Abstract
Background
Colorectal cancer (CRC) and cardiovascular disease (CVD) are major global health burdens, sharing common risk factors like obesity, smoking, and diabetes. While CRC’s impact on CVD has been studied, the reverse association remains less explored. The study aims to investigate the relationship between CVD and the morbidity and prognosis of CRC.
Materials
This study was conducted across 9 databases (PubMed, Embase, Cochrane, Scopus, Web of Science, CINAHL complete, SPORT Discus, PsycINFO and CNKI) from January 1, 2000, to February 27, 2025. The prognosis were diagnosis of colorectal cancer or postoperative prognosis (including death and complications). This study was analyzed by Stata (V. 16.0) software.
Results
A total of 23 studies were included: 11 studies (5,462,791 patients) from 6 countries or regions assessed morbidity risk, and 12 studies (307,857 patients) from 9 countries evaluated postoperative prognosis. Cardiovascular disease (CVD) was associated with increased risks for colorectal cancer (CRC) (RR 1.24, 95%CI 1.04 to 1.48) (CI, confidence intervals) (RR, relative risk), postoperative arrhythmia (RR 3.05, 95%CI 1.27 to 7.35), and all-cause mortality (RR 1.56, 95%CI 1.26 to 1.94). Subgroup analysis shows that heart failure (HF) may be a risk factor for CRC (RR 1.39, 95%CI 1.03 to 1.89) and all-cause mortality (RR 1.68, 95%CI 1.49 to 1.90). Among female patients, HF (RR 2.32, 95%CI 1.39 to 3.88) and heart failure with preserved ejection fraction (HFpEF) (RR 2.54, 95%CI 1.52 to 4.24) show associations with CRC.
Conclusions
CVD was associated with increased risks of CRC incidence and all-cause mortality in CRC patients, with HF specifically linked to both outcomes. However, no conclusive associations emerged between CVD and postoperative complications beyond arrhythmia. Further investigation into physiological mechanism is required.
Prospero id:
CRD420251020352.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-025-14905-3.
Keywords: Cardiovascular disease, Colorectal cancer, Morbidity, Prognosis
Background
Colorectal cancer (CRC), the third most common cancer worldwide (accounting for 10% of all cancers), is the second leading cause of cancer-related death [1–4]. Globally, the incidence and mortality of CRC doubled between 1990 and 2021, posing a major challenge to human health [5]. There are significant geographical and gender differences in CRC, with increasing incidence rates reported in developing countries with economies in transition, while developed countries such as Europe and the United States tend to show decreasing or stable incidence rates [6]. The CRC burden is higher in men than women, with higher morbidity and mortality rates [7, 8]. Age, genetics, inflammatory bowel disease, metabolic syndrome, obesity, dietary imbalances (excessive intake of red and processed meats, inadequate dietary), behavioral patterns (sedentary lifestyle, physical inactivity), and exposure to addictive substances (cigarette smoking, alcohol abuse) contribute to colorectal carcinogenesis [3, 9]. The mechanism of CRC may be related to the intestinal microbiota, aberrant signaling pathways, and genetic susceptibility [7, 10]. The common screening methods include colonoscopy and fecal testing [2, 11, 12]. Currently, the common treatment for CRC is colorectal radical surgery. Postoperative complications, mainly anastomotic leakage, pose great challenge to patients’ postoperative recovery [13, 14].
Cardiovascular disease (CVD) is a major challenge in global public health, including organic cardiac diseases such as coronary heart disease (CHD), atrial fibrillation (AF) and heart failure (HF), as well as systemic vascular disease such as strokes and peripheral vascular diseases [15]. These cause a number of deaths and disabilities globally, and increase the economic costs for the world, especially in developing countries [16]. According to the World Heart Federation, CVD causes more than 17.3 million deaths per year (about 31% of total deaths). The death is expected to rise to 23 million cases by 2030 [17]. World Health Organization data shows that 85% of cardiovascular-related death is attributed to myocardial infarctions and strokes, highlighting its threat to human health [18]. CVD significantly impacts chronic diseases, including diabetes mellitus [19], chronic kidney disease (CKD) [20, 21] and cancer [22]. The risk of development of these diseases and poor prognosis are related to CVD. For example, CVD especially HF is the leading cause of death in patients with type 2 diabetes [19].
As 2 major global health burdens, CVD and CRC share common risk factors. For example, a study showed that obesity, high body mass index (BMI), cigarette smoking, diabetes mellitus and hypercholesterolemia are independent risk factors for not only CVD but also CRC [23]. The interaction between CVD and CRC has received attention in recent years. Most of the current studies focus on the impact of CRC on CVD. Previous studies found that patients with CRC had an increased risk of CVD, especially CHD [24] and HF [15]. And another study found that CRC patients had higher risk for major adverse cardiac events (MACE) and all-caused long-term mortality within 30 days after surgery [25]. And another study found that CRC patients had similar high risk of cardiovascular-specific mortality compared to ones with no cancers [26]. There are fewer studies on the impact of CVD on CRC. Some studies showed that CVD was positively associated with morbidity [27] and poor prognosis [8] of CRC. The main studies are about single subtypes of CVD rather than the whole CVD. And the prognosis studies are limited to several complications, death and so on. There is a lack of systematic assessment between CVD and CRC. This study aimed to systematically assess the association between these 2 diseases as well as prognosis of patients with both diseases, by integrating the existing evidence, and to provide a clearer evidence-based basis for clinical co-morbidity management.
