Abstract
Objective
To evaluate the clinical efficacy of integrating psychological support with multidisciplinary rehabilitation nursing in managing type 2 diabetes mellitus (T2DM), self-management capacity.
Methods
A prospective cohort study was conducted on 113 T2DM patients admitted to our hospital between May 2020 and February 2021. Participants were randomized into a control group (n = 49, standard care) and a study group (n = 64, psychological support + multidisciplinary rehabilitation). Outcomes were assessed using: Glycemic markers: Fasting blood glucose (FBG), 2-hour postprandial blood glucose (2hPBG). Functional and quality-of-life metrics: Physiological Function (PF), Role Physical (RP), Activities of Daily Living (ADL). Psychological scales: Visual Analogue Scale (VAS) for pain, Hamilton Depression (HAMD) and Anxiety (HAMA) Scales. Behavioral outcomes: Self-management ability and self-efficacy scores.
Results
Baseline comparisons showed no significant differences between groups (P > 0.05) for all measured parameters. Post-intervention analysis revealed that the study group demonstrated statistically significant improvements in FBG, 2hPBG, PF, RP, ADL, VAS, self-management, self-efficacy, anxiety (HAMA), and depression (HAMD) scores compared to controls (all P < 0.05).
Conclusion
Psychological support combined with multidisciplinary rehabilitation nursing significantly alleviates anxiety and depression in T2DM patients, while enhancing self-efficacy and self-management capacity. This integrative approach highlights its value as an adjunctive therapy for comprehensive diabetes care, encompassing mental health, glycemic control, functional status, and quality of life. Further research is warranted to explore its long-term metabolic and functional impacts.
Keywords: Psychological support, Multidisciplinary rehabilitation nursing, Type 2 diabetes, Daily living ability, Quality of life
Introduction
Diabetes mellitus is a chronic, systemic, metabolic disease characterized by impaired insulin secretion. With population aging, lifestyle transitions, and economic development, its prevalence continues to rise worldwide [1]. Epidemiological data indicate that more than 85% of patients have type 2 diabetes mellitus (T2DM), which predominantly affects middle-aged and older adults. T2DM is often insidious in onset with few early symptoms, but may progress to complications such as diabetic nephropathy, foot ulcers, and neuropathy [2–4]. Because diabetes is incurable and requires lifelong self-management, patients frequently endure persistent treatment-related stress that adversely affects mental health. Recent studies show that such chronic stress is closely associated with anxiety, depression, and suboptimal glycemic control [5]. Multidisciplinary rehabilitation nursing involves individualized care plans jointly developed by experts from different disciplines to address both physical and psychological needs. Psychological support consists of targeted interventions tailored to patients’ circumstances to stabilize emotions, enhance treatment adherence, and reduce complications [6, 7]. In recent years, care models integrating psychological support with multidisciplinary, guideline-directed rehabilitation have shown promising results in various clinical settings. For example, a randomized controlled trial by Xiaojuan Wan and colleagues in stroke survivors demonstrated that the addition of psychological support significantly improved quality of life [8]. Despite growing attention, the combined application of this approach in T2DM remains limited, and comprehensive evaluations of its overall impact on patient outcomes are lacking. Therefore, this study aimed to evaluate the effects of combining psychological support with multidisciplinary rehabilitation nursing in patients with T2DM, particularly on anxiety and depression, with the goal of informing the optimization of comprehensive diabetes management.
Participants and methods
Participants
Study population: Case enrollment and data collection spanned 9 months (May 2020 - February 2021). A total of 113 inpatients with type 2 diabetes mellitus (T2DM) were included; all had a confirmed diagnosis and were admitted to our hospital for standardized inpatient treatment. Using a computer-generated random number table, patients were randomized into a control group (n = 49) and an intervention group (n = 64). In the control group, there were 25 men and 24 women, aged 40–80 years, with a mean age of (60.31 ± 10.68) years; disease duration was 2–25 years, with a mean duration of (15.32 ± 5.14) years. In the intervention group, there were 30 men and 34 women, aged 45–85 years, with a mean age of (65.10 ± 11.31) years; disease duration was 3–30 years, with a mean duration of (16.34 ± 5.10) years. Baseline characteristics did not differ significantly between groups (P>0.05), indicating good comparability.
