, the intracellular Na+ activity was 18.5 +/- 9.9 mM with a short-circuit current of 4.5 +/- 1.3 microA/cm2 (mean +/- S.D.). 4. There was a correlation between short-circuit current and intracellular Na+ activity which could be fitted by a saturating hyperbolic relationship. 5. Treatment of the issue with ouabain and amiloride produced an increase and a decrease, respectively, in the intracellular Na+ activity. 6. Treatment with aldosterone produced a large increase in short-circuit current with a substantial increase in intracellular Na+ activity. 7. Intracellular Na+ activity does not seem to affect apical membrane permeability directly.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., Russell J. M., Brodwick M. S., Keifer D. W., Roos A. Influence of cyclic AMP on intracellular pH regulation and chloride fluxes in barnacle muscle fibers. Nature. 1978 Nov 30;276(5687):511–513. doi: 10.1038/276511a0. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Mullins L. J. Effects of membrane potential on sodium and potassium fluxes in squid axons. Ann N Y Acad Sci. 1974;242(0):406–433. doi: 10.1111/j.1749-6632.1974.tb19106.x. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Mullins L. J. Sodium fluxes in internally dialyzed squid axons. J Gen Physiol. 1968 Aug;52(2):181–211. doi: 10.1085/jgp.52.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civan M. M. Intracellular activities of sodium and potassium. Am J Physiol. 1978 Apr;234(4):F261–F269. doi: 10.1152/ajprenal.1978.234.4.F261. [DOI] [PubMed] [Google Scholar]
- Eaton D. C. Potassium ion accumulation near a pace-making cell of Aplysia. J Physiol. 1972 Jul;224(2):421–440. doi: 10.1113/jphysiol.1972.sp009903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D. C., Russell J. M., Brown A. M. Ionic permeabilities of an Aplysia giant neuron. J Membr Biol. 1975;21(3-4):353–374. doi: 10.1007/BF01941076. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay R. P., Garrahan P. J. The interaction of sodium and potassium with the sodium pump in red cells. J Physiol. 1973 Jun;231(2):297–325. doi: 10.1113/jphysiol.1973.sp010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Karlish S. J. The sodium pump. Annu Rev Physiol. 1975;37:13–55. doi: 10.1146/annurev.ph.37.030175.000305. [DOI] [PubMed] [Google Scholar]
- Graf J., Giebisch G. Intracellular sodium activity and sodium transport in necturus gallbladder epithelium. J Membr Biol. 1979 Jun 7;47(4):327–355. doi: 10.1007/BF01869743. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J Gen Physiol. 1967 Jan;50(3):583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler J. S., Preston A. S., Orloff J. Effect of ADH, aldosterone, ouabain, and amiloride on toad bladder epithelial cells. Am J Physiol. 1972 May;222(5):1071–1074. doi: 10.1152/ajplegacy.1972.222.5.1071. [DOI] [PubMed] [Google Scholar]
- Helman S. I., Nagel W., Fisher R. S. Ouabain on active transepithelial sodium transport in frog skin: studies with microelectrodes. J Gen Physiol. 1979 Jul;74(1):105–127. doi: 10.1085/jgp.74.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOEFOED-JOHNSEN V., USSING H. H. The nature of the frog skin potential. Acta Physiol Scand. 1958 Jun 2;42(3-4):298–308. doi: 10.1111/j.1748-1716.1958.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Pidoplichko V. I. Potential-dependent membrane current during the active transport of ions in snail neurones. J Physiol. 1972 Oct;226(2):373–392. doi: 10.1113/jphysiol.1972.sp009989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraig R. P., Nicholson C. Sodium liquid ion exchanger microelectrode used to measure large extracellular sodium transients. Science. 1976 Nov 12;194(4266):725–726. doi: 10.1126/science.982036. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Diamond J. M. Na+ transport by rabbit urinary bladder, a tight epithelium. J Membr Biol. 1976 Aug 27;28(1):1–40. doi: 10.1007/BF01869689. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Eaton D. C., Clausen C., Diamond J. M. Nystatin as a probe for investigating the electrical properties of a tight epithelium. J Gen Physiol. 1977 Oct;70(4):427–440. doi: 10.1085/jgp.70.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. A., Eaton D. C., Diamond J. M. The mechanism of Na+ transport by rabbit urinary bladder. J Membr Biol. 1976 Aug 27;28(1):41–70. doi: 10.1007/BF01869690. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Wills N. K., Eaton D. C. Basolateral membrane potential of a tight epithelium: ionic diffusion and electrogenic pumps. J Membr Biol. 1978 Jun 28;41(2):117–148. doi: 10.1007/BF01972629. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Wills N. K. Intracellular ion activities and their relationship to membrane properties of tight epithelia. Fed Proc. 1979 Dec;38(13):2739–2742. [PubMed] [Google Scholar]
- Lindemann B. Letter: Impalement artifacts in microelectrode recordings of epithelial membrane potentials. Biophys J. 1975 Nov;15(11):1161–1164. doi: 10.1016/S0006-3495(75)85892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACROBBIE E. A., USSING H. H. Osmotic behaviour of the epithelial cells of frog skin. Acta Physiol Scand. 1961 Nov-Dec;53:348–365. doi: 10.1111/j.1748-1716.1961.tb02293.x. [DOI] [PubMed] [Google Scholar]
- Russell J. M., Boron W. F. Role of choloride transport in regulation of intracellular pH. Nature. 1976 Nov 4;264(5581):73–74. doi: 10.1038/264073a0. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Frömter E. The potential and resistance profile of Necturus gallbladder cells. Pflugers Arch. 1977 Oct 19;371(1-2):109–117. doi: 10.1007/BF00580778. [DOI] [PubMed] [Google Scholar]
- TOSTESON D. C., HOFFMAN J. F. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J Gen Physiol. 1960 Sep;44:169–194. doi: 10.1085/jgp.44.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular pH of snail neurones measured with a new pH-sensitive glass mirco-electrode. J Physiol. 1974 Apr;238(1):159–180. doi: 10.1113/jphysiol.1974.sp010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnheim K., Frizzell R. A., Schultz S. G. Effects of anions on amiloride-sensitive, active sodium transport across rabbit colon, in vitro. Evidence for "trans-inhibition" of the Na entry mechanism. J Membr Biol. 1977 Oct 3;37(1):63–84. doi: 10.1007/BF01940924. [DOI] [PubMed] [Google Scholar]
- Wills N. K., Lewis S. A. Intracellular Na+ activity as a function of Na+ transport rate across a tight epithelium. Biophys J. 1980 Apr;30(1):181–186. doi: 10.1016/S0006-3495(80)85086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