Methods
Search strategy
This study followed the guidelines for systematic evaluation and meta-analysis (PRISMA). A total of 9 databases, including PubMed, Embase, Cochrane Library, Scopus, Web of Science, CINAHL Complete, SPORT Discus, PsycINFO and CNKI, were independently searched by 2 researchers. The search period spanned from January 1, 2000, to February 27, 2025. The search strategy combined MeSH terms with free words, including: (1) CVD-related terms “cardiovascular abnormalities OR congenital heart defects OR vascular malformations OR cardiovascular infections OR endocarditis bacterial OR heart diseases OR cardiac arrhythmias OR carcinoid heart disease OR cardiac conduction system disease OR cardiac output OR cardiac tamponade OR cardiomegaly OR cardiomyopathies OR cardiotoxicity OR endocarditis OR heart aneurysm OR heart arrest OR congenital heart defects OR heart failure OR heart neoplasms OR heart rupture OR heart valve diseases OR myocardial ischemia OR myocardial stunning OR pericardial effusion OR pericarditis OR post-cardiac arrest syndrome OR postpericardiotomy syndrome OR pulmonary heart disease OR rheumatic heart disease OR ventricular dysfunction OR ventricular outflow OR obstruction OR peripartum cardiomyopathy OR myocardial ischemia” (2)CRC-related terms “colorectal neoplasm OR colorectal tumors OR colorectal tumor OR colorectal cancer OR colorectal cancers OR colorectal carcinoma OR colorectal carcinoma”. We used the Boolean operator “AND” to connect the 2 sets of keywords and we limited the language of the document to English and Chinese.
Inclusion and exclusion criteria
Criteria for inclusion included: (1) Observational studies exploring the effect of CVD on CRC morbidity and postoperative prognosis. (2) Studies reporting hazard ratio (HR), relative risk ratio (RR) or odds ratio (OR) and their 95% confidence intervals (CI), or studies providing data that can be converted to these above effect values. (3) Studies reporting a definitive diagnosis of CVD. (4) Studies with effect values that refer to non-CVD patients. Criteria for exclusion included: (1) Descriptive studies, reviews, meta-analyses, case reports, conference abstracts, commentaries. (2) Studies that did not differentiate between CVD and other diseases. (3) Studies that did not differentiate between CRC and other cancers. (4) Studies with incomplete data such as lack of an effect value or inability to calculate an effect value based on data provided. (5) Studies that explored CRC as an indicator of intervention on CVD risk of morbidity or postoperative prognosis.
Data collection and quality assessment
Two investigators independently extracted and cross-checked data on: first author, year of publication, country, duration of study, cases (number of patients), subjects (sample size), type of study, type of CVD, prognosis indicator or number of morbidities, effect value. In the event of disagreement over the inclusion of specific literature in the study, it was first resolved by negotiation between the 2 investigators, and if disagreement remained, a third person, usually the corresponding author, made a direct decision on inclusion. When retrieved for meta-analyses, references were included within the literature to be screened. The quality of cohort studies was assessed using the Newcastle-Ottawa Scale (NOS), with high-quality studies scoring 7–9, moderate-quality studies scoring 4–6, and low-quality studies scoring less than 1–3.
Statistical analysis
Stata 16.0 software was used for analysis. Continuous variables were shown as mean ± standard deviation (SD), and categorical variables were shown as specific numbers and proportions. If effect value was provided in the literature, they could be directly included in the study. Categorical variables were used as effect sizes with RR. OR and HR were converted to RR (when the control incidence rate P0 ≥ 10%, RR = OR/(1 - P0 + P0 * OR). When P0 < 10%, OR was approximated to be seen as RR. HR was directly approximated to be seen as RR. Continuous variables were expressed as mean difference (MD). The effect value and MD were calculated with 95% CI. DerSimonian-Laird random effects model was used. Heterogeneity was assessed using the Q-test with I² values: heterogeneity was considered to be high when I²>50% using the random effects model. P < 0.05 was considered statistically significant. The effect of various subgroups of CVD and gender on CRC patients are analyzed. Publication bias was assessed by funnel plot symmetry and de-monotonic sensitivity analyses.
Results
Study selection
A total of 10,033 studies were retrieved from the identification (89 studies in PubMed, 117 studies in Embase, 913 studies in Cochrane, 0 studies in Scopus, 8,509 studies in Web of Science, 309 studies in CINAHL complete and SPORT Discus, 88 studies in PsycInfo, 8 studies in CNKI). In the screening stage, after exclusion of duplicate records, 9609 studies were included, and after an initial screening of titles and abstracts of the articles, 83 studies were retained for full-text reading assessment. In the eligibility stage, 27 studies were included, and after excluding studies that could not be matched, a final total of 23 studies were included [28–50]. In the included stage, 11 studies were included in the study of the effect of CVD on the occurrence of CRC, and 12 were included in the study of the effect of CVD on the prognosis of patients with CRC. (Fig. 1)
Fig. 1.
Flowchart of studies screening
Basic characteristics of the included studies and quality assessment
The 23 studies involving 216,302 patients were included in this study, with publication years ranging from 2001 to 2025 and study years ranging from 1988 to 2023. 14 cohort studies (including 11 retrospective and 3 prospective studies) and 9 case-control studies were included. 1 study was published in Israel, 2 in Denmark, 1 in Netherland, 1 in United Kingdom (Northern Ireland), 1 in Canada, 1 in Japan, 1 in Australia, 1 in Singapore, 1 in Italy, 6 in the United States of America, and 7 in China (2 in Hong Kong and 1 in Taiwan). Specific information and NOS scores for each study are shown. (Table 1)
Table 1.