Inclusion and exclusion criteria
Inclusion criteria. Good treatment adherence; no history of psychiatric disorders or family history of psychiatric disorders; intact verbal communication ability; the patient and family were made fully informed about the study and signed written informed consent.Exclusion criteria. Concomitant acute infectious disease; acute cerebral infarction; acute myocardial infarction; acute heart failure; cognitive impairment; gestational diabetes mellitus; severe cerebrovascular accident; or severe cardiac arrhythmias.
Nursing interventions
Control group: multidisciplinary rehabilitation nursing
Establish a psychology, nutrition, rehabilitation-exercise, and home-care subteam, each staffed by three nurses.
Invite senior nursing experts to provide standardized, competency-based training for all subteam members.
At admission, the charge nurse conducts an initial assessment; based on the patient’s condition, each subteam develops an individualized rehabilitation nursing plan. The plan is dynamically adjusted during hospitalization in response to changes in the patient’s status.
After discharge, the charge nurse performs scheduled telephone follow-ups to document health status, rehabilitation adherence, and psychological state. For patients who cannot be reached or decline follow-up, secondary contact attempts are made via family members, community health services, or text messages to minimize attrition. The home-care subteam refines individualized plans according to patient feedback and provides home visits for those requiring additional support.
During the study period, both the intervention and control groups received ongoing psychological support after discharge, including telephone counseling, emotional guidance, and referral to specialist psychiatric services when necessary.
Intervention group: psychological-support nursing in addition to the above
On the basis of the control regimen, the intervention group received structured psychological-support nursing. The intervention began on the day of admission and was delivered by 10 licensed psychological counselors from the Department of Psychiatry who had prior experience in psychological support and had completed study-specific standardized training. Psychological support continued throughout hospitalization and was maintained after discharge via scheduled telephone follow-ups and face-to-face counseling at outpatient visits; the total intervention period lasted up to 9 months.
One-to-one counseling and supportive orientation. Nurses with psychological-support experience conducted individualized counseling. At admission, staff greeted patients proactively and warmly, oriented them to the ward environment, facilitated familiarity with routines, and encouraged appropriate peer connections to reduce anxiety and foster confidence in coping with their condition.
Emotion regulation and targeted coping strategies.
Nurses taught patients how to express emotions appropriately and relieve psychological stress.
For introverted patients, nurses encouraged keeping a brief daily log of pleasant or satisfying events and reviewing it before bedtime to enhance well-being.
For irritable patients, nurses recommended channeling emotions through exercise, singing, or other recreational activities.
For anxious inpatients, nurses guided music-assisted relaxation: sitting quietly, closing the eyes, listening to music, and practicing deep breathing in synchrony with the rhythm → music-assisted relaxation exercises, which involved sitting quietly, closing the eyes, listening to music, and practicing deep breathing in synchrony with the rhythm.
For patients with pronounced loneliness, nurses provided sustained companionship and support, and coached family members to offer increased presence and emotional engagement.
Outcome assessment
This study was designed to evaluate the short-term effects of the intervention on patients’short-term physiological and psychological responses. Accordingly, outcomes were assessed 1–2 days after the intervention to capture immediate rather than long-term changes. The American Diabetes Association (ADA) Standards of Care issued by the Professional Practice Committee in 2025 also recommend that patients complete self-management education and glucose monitoring before hospital discharge [9]. In addition, a systematic review and meta-analysis on the effects of psychological interventions in patients with type 2 diabetes reported a significant short-term reduction in diabetes-related distress, whereas no clear long-term benefits were observed for HbA1c or diabetes-related distress [10]. All instruments used in this study were validated Chinese-language versions, including the Short Form-36 (SF-36) [11], Visual Analogue Scale (VAS) [12], the Hamilton Depression Rating Scale (HAMD) [13, 14], the Hamilton Anxiety Rating Scale (HAMA) [13, 15], and the Chinese version of the Summary of Diabetes Self-Care Activities (SDSCA) [16]. These instruments have undergone cultural adaptation and demonstrated established reliability and validity, making them appropriate for use in Chinese populations.
Fasting blood glucose (FBG) and 2-hour postprandial blood glucose (2hPBG)
On the day before the intervention and on day 2 after the intervention, both groups underwent a 10-hour overnight fast followed by venous blood sampling; a second venous sample was drawn 2 h after a standardized meal. Serum was separated and glucose measured using a Kyoto glucometer(Manufacturer: Kyoto Science Co., Ltd., Model: GT-7110).