The basic information, sample size and NOS score of included studies. CVD, cardiovascular disease; NOS, the Newcastle-Ottawa scale; CHD, coronary heart disease; AMI, acute myocardial infarction; HF, heart failure; hfref, heart failure with reduced ejection fraction; hfpef, heart failure with preserved ejection fraction; AF, atrial fibrillation; IHD, ischemic cardiomyopathy; SVT, supraventricular tachycardia
| Author | Year | Country | Study date | Cases/Subjects | Study type | CVD subtype | NOS | Research direction |
|---|---|---|---|---|---|---|---|---|
| Reicher-Reiss H | 2001 | Israel | 1990–1996 | 603/10,923 | Retrospective cohort study | CHD in male | 6 | Risk of CRC |
| Chan AOO | 2007 | Hong Kong(China) | 2004–2006 | 9/415 | Prospective cohort study | CHD | 8 | Risk of CRC |
| Chou SH | 2023 | Taiwan(China) | 2001–2012 | 2,076/303,471 | Retrospective cohort study | AMI | 5 | Risk of CRC |
| Leedy D | 2021 | USA | 1993–2015 | 854/146,817 | Prospective cohort study |
HF (including HFpEF and HFrEF) in female |
8 | Risk of CRC |
| Wassertheil-Smoller S | 2017 | USA | 1994–1998 | 76,252/93,676 | Prospective cohort study | AF in female | 7 | Risk of CRC |
| Bai T | 2024 | USA | 2005–2018 | 11,452/15,147,126 | Case-control |
CVD, CHD, HF, Stroke, Heart attack, Angina pectoris |
5 | Risk of CRC |
| Chen QF | 2025 | China | 2009–2023 | 631/33,033 | Retrospective cohort study | HF | 7 | Risk of CRC |
| Banke A | 2016 | Denmark | 2002–2009 | 120/4,959,275 | Retrospective cohort study | HF | 7 | Risk of CRC |
| Schwartz B | 2020 | Denmark | 1997–2017 | 4,494/1,004,759 | Retrospective cohort study | HF | 5 | Risk of CRC |
| Selvaraj | 2018 | USA | 1982–2011 | 2,627/28,341 | Retrospective cohort study | HF | 4 | Risk of CRC |
| Chan AO | 2006 | Hong Kong(China) | 1997–2000 | 32/1,382 | Case-control | CHD | 5 | Risk of CRC |
| Gross CP | 2006 | USA | 1993–1999 | 5,590/29,733 | Retrospective cohort study | HF, AF | 7 | Postoperative prognosis |
| Lemmens VE | 2005 | Netherlands | 1995–2001 | 388/6,931 | Case-control | CVD | 5 | Postoperative prognosis |
| O’Neill C | 2022 | Northern Ireland | 2011–2019 | 4,656/35,000 | Case-control | CVD | 7 | Postoperative prognosis |
| Cuthbert CA | 2018 | Canada | 2004–2017 | 1,668/12,265 | Retrospective cohort study | CVD | 7 | Postoperative prognosis |
| Watanabe Y | 2005 | Japan | 1988–1990 | 799/110,792 | Retrospective cohort study | Stroke and AMI | 6 | Postoperative prognosis |
| Flynn DE | 2020 | Australia | 2010–2018 | 66/533 | Retrospective cross-sectional study | IHD, AF, HF | 5 | Postoperative prognosis |
| Koo CY | 2021 | Singapore | 2013–2015 | 86/412 | Retrospective cohort study | CHD | 6 | Postoperative prognosis |
| Kobo O | 2022 | USA | 2016–2018 | 103,224/20,737,247 | Case-control | AF, AMI, Stroke, HF, Cardiac arrest, SVT | 6 | Postoperative prognosis |
| Basso C | 2022 | Italy | 2013–2015 | 408/8,447 | Retrospective cohort study | HF | 5 | Postoperative prognosis |
| Sicheng Zhou | 2019 | China | 2007–2018 | 128/313 | Case-control | CVD | 5 | Postoperative prognosis |
| Yiming Li | 2023 | China | 2017–2022 | 68/132 | Case-control | CVD | 5 | Postoperative prognosis |
| Xuejin Zhang | 2018 | China | 2015–2016 | 71/132 | Case-control | CVD | 5 | Postoperative prognosis |
Baseline characteristics of the CVD and non-CVD groups
After pooling and analyzing the baseline data of the patients of the 23 studies, we found that diabetes (RR = 1.88[1.22, 2.90]; P = 0.0042), hypertension (RR = 1.35[1.09, 1.68]; P = 0.0058), CKD (RR = 3.03[1.18, 7.77]; P = 0.02) may increase the risk of CVD, especially CKD has 3 times risks for CVD. We also found that education stage (upon college) (RR = 0.78[0.73, 0.83]; P < 0.0001) may decrease the risk of CVD. These factors mentioned above may be the confounding factor for the CVD’s effect to CRC. As for gender (RR = 1.02[0.93, 1.11]; P = 0.72), alcohol drinking (RR = 0.87[0.70, 1.08]; P = 0.21), smoking (RR = 1.13[0.92, 1.40]; P = 0.22), family history of CRC (RR = 0.94[0.80, 1.12]; P = 0.49), marriage (RR = 0.97[0.85, 1.11]; P = 0.66) and pathological stage (RR = 0.94[0.74, 1.19]; P = 0.61) are not sure if there is a confounding factor. Other characteristics, including age (MD = 0.34, 95% CI −0.17 to 0.84), BMI (MD = 0.09, 95% CI −0.08 to 0.26), number of lymph node dissection (MD= −0.02[−0.31, 0.27]) and tumor location (MD = 0.26, 95% CI −0.11 to 0.63), were no statistical difference between the two groups (P > 0.05). (Table 2)
Table 2.
The baseline of included studies
| Characteristic | CVD | Studies | RR/MD (95% CI) | Heterogeneity | |
|---|---|---|---|---|---|
| Age, mean (SD), y | |||||
| 64.7 (12.8) | Yes | 11 | MD = 0.34[−0.17, 0.84]; P = 0.19 | I²=100% | |
| 46.6 (16.1) | No | 11 | |||
| BMI, mean (SD), kg/m2 | |||||
| 24.8 (4.2) | Yes | 5 | MD = 0.09[−0.08, 0.26]; P = 0.30 | I²=98% | |
| 26.4 (5.1) | No | 5 | |||
| Gender, number (%) | |||||
| Male | 8,359,011 (4.9) | Yes | 8 | RR = 1.02[0.93, 1.11]; P = 0.22 | I²=99% |
| Female | 6,974,096 (4.1) | Yes | 8 | ||
| Male | 73,154,350 (43.2) | No | 8 | ||
| Female | 80,943,661 (47.8) | No | 8 | ||
| Smoke, number (%) | |||||
| Yes | 8,797 (9.0) | Yes | 4 | RR = 1.13[0.92, 1.40]; P = 0.22 | I²=99% |
| No | 13,856 (14.1) | Yes | 4 | ||
| Yes | 59,056 (60.3) | No | 4 | ||
| No | 16,263 (16.6) | No | 4 | ||
| Alcohol, number (%) | |||||
| Yes | 5,551 (9.0) | Yes | 2 | RR = 0.87[0.70, 1.08]; P = 0.21 | I²=99% |
| No | 12,591 (20.5) | Yes | 2 | ||
| Yes | 28,162 (45.9) | No | 2 | ||
| No | 15,070 (24.6) | No | 2 | ||
| Diabetes, number (%) | |||||
| Yes | 40,239 (6.7) | Yes | 6 | RR = 1.88[1.22, 2.90]; P = 0.0042 | I²=99% |
| No | 119,150 (19.8) | Yes | 6 | ||
| Yes | 59,451 (9.9) | No | 6 | ||
| No | 382,289 (63.6) | No | 6 | ||
| Hypertension, number (%) | |||||
| Yes | 73,145 (12.2) | Yes | 5 | RR = 1.35[1.09, 1.68]; P = 0.0058 | I²=99% |
| No | 86,000 (14.3) | Yes | 5 | ||
| Yes | 160,703 (26.8) | No | 5 | ||
| No | 279,892 (46.7) | No | 5 | ||
| CKD, number (%) | |||||
| Yes | 15,960 (1.5) | Yes | 2 | RR = 3.03[1.18, 7.77]; P = 0.02 | I²=99% |
| No | 168,395 (16.2) | Yes | 2 | ||
| Yes | 14,366 (1.4) | No | 2 | ||
| No | 839,071 (80.9) | No | 2 | ||
| Family History of CRC, number (%) | |||||
| Yes | 136 (0.5) | Yes | 2 | RR = 0.94[0.80, 1.12]; P = 0.49 | I²=0 |
| No | 1,490 (5.2) | Yes | 2 | ||
| Yes | 2,515 (8.7) | No | 2 | ||
| No | 24,614 (85.6) | No | 2 | ||
| Get married, number (%) | |||||
| Yes | 8,905,798 (5.3) | Yes | 2 | RR = 0.97[0.85, 1.11]; P = 0.66 | I²=99% |
| No | 6,245,704 (3.7) | Yes | 2 | ||
| Yes | 86,855,102 (51.6) | No | 2 | ||
| No | 66,462,936 (51.6) | No | 2 | ||
| Education upon College number (%) | |||||
| Yes | 7,375,136 (4.4) | Yes | 2 | RR = 0.78[0.73, 0.83]; P < 0.0001 | I²=89% |
| No | 7,776,366 (4.6) | Yes | 2 | ||
| Yes | 98,471,081 (58.5) | No | 2 | ||
| No | 54,846,957 (32.5) | No | 2 | ||
| Pathological Stage, number (%) | |||||
| Ⅰ+Ⅱ | 111 (21.3) | Yes | 3 | RR = 0.94[0.74, 1.19]; P = 0.61 | I²=31% |
| Ⅲ+Ⅳ | 156 (30.0) | Yes | 3 | ||
| Ⅰ+Ⅱ | 113 (21.7) | No | 3 | ||
| Ⅲ+Ⅳ | 140 (27.0) | No | 3 | ||
| Number of Lymph Node Dissection, mean (SD) | |||||
| 17.6 (4.6) | Yes | 3 | MD= −0.02[−0.31, 0.27]; P = 0.89 | I²=63% | |