Physical function (PF) and role-physical (RP)
On the day before the intervention and on day 2 after the intervention, PF and RP were assessed using the Short Form-36 (SF-36). Each domain is scored on a 0–100 scale, with higher scores indicating better PF or RP status.
Activities of daily living (ADL) and visual analogue scale (VAS)
On the day before the intervention and on day 2 after the intervention, ADL was evaluated using a hospital-developed rating scale; a score of 80 indicates relatively strong activities of daily living, and higher scores reflect better ADL performance. Pain was assessed with a VAS, where 10 denotes unbearable pain and higher scores indicate greater pain intensity.
Hamilton depression rating scale (HAMD) and Hamilton anxiety rating scale (HAMA)
On the day before the intervention and on day 2 after the intervention, depressive and anxiety symptoms were evaluated using HAMD and HAMA, respectively. The maximum score for each scale is 35, with higher scores indicating more severe depression or anxiety.
Self-management level and self-efficacy scale
On the day before the intervention and on day 1 after the intervention, self-management was assessed using the validated Chinese version of the Summary of Diabetes Self-Care Activities (SDSCA). The maximum score is 77, with higher scores indicating better self-management. For the self-efficacy scale, a score of 40 represents the strongest self-efficacy, and higher scores indicate stronger self-efficacy.
Statistical analysis
Data were analyzed using SPSS 19.0. Continuous variables are presented as mean ± standard deviation; within-group comparisons were performed with the paired t test. Categorical variables are expressed as percentages(%), and between-group comparisons were conducted with the χ² test. A two-sided P < 0.05 was considered statistically significant.
Results
Comparison of fasting blood glucose (FBG) and 2-hour postprandial blood glucose (2hPBG) between the two groups
As shown in Table 1, before the nursing intervention, there were no statistically significant differences in FBG and 2hPBG between groups (FBG: P > 0.05, 95% CI − 0.5 to 0.70, T = 0.329; 2hPBG: P > 0.05, 95% CI − 0.35 to 1.37, T = 1.178). After the nursing intervention, FBG and 2hPBG levels decreased in both groups and were lower in the intervention group than in the control group (FBG: P < 0.05, 95% CI 1.81 to 2.67, T = 10.670; 2hPBG: P < 0.05, 95% CI 1.54 to 2.44, T = 8.668), with statistically significant differences.
Table 1.
Comparison of FBG and 2hPBG between the two groups [()]
| Group | Number of cases (n) | FBG | 2hPBG | ||
|---|---|---|---|---|---|
| Care before | After the nursing | Care before | After the nursing | ||
| The control group | 49 | 11.23 + / − 1.59 | 8.59 + / − 1.21 | 12.03 + / − 2.24 | 9.35 + / − 1.11 |
| The team | 64 | 11.13 + / − 1.61 | 6.35 + / − 1.02 | 11.52 + / − 2.31 | 7.36 + / − 1.28 |
| T value | 0.329 | 10.670 | 1.178 | 8.668 | |
| P values | 0.743 | 0.001 | 0.241 | 0.001 | |
| 95%cl | -0.5,0.70 | 1.81,2.67 | -0.35,1.37 | 1.54,2.44 | |
Comparison of physical functioning (PF) and role-physical (RP) between the two groups
As shown in Table 2, before the nursing intervention, there were no significant differences in PF (P > 0.05, 95% CI − 4.96 to 4.62, T = 0.070) or RP (P > 0.05, 95% CI − 2.98 to 3.56, T = 0.173) between groups. After the intervention, PF (P = 0.016, 95% CI 1.00 to 9.20, T = 2.442) and RP (P = 0.006, 95% CI 1.14 to 6.20, T = 2.787) scores increased in both groups, with higher scores in the intervention group; the differences were statistically significant.
Table 2.