| 17.7 (4.0) | No | 3 | |||
| Location, number (%) | |||||
| Colon | 1,011 (10.9) | Yes | 3 | RR = 1.13[1.09, 1.16]; P = 0 | I²=0 |
| Rectal | 334 (3.6) | Yes | 3 | ||
| Colon | 5,476 (59.0) | No | 3 | ||
| Rectal | 2,456 (26.5) | No | 3 | ||
Abbreviations: SD, Standard Deviation; CVD, cardiovascular disease; BMI, Body Mass Index; CKD, Chronic Kidney Disease
Risk of colorectal cancer in cardiovascular disease and non-cardiovascular disease groups
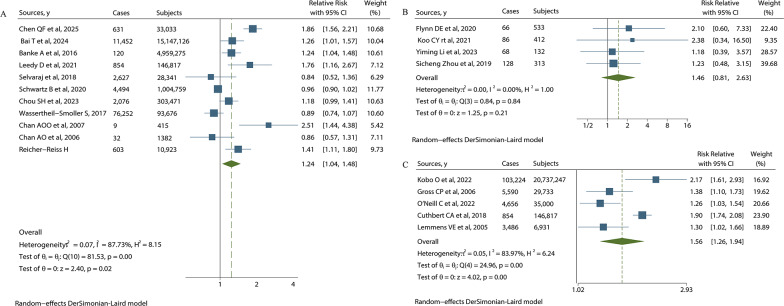
The study found that CVD is a risk factor for CRC morbidity (RR = 1.24, 95%CI 1.04 to 1.48). For postoperative complications, CVD has a significant effect on arrhythmia in CRC patients (RR = 3.05, 95%CI 1.27 to 7.35), whereas it doesn’t have a significant effect on the total postoperative complications (RR = 1.46, 95%CI 0.81 to 2.63). CVD increases all-cause mortality in CRC patients (RR = 1.56, 95%CI 1.26 to 1.94). There is no significant difference in the effect of CVD on perioperative metrics such as surgical time (RR = 0.10, 95%CI −0.07 to 0.27), surgical blood loss (RR = 0.16, 95%CI −0.09 to 0.41), postoperative exhaust time (RR = 0.04, 95%CI −0.16 to 0.24) and postoperative hospital stay (RR = −0.03, 95%CI −0.29 to 0.23). And there is no significant difference in the effect of CVD on the higher complication grade of postoperative Clavien-Dindo (RR = 0.56, 95%CI 0.27 to 1.18), incision infection (RR = 1.19, 95%CI 0.63 to 2.24), anastomotic leakage (RR = 2.1, 95%CI 0.83 to 5.37), intestinal obstruction (RR = 0.98, 95%CI 0.38 to 2.54), abdominal infection (RR = 0.96, 95%CI 0.30 to 3.10), abdominal bleeding (RR = 0.97, 95%CI 0.18 to 5.37), HF (RR = 2.45, 95%CI 0.47 to 12.87) and acute coronary syndrome (ACS) (RR = 3.32, 95%CI 0.89 to 12.35). (Fig. 2)
Fig. 2.
Forest plot of CVD’s effects on CRC: (A) shows CVD’s effect on morbidity of CRC. (B) shows CVD’s effect on postoperative complication of CRC. (C) shows CVD’s effect on all-cause mortality of CRC
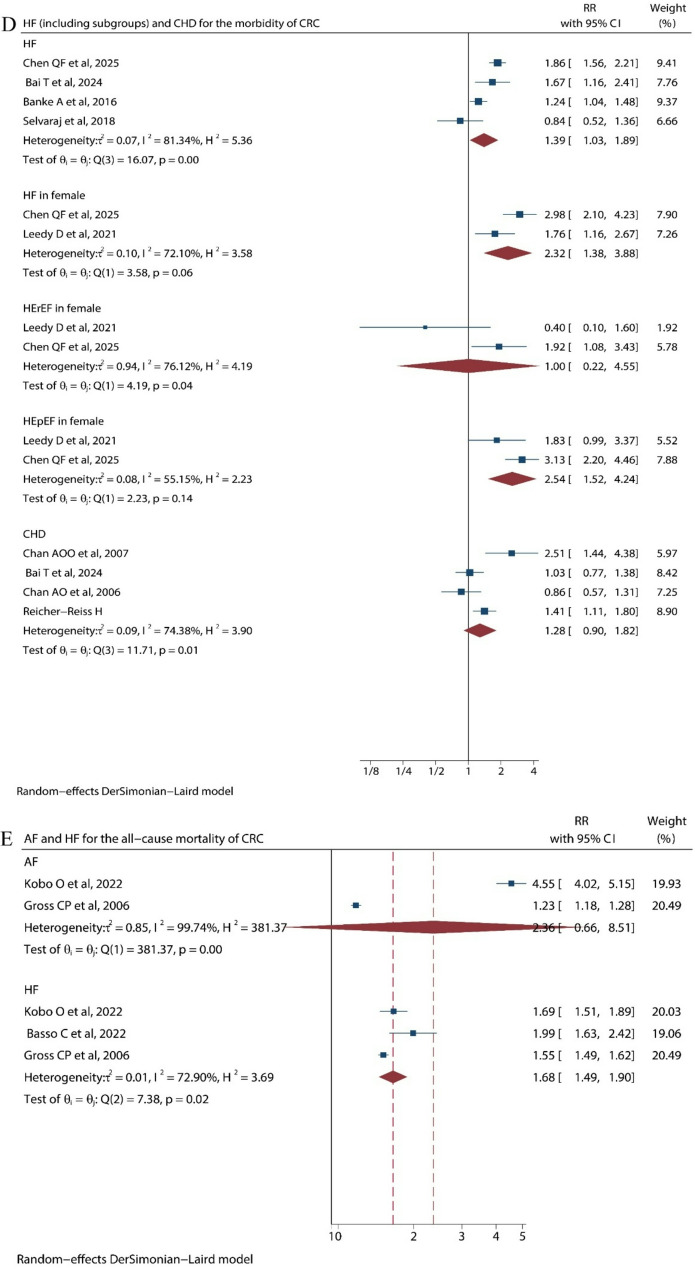
Further analysis of subgroup of CVD shows that HF was a risk factor for CRC incidence (RR = 1.39, 95%CI 1.03 to 1.89) and all-cause mortality (RR = 1.68, 95%CI 1.49 to 1.90), while CHD (RR = 1.26, 95%CI 0.74 to 2.14) and AF (RR = 2.36, 95%CI 0.66 to 8.51) don’t have significant effect on all-cause mortality of CRC. HF, as a kind of CVD, also has subtypes based on ejection fraction. These are heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF). Among female patients, HF (RR = 2.32, 95% CI 1.39 to 3.88) and HFpEF (RR = 2.54, 95% CI 1.52 to 4.24) were the risk factors for CRC development, whereas it is indefinite for male patients due to insufficient literature (Fig. 3) (Table 3).