Comparison of PF and RP between the two groups [(), (score)]
| Group | Number of cases (n) | PF | RP | ||
|---|---|---|---|---|---|
| Care before | After the nursing | Care before | After the nursing | ||
| The control group | 49 | 80.64 + / − 13.01 | 82.21 + / − 11.04 | 80.35 + / − 8.64 | 81.64 + / − 6.37 |
| The team | 64 | 80.47 + / − 12.67 | 87.31 + / − 10.97 | 80.64 + / − 9.01 | 85.31 + / − 7.34 |
| T value | 0.070 | 2.442 | 0.173 | 2.787 | |
| P values | 0.944 | 0.162 | 0.863 | 0.006 | |
| 95%cl | −4.96,4.62 | 1.00,9.20 | −2.98,3.56 | 1.14,6.20 | |
Activities of daily living (ADL) and visual analogue scale (VAS) scores
As shown in Table 3, before the intervention, there were no significant differences between groups in ADL and VAS scores (ADL: P = 0.821, 95% CI − 1.46 to 1.82, T = 0.227; VAS: P = 0.732, 95% CI − 0.33 to 0.47, T = 0.343). After the intervention, ADL increased and VAS decreased in both groups; compared with the control group, improvements were greater in the intervention group (ADL: P < 0.001, 95% CI 3.12 to 6.16, T = 6.567; VAS: P = 0.015, 95% CI − 0.59 to − 0.07, T = 2.480), with statistically significant differences.
Table 3.
Comparison of ADL scores and VAS scores between the two groups [(), (points)])]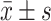
| Group | Number of cases (n) | Ability of daily living score | VAS score | ||
|---|---|---|---|---|---|
| Care before | After the nursing | Care before | After the nursing | ||
| The control group | 49 | 35.97 + / − 4.37 | 64.21 + / − 3.79 | 3.14 + / − 1.04 | 2.31 + / − 0.74 |
| The team | 64 | 36.15 + / − 4.01 | 68.85 + / − 3.67 | 3.21 + / − 1.10 | 1.98 + / − 0.67 |
| T value | 0.227 | 6.567 | 0.343 | 2.480 | |
| P values | 0.821 | 0.001 | 0.732 | 0.015 | |
| 95%cl | -1.46,1.82 | 3.12,6.16 | -0.33,0.47 | -0.59,-0.07 | |
Comparison of HAMD and HAMA between the two groups
As shown in Table 4, before the intervention, there were no significant differences in HAMD and HAMA between groups (HAMD: P = 0.312, 95% CI − 0.64 to 1.98, T = 1.015; HAMA: P = 0.763, 95% CI − 1.89 to 1.39, T = 0.302). After the intervention, both HAMD and HAMA scores decreased and were lower in the intervention group (HAMD: P < 0.001, 95% CI − 6.84 to − 4.76, T = 11.070; HAMA: P < 0.001, 95% CI − 6.03 to − 3.63, T = 7.980), with statistically significant differences.
Table 4.
Comparison of HAMD and HAMA between the two groups [(), (points)] 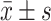
| Group | Number of cases (n) | HAMD | HAMA | ||
|---|---|---|---|---|---|
| Care before | After the nursing | Care before | After the nursing | ||
| The control group | 49 | 25.31 + / − 3.21 | 19.04 + / − 2.34 | 27.24 + / − 4.37 | 20.87 + / − 3.16 |
| The team | 64 | 25.98 + / − 3.67 | 13.24 + / − 3.04 | 26.99 + / − 4.36 | 16.04 + / − 3.21 |
| T value | 1.015 | 11.070 | 0.302 | 7.980 | |
| P values | 0.312 | 0.001 | 0.763 | 0.001 | |
| 95%cl | -0.67,2.01 | -6.71,-4.89 | -1.78,1.28 | -5.99,-3.67 | |
Self-management level and self-efficacy
As shown in Table 5, before the intervention, there were no significant differences between groups in self-management level or self-efficacy (self-management: P = 0.676, 95% CI − 1.01 to 1.55, T = 0.419; self-efficacy: P = 0.545, 95% CI − 0.59 to 1.11, T = 0.607). After the intervention, both measures increased in both groups and were higher in the intervention group (self-management: P < 0.001, 95% CI 10.87 to 13.89, T = 16.290; self-efficacy: P < 0.001, 95% CI 6.32 to 9.34, T = 10.280), with statistically significant differences.
Table 5.
Comparison of self-management level and quality of life between the two groups [(), (points)] 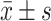
| Group | Number of cases (n) | Level of self-management | Self-efficacy scale | ||
|---|---|---|---|---|---|
| Care before | After the nursing | Care before | After the nursing | ||
| The control group | 49 | 31.38 + / − 3.24 | 45.98 + / − 3.64 | 22.31 + / − 2.13 | 27.41 + / − 3.98 |
| The team | 64 | 31.65 + / − 3.51 | 58.36 + / − 4.26 | 22.57 + / − 2.35 | 35.24 + / − 4.04 |
| T value | 0.419 | 16.290 | 0.607 | 10.280 | |
| P values | 0.676 | 0.001 | 0.545 | 0.001 | |
| 95%cl | -0.83,1.37 | 10.92,13.84 | -0.51,1.03 | 6.39,9.27 | |
Patient satisfaction
As shown in Table 6, overall satisfaction was 89.80% in the control group and 98.44% in the intervention group. Between-group comparison showed a statistically significant difference in overall satisfaction (χ²=4.122, P = 0.042; difference in proportions = 0.086, 95% CI − 0.004 to 0.176). By category, the proportion not satisfied was significantly lower in the intervention group than in the control group (1.56% vs. 10.20%, χ²=4.122, P = 0.042; difference in proportions = − 0.086, 95% CI − 0.176 to 0.004), whereas there were no significant differences for the more satisfied category (difference in proportions = − 0.068, 95% CI − 0.253 to 0.117; χ²=0.517, P = 0.472) or the satisfied category (difference in proportions = 0.154, 95% CI − 0.029 to 0.338; χ²=2.644, P = 0.104).
Table 6.
Comparison of satisfaction between the two groups [N, %]
| Group | Number of cases (n) | The more satisfied | Satisfied with the | Not satisfied with | Total satisfaction |
|---|---|---|---|---|---|
| The control group | 49 | 24 (48.98) | 20 (40.82) | 5 (10.20) | 44 (89.80) |
| The team | 64 | 27 (42.19) | 36 (56.25) | 1 (1.56) | 63 (98.44) |
| x2value | 0.517 | 2.644 | 4.122 | 2.517 | |
| P values | 0.472 | 0.104 | 0.042 | 0.042 | |
| 95%cl | −0.32,0.19 | -0.11,0.39 | -0.21,0.04 | 0.32,0.21 |
Discussion
Type 2 diabetes mellitus (T2DM) is a chronic, systemic metabolic disease caused by multiple interacting factors, characterized by the progressive decline of pancreatic β-cell secretory function and reduced insulin sensitivity in peripheral tissues [17]. Clinically, patients often present with fatigue, susceptibility to infection, and cognitive impairment, and may develop cardiovascular, cerebrovascular, gastrointestinal, and psychological comorbidities, all of which complicate long-term glycemic control and clinical management [18–21]. Fasting blood glucose (FBG) and 2-hour postprandial blood glucose (2hPBG) remain among the most commonly used monitoring indices for diabetes. Current evidence indicates that emotional fluctuations can affect glycemic variability, potentially via stress-related catecholamine release and/or reductions in peripheral insulin sensitivity that aggravate insulin resistance [22, 23]. Within the SF-36, the Physical Functioning (PF) domain reflects basic physical activities and overall organ functional status, whereas Role-Physical (RP) evaluates the extent to which physical health limits daily role performance. A Chinese version of the SF-36 has been developed and validated, demonstrating domain performance comparable to the U.S. version [11]. The Visual Analogue Scale (VAS) is a widely used tool for subjective pain assessment; by marking pain intensity on a calibrated line, it captures patients’ perceived pain in a simple, intuitive manner and has been recognized as appropriate for recording and researching pain in Chinese populations [24, 25]. The Hamilton Depression Rating Scale (HAMD) and Hamilton Anxiety Rating Scale (HAMA) are standard instruments for evaluating depressive and anxiety symptoms. The Chinese versions used in this study have demonstrated good reliability and validity in empirical studies and serve as dependable tools for assessing depression and anxiety among Chinese patients [26–28]. Likewise, the Summary of Diabetes Self-Care Activities (SDSCA) has been validated in Chinese patients with T2DM, showing good reliability and stability [29]. From a treatment perspective, psychological support can identify and alleviate negative emotions such as anxiety and depression, enhance treatment adherence and life satisfaction, and thereby facilitate glycemic control and complication prevention [30].