Fig. 3.
Forest plot of cardiovascular disease subgroups on colorectal cancer: (D) shows subgroups’ effect on morbidity of CRC. (E) shows subgroups’ effect on all-cause mortality of CRC
Table 3.
Effect of CVD on CRC
| Characteristics | Studies | Cases/Subjects | Relative Risk/Mean Difference (95% CI) | Model | Heterogeneity |
|---|---|---|---|---|---|
|
Risk of CRC CVD† HF† HF in female† HFrEF in female HFpEF in female† CHD Postoperative prognosis Surgical time/min Surgical blood loss/ml Postoperative exhaust time/day Postoperative hospital stay/day All complications Clavien-Dindo complication grade Incision Infection Anastomotic Leakage Intestinal Obstruction Abdominal Infection Abdominal Bleeding HF ACS Arrhythmias† All-cause mortality in CVD† All-cause mortality in AF All-cause mortality in HF† |
11 4 2 2 2 3 3 2 2 2 4 2 2 2 2 2 2 2 3 3 5 2 3 |
99,150/21,729,218 15,174,575/153,279,600 10,805/148,807 70/1415 173/1312 15,147,500/153,237,790 267/253 196/192 196/192 196/192 348/1,390 196/192 196/192 196/192 196/192 196/192 196/192 196/192 267/253 267/253 117,810/20,955,728 2,061,081/18,531,591 1,530,786/19,112,649 |
RR = 1.24[1.04, 1.48]; P = 0.02 RR = 1.39[1.03, 1.89]; P = 0.0307 RR = 2.32[1.39, 3.88]; P = 0.0014 RR = 1.00[0.22, 4.55]; P = 0.9996 RR = 2.54[1.52, 4.24]; P = 0.0004 RR = 1.26[0.74, 2.14]; P = 0.4032 MD = 0.10[−0.07, 0.27]; P = 0.246 MD = 0.16[−0.09, 0.41]; P = 0.1984 MD = 0.04[−0.16, 0.24]; P = 0.41 MD= −0.03[−0.29, 0.23]; P = 0.8724 RR = 1.46[0.81, 2.63]; P = 0.21 RR = 0.56[0.27, 1.18]; P = 0.1295 RR = 1.19[0.63, 2.24]; P = 0.5857 RR = 2.1[0.83, 5.37]; P = 0.1194 RR = 0.98 [0.38, 2.54]; P = 0.9674 RR = 0.96[0.30, 3.10]; P = 0.9473 RR = 0.97[0.18, 5.37]; P = 0.9721 RR = 2.45[0.47, 12.87]; P = 0.2904 RR = 3.32[0.89, 12.35]; P = 0.073 RR = 3.05[1.27, 7.35]; P = 0.0127 RR = 1.56[1.26, 1.94]; P < 0.01 RR = 2.36[0.66, 8.51]; P = 0.1887 RR = 1.68[1.49, 1.90]; P < 0.00001 |
DL DL DL DL DL DL DL DL DL DL DL DL DL DL DL DL DL DL DL DL DL DL DL |
I2 = 88%; P < 0.01 I2 = 81%; P = 0.0011 I2 = 72%; P = 0.0583 I2 = 76%; P = 0.0407 I2 = 55%; P = 0.1354 I2 = 80; P = 0.0070 I2 = 0; P = 0.7753 I2 = 31; P = 0.2281 I2 = 0; P = 0.9788 I2 = 37; P = 0.2082 I2 = 0; P = 0.84 I2 = 0; P = 0.6776 I2 = 0; P = 0.9763 I2 = 0; P = 0.2916 I2 = 0; P = 0.6333 I2 = 0; P = 0.5186 I2 = 0; P = 0.4401 I2 = 0; P = 0.7828 I2 = 0; P = 0.8090 I2 = 0; P = 0.8547 I2 = 84; P < 0.01 I2 = 100%; P < 0.0001 I2 = 73%; P = 0.025 |
Significant results are marked with an dagger†
CI, confidence intervals; HF, Heart Failure; HFrEF, Heart Failure with Reduced Ejection Fraction; HFpEF, Heart Failure with Preserved Ejection Fraction; AMI, Acute Myocardial Infarction; CHD, Coronary Heart Disease; AF, Atrial Fibrillation; CVD, cardiovascular disease; ACS, Acute Coronary Syndrome
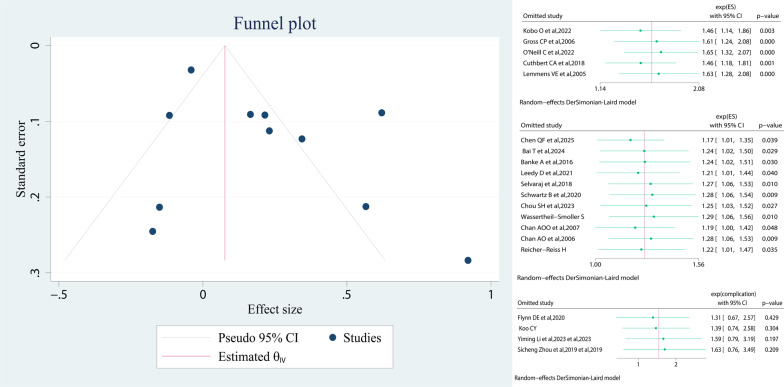
Analysis of sensitivity and publication bias
Sensitivity analysis was performed by excluding 1 study at a time. We did sensitivity analysis for the effect values of CRC morbidity, postoperative complications, and all-cause mortality, showing that excluding any of the studies did not significantly change the results. Publication bias of included studies was based on visual inspection of funnel plots. Our funnel plot for the risk of CVD on CRC morbidity was symmetrical and no significant publication bias was found (Fig. 4).
Fig. 4.
Publication bias funnel plot and sensitivity analysis
Discussion
Our study investigated the association of CVD with CRC morbidity and short-term and long-term prognosis. The analysis leads to the following conclusions that CVD (especially HF) has some association with the risk of developing CRC and all-cause mortality. But the effect on postoperative complications is only found in arrhythmias and inconclusive in other complications. Further subgroup analysis may reveal heterogeneity of disease subtypes and genders in the effect.