In the present study, there were no significant between-group differences at baseline in FBG, 2hPBG, PF, RP, activities of daily living, or VAS pain scores (P>0.05), indicating good comparability. After the intervention, both FBG and 2hPBG decreased significantly in both groups, with greater reductions in the intervention group than in the control group (P<0.05), suggesting that the addition of psychological support to routine multidisciplinary nursing was more effective in improving glycemic control. Concurrently, PF and RP scores improved significantly from baseline in both groups, with larger gains in the intervention group (P<0.05), indicating a positive effect on physical functioning and daily role performance. Notably, upon completion of the intervention, the intervention group exhibited higher activities-of-daily-living scores and lower VAS pain scores than the control group (P<0.05), implying that integrating psychological support with multidisciplinary rehabilitation nursing not only enhanced self-care capacity but also alleviated pain. Furthermore, HAMD and HAMA scores were significantly lower in the intervention group than in the control group (P<0.05), demonstrating the intervention’s effectiveness in mitigating negative affect—findings consistent with prior research. Finally, patient-reported satisfaction was higher in the intervention group (98.44% vs. 89.80%, P<0.05), further supporting the superiority of this care model in improving overall treatment experience and adherence.
Persistent psychological stress—such as depression and anxiety—has been shown to increase the risk of type 2 diabetes mellitus (T2DM), and higher rates of anxiety and depressive symptoms have been observed among individuals with T2DM compared with the general population [31, 32]. Such psychological burdens are associated with elevated inflammatory markers, worsened glycemia, and related complications [33]. Accordingly, effective psychological interventions are crucial in diabetes care.For example, Na Dong et al. reported that cognitive behavioral therapy produced sustained benefits for long-term glycemic control in patients with diabetes [34]. Qasir Abbas et al. found that psychological interventions significantly alleviated depressive and anxiety symptoms in patients with T2DM while improving quality of life and treatment adherence [35]. Moreover, a meta-analysis including 45 randomized controlled trials confirmed that psychological interventions significantly improved glycemic levels and depressive symptoms in T2DM [36].Building on this evidence, our study integrates psychological interventions into a multidisciplinary rehabilitation program tailored specifically for T2DM. Unlike prior work that evaluated psychological strategies in isolation, we combined psychological support with multidisciplinary rehabilitation nursing. This comprehensive model not only substantially improved mental health but also enhanced glycemic control and functional behaviors, offering a more holistic and sustainable strategy for the management of T2DM.
First, this study had a relatively small sample size and was conducted at a single center, which may have reduced statistical power and limited the generalizability of the findings. Future research should expand the sample and implement multicenter, large-scale randomized controlled trials across different regions and healthcare institutions to enhance representativeness and external validity.Second, we performed comprehensive assessments of scales and laboratory indicators primarily within 1–2 days after the intervention, without dynamic monitoring during longer-term follow-up. As a result, we could not characterize trajectories of intervention effects over time. Future studies should incorporate multiple time-point evaluations during extended follow-up to more fully elucidate the persistence and evolution of intervention effects.Third, although instruments such as the SF-36, activities of daily living, VAS pain score, HAMD, HAMA, and self-management measures have demonstrated good reliability and validity domestically and internationally, they rely on patient self-report and may be subject to response bias. While we complemented these with objective indices (e.g., blood glucose), the range of objective measures remained limited. It would be valuable to add more objective monitoring modalities—such as wearable devices for activity and physiological parameters—to improve objectivity and comprehensiveness [37, 38]. Fourth, we did not fully control for potential confounders, such as socioeconomic status and coexisting chronic diseases, which may have influenced outcome assessments. Future work should collect more comprehensive baseline data, apply multivariable adjustment, and conduct stratified analyses to determine the applicability of the combined psychological support plus multidisciplinary rehabilitation model across different patient subgroups.
Conclusion
In summary, our study demonstrated that the addition of structured psychological support to multidisciplinary rehabilitation nursing significantly improved glycemic control, physical functioning (PF), role-physical functioning (RP), activities of daily living, pain perception, emotional status (depression and anxiety), self-management, and self-efficacy, while also enhancing patient satisfaction. These findings suggest that clinical care for T2DM should comprehensively address patients’ physiological, psychological, and behavioral needs. Integrating these dimensions into routine management may improve accessibility, strengthen adherence, and ensure the long-term effectiveness of interventions, thereby promoting recovery and enhancing overall quality of life.
Acknowledgements
Not applicable.
Author contributions
Xueqin Lu wrote the main manuscript. Haojin Yang prepared the data collection. Xiaoyun Ying prepared figure and tables. Sisi Lin analyse and interpret of results. All authors reviewed the results and approved the final version of the manuscript.
Funding
This study was supported by the Wenzhou City Science and Technology Bureau project Fund(No.Y20180055).
Data availability
The experimental data used to support the findings of this study are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee in Clinical Research (ECCR) of the First Affiliated Hospital Of Wenzhou Medical University (approval number: 2023115). Informed consent was obtained from all the participants. All methods were carried out in accordance with Declaration of Helsinki.
Consent for publication
All the authors confirming that WRITTEN INFORMED consent was obtained from all subjects and/or their legal guardian(s).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global regional, national burden of diabetes. From 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021 [J]. Lancet. 2023;402(10397):203–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho J, Woo S, Hwang SH, et al. A multimodal predictive model for chronic kidney disease and its association with vascular complications in patients with type 2 diabetes: model development and validation study in South Korea and the U.K [J]. Diabetes Care. 2025;48(9):1562–70. [DOI] [PubMed] [Google Scholar]
- 3.Tjaden AH, Braffett BH, Butera NM, et al. Sociodemographic, clinical, and psychosocial predictors of short- and long-term study retention in diabetes prevention program (DPP) outcomes study (DPPOS) [J]. Diabetes Care; 2025. [DOI] [PMC free article] [PubMed]
- 4.O’hara LM, Lydecker AD, Robinson GL, et al. Understanding patient preferences regarding limb salvage for diabetic foot ulcers: A discrete choice experiment [J]. Diabetes Care. 2025;48(9):1517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poole L, Hackett RA. Diabetes distress: the psychological burden of living with diabetes [J]. Lancet Diabetes Endocrinol. 2024;12(7):439–41. [DOI] [PubMed] [Google Scholar]
- 6.Nishikawa H, Fukunishi S, Asai A et al. Sarcopenia, frailty and type 2 diabetes mellitus (Review) [J]. Mol Med Rep. 2021;24(6). [DOI] [PubMed]
- 7.Chen CW, Wang TJ, Liu CY, et al. Effectiveness of a nurse practitioner-led collaborative health care model on self-care, functional status, rehospitalization and medical costs in heart failure patients: A randomized controlled trial [J]. Int J Nurs Stud. 2025;162:104980. [DOI] [PubMed] [Google Scholar]
- 8.Wan X, Chan DNS, Chau JPC, et al. Effects of a nurse-led peer support intervention on psychosocial outcomes of stroke survivors: A randomised controlled trial [J]. Int J Nurs Stud. 2024;160:104892. [DOI] [PubMed] [Google Scholar]
- 9.16. Diabetes care in the hospital: standards of care in Diabetes-2025 [J]. Diabetes Care. 2025;48(1 Suppl 1):S321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zu W, Zhang S, Du L, et al. The effectiveness of psychological interventions on diabetes distress and glycemic level in adults with type 2 diabetes: a systematic review and meta-analysis [J]. BMC Psychiatry. 2024;24(1):660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Wang HM, Shen Y. Chinese SF-36 health survey: translation, cultural adaptation, validation, and normalisation [J]. J Epidemiol Community Health. 2003;57(4):259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhuo L, Gao S, Jin R, et al. VAS value set still has its own application value compare with TTO value set of EQ-5D-3L in Chinese population [J]. BMC Med Res Methodol. 2025;25(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang XM, Ma HY, Zhong J, et al. A Chinese adaptation of six items, self-report Hamilton depression scale: factor structure and psychometric properties [J]. Asian J Psychiatr. 2022;73:103104. [DOI] [PubMed] [Google Scholar]
- 14.Sun XY, Li YX, Yu CQ, et al. Reliability and validity of depression scales of Chinese version: a systematic review] [J]. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38(1):110–6. [DOI] [PubMed] [Google Scholar]
- 15.Du C, Chen J, Ma X, et al. Testing the validity and reliability of the Chinese version of the Staden schizophrenia anxiety rating scale [J]. Front Psychiatry. 2022;13:992745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Q, Liu Y, Lin K, et al. Diabetes self-management and its related factors among Chinese young adults with type 2 diabetes mellitus [J]. Nurs Open. 2023;10(9):6125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanaley JA, Colberg SR, Corcoran MH, et al. Exercise/physical activity in individuals with type 2 diabetes: a consensus statement from the American college of sports medicine [J]. Med Sci Sports Exerc. 2022;54(2):353–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khosla S, Samakkarnthai P, Monroe DG, et al. Update on the pathogenesis and treatment of skeletal fragility in type 2 diabetes mellitus [J]. Nat Rev Endocrinol. 2021;17(11):685–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salib A, Cayabyab F, Yoshihara E. Stem cell-derived islets for type 2 diabetes [J]. Int J Mol Sci. 2022;23(9). [DOI] [PMC free article] [PubMed]