Several studies have shown that patients diagnosed with CRC are at high risk of developing CVD [51–56], CRC survivors have 1.37 times higher risk of CVD and a 1.52 times higher risk of HF than those without cancer [57], and patients with HF who have comorbid cancers are at a higher risk of death [58]. Regarding the impact of CVD on CRC, patients with intermediate- and high-risk CVD have a higher risk of having advanced CRC [59], and negative effects on overall health-related quality of life (HRQoL), physical, role functioning, fatigue and dyspnea. CVD and CRC share common risk factors, for example, BMI, smoking, alcohol drinking, diabetes, hypertension, hyperlipidemia and so on. And CVD’s risk factors can increase the risk of CRC [60, 61].
Our findings demonstrate a clear clinical association between CVD and CRC, and there are various views on how CVD induces CRC. Previously mentioned CVD and CRC share some of the risk factors, and the specific mechanism of action may be related to the inflammation induced by these risk factors, such as some hormones (e.g., leptin), cytokines, growth factors and other metabolic responses are associated with both diseases [62]. Another possible mechanism is reverse cardiac oncology, where it has been suggested that the expression profiles of CVD-associated miRNA undergoes changes that may affect tumor formation [63, 64] There is also a possibility that intestinal hypoperfusion due to CVD may disrupt the mucosal barrier and increase susceptibility to carcinogens [65]. The pathogenesis of some specific cardiovascular diseases and cancers is being progressively investigated, such as the impaired lipid metabolism closely related to coronary atherosclerotic plaques in coronary heart disease, which may be involved in tumor progression [66], and the involvement of microRNAs as key post-transcriptional regulators in lipid metabolmis. Biological evidence suggests that hypoxia-inducible factor 1 (HIF-1), produced in response to myocardial infarction, stimulates tumor growth by promoting the expression of antiapoptotic factors and angiogenesis [67]. There are also articles mentioning chronic inflammation, altered immune response, activation of stromal and vascular cells, and underlying signaling pathways leading to pathological tissue remodeling as potential mechanisms for cardiovascular disease-induced colorectal cancer and for practical applications such as drug development [68]. In addition, the hypoxic state of the body induced by cardiovascular disease may induce cancer by regulating the tumor microenvironment through many signaling pathways in apoptosis, autophagy, DNA damage, mitochondrial activity, p53, and drug efflux [69]. On the other side, as for how CRC affects CVD, it has been found that cancer cell metabolism can extend beyond the tumor microenvironment to affect the cardiovascular system. For example, a cancer metabolite, D2-HG, is released into the blood stream and may cause a toxic response in the cardiovascular system [70]. CRC patients with comorbid CVD have a significantly higher risk of postoperative arrhythmias, which may be related to cardiac overload due to postoperative electrolyte disturbances and excessive fluid-based medications, which are also amplified by comorbid CVD [71]. HFpEF is more prevalent in females than in males, and HFpEF is associated with the development of CRC in females. These suggest that during menopause, decreasing estrogen may be a risk factor for HF, but the mechanism of HFpEF is currently unclear [72]. The role of HFrEF remains unclear and may be limited by sample size or disease heterogeneity.