- 20.Giovenzana A, Carnovale D, Phillips B, et al. Neutrophils and their role in the aetiopathogenesis of type 1 and type 2 diabetes [J]. Diabetes Metab Res Rev. 2022;38(1):e3483. [DOI] [PubMed] [Google Scholar]
- 21.Shan Z, Fa WH, Tian CR, et al. Mitophagy and mitochondrial dynamics in type 2 diabetes mellitus treatment [J]. Aging. 2022;14(6):2902–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vedantam D, Poman DS, Motwani L, et al. Stress-induced hyperglycemia: consequences and management [J]. Cureus. 2022;14(7):e26714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franc S, Bensaid S, Schaepelynck P, et al. Impact of chronic emotions and psychosocial stress on glycemic control in patients with type 1 diabetes. Heterogeneity of glycemic responses, biological mechanisms, and personalized medical treatment [J]. Diabetes Metab. 2023;49(6):101486. [DOI] [PubMed] [Google Scholar]
- 24.Aun C, Lam YM, Collett B. Evaluation of the use of visual analogue scale in Chinese patients [J]. Pain. 1986;25(2):215–21. [DOI] [PubMed] [Google Scholar]
- 25.Ma K, Jiang W, Wang Y-X et al. Expert consensus on the application of pain evaluation questionnaires in China(2020) [J]. Chin J Pain Med. 2020;16:177. [Google Scholar]
- 26.Zimmerman M, Martinez JH, YOUNG D, et al. Severity classification on the Hamilton depression rating scale [J]. J Affect Disord. 2013;150(2):384–8. [DOI] [PubMed] [Google Scholar]
- 27.Zheng YP, Zhao JP, Phillips M, et al. Validity and reliability of the Chinese Hamilton depression rating scale [J]. Br J Psychiatry. 1988;152:660–4. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Zhu E, Ai P, et al. The potency of psychiatric questionnaires to distinguish major mental disorders in Chinese outpatients [J]. Front Psychiatry. 2022;13:1091798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y, Liao L, Sun M, et al. Self-care practices of Chinese individuals with diabetes [J]. Exp Ther Med. 2013;5(4):1137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yun JS, Ko SH. Current trends in epidemiology of cardiovascular disease and cardiovascular risk management in type 2 diabetes [J]. Metabolism. 2021;123:154838. [DOI] [PubMed] [Google Scholar]
- 31.Alajmani DSA, Alkaabi AM, Alhosani MW, et al. Prevalence of undiagnosed depression in patients with type 2 diabetes [J]. Front Endocrinol (Lausanne). 2019;10:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maimaitituerxun R, Chen W, Xiang J, et al. Prevalence of anxiety and associated factors among inpatients with type 2 diabetes mellitus in china: A Cross-Sectional study [J]. Psychiatr Q. 2023;94(3):371–83. [DOI] [PubMed] [Google Scholar]
- 33.Hackett RA, Steptoe A. Type 2 diabetes mellitus and psychological stress - a modifiable risk factor [J]. Nat Rev Endocrinol. 2017;13(9):547–60. [DOI] [PubMed] [Google Scholar]
- 34.Dong N, Wang X, Yang L. The short- and long-term effects of cognitive behavioral therapy on the glycemic control of diabetic patients: a systematic review and meta-analysis [J]. Biopsychosoc Med. 2023;17(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbas Q, Latif S, Ayaz Habib H, et al. Cognitive behavior therapy for diabetes distress, depression, health anxiety, quality of life and treatment adherence among patients with type-II diabetes mellitus: a randomized control trial [J]. BMC Psychiatry. 2023;23(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapman A, Liu S, Merkouris S, et al. Psychological interventions for the management of glycemic and psychological outcomes of type 2 diabetes mellitus in china: A systematic review and meta-analyses of randomized controlled trials [J]. Front Public Health. 2015;3:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung W, Vo K, Clough M, et al. The use of wearable devices on physical activity levels among individuals living with diabetes: 2017 BRFSS [J]. Prim Care Diabetes. 2024;18(4):466–9. [DOI] [PubMed] [Google Scholar]
- 38.Zhu B, Zhu L, Wang H, et al. Fully printed multimodal sensing system for continuous health monitoring during exercise [J]. Biosens Bioelectron. 2025;288:117840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The experimental data used to support the findings of this study are available from the corresponding author upon request.