Explorations about the interrelationship between CVD and CRC have proposed new treatment modalities, such as aspirin, a common antiplatelet drug, which has become a hotspot of research on the prevention of CRC, even though the protective effect of aspirin against CRC is still controversial [73, 74]. Statins and estrogen replacement therapy may have a positive effect on preventing the development of CRC and CVD and improving prognosis [62]. Statins may reduce the risk of colorectal cancer and death by regulating intestinal flora through an increase in tryptophan metabolites [75]. Beta-adrenergic receptors antagonized by beta-blockers provide a survival benefit to breast and lung cancer patients by reducing norepinephrine secretion and migratory effects on tumors [76]. Cardiovascular disease drugs combined with anticancer drugs represented by diterpenoid drugs [77] may be the future direction of anticancer.
This study is the first meta-analysis of the impact of CVD on CRC morbidity as well as prognosis and includes subgroup analysis of gender and cardiovascular subtypes. The study assesses the quality of the literature according to NOS, counts the sample size, and assesses publication bias and sensitivity analyses. Limitations of this study: First, the heterogeneity of the included studies is high (I² values are mostly more than 50%), which may stem from differences in the definition of CVD, follow-up time, and control of confounders among different studies. Second, the only developing country included in this study is China, and the others were developed countries, which may be biased for studying the global spectrum of the disease. Third, regarding the results of the studies on perioperative metrics and postoperative complications, there were only 3 Chinese studies included in the study, with small sample sizes and weak representativeness of geographical heterogeneity. Fourth, most of the studies were retrospective, and confounding bias may occur (e.g., not adjusted for diet, exercise, or genetic factors). Fifth, We included only literature after January 1, 2000 in order to reduce the heterogeneity in people’s chronological lifestyle habits in the older studies and to improve the quality of the included literature, but setting the start date of the search at 2000 lacked a basis, which may have resulted in a selection bias, and also resulted in a smaller number of included literature, which did not allow for more in-depth subgroup analyses to be conducted. For example, most of the available studies focused on all-cause mortality and lacked analyses of CVD’s specific mortality or CRC recurrence. Sixth, this article was limited to the number of included literature to better reflect the effect of cardiovascular disease subtypes on colorectal cancer from subgroup analyses, and it was not matched with a suitable article from the baseline data to verify whether the factors of tumor site, drug intervention, time of illness, and lifestyle habits play a role in interfering with the role of cardiovascular disease in colorectal cancer. For example, tumor site factors may have an impact in the prognosis of cardiovascular disease on colorectal cancer, such as the higher chance of lower gastrointestinal bleeding in rectal cancer than in other sites of colorectal cancer [78], and subgroup analyses of tumor sites, to exclude interferences, may serve as a direction for further research. Seventh, the paper’s treatment of the transformation of effect sizes is not quite right; the risk of illness does not change over time as a condition for the transformation of HR into RR, and the paper lacks persuasiveness on this point. Future studies should explore the molecular interaction mechanism between CVD and CRC through prospective cohort design and the combination of multi-omics technology (e.g., metabolomics, intestinal flora analysis). In addition, the development of individualized CRC screening guidelines for patients with CVD, enhanced CRC screening and optimization of perioperative cardiac monitoring may improve prognosis.
Conclusion
This study suggests that CVD (e.g., HF) is probably a significant risk factor for CRC morbidity and all-cause mortality but has a uncertain impact on postoperative complications (Only the risk of arrhythmia was observed to be increased). Enhanced CRC screening for patients with CVD and targeted management of perioperative cardiac risk are needed in clinical practice. In the future, the mechanistic associations between specific cardiovascular subtypes and CRC need to be further elucidated and interdisciplinary intervention strategies explored.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledgment to all the authors in this article.
Abbreviations
- CVD
Cardiovascular disease
- CRC
Colorectal cancer
- RR
Relative risk
- CI
Confidence intervals
- HF
Heart Failure
- HFpEF
Heart Failure with Preserved Ejection Fraction
- HFrEF
Heart Failure with Reduced Ejection Fraction
- CHD
Coronary Heart Disease
- AF
Atrial Fibrillation
- CKD
Chronic kidney disease
- BMI
Body Mass Index
- MACE
Major adverse cardiac events
- HR
Hazard ratio
- RR
RR: Relative risk ratio
- OR
Odds ratio
- NOS
The Newcastle-Ottawa Scale
- SD
Standard deviation
- MD
Mean difference
- ACS
Acute coronary syndrome
Author contributions
Ling-Yun Xie designed research directions, Si-Qi Li and Lang Wang independently searched studies and collected data, Si-Qi Li wrote the first draft, Lang Wang checked raw data, Xiao-Yu Liu revised the manuscript, Xu-Rui Liu used software for analysis, all authors read and approved the final manuscript.
Funding
Natural Science Foundation of Chongqing, Grand/Award Number: cstc2019jcyj-msxmX0054; the Science and Technology Planning Project of Yuzhong District of Chongqing City, Grand/Award Number: 20210115.
Data availability
All data, from 23 articles involving 5,770,648 patients, were obtained from the published literature mentioned (feasible Doi attached) or websites (URLs attached) in the ‘References’ section.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Si-Qi Li and Lang Wang contributed equally to this work and share first authorship.
References
- 1.World Health Organization. Colorectal cancer [Internet]. 2023 [cited 2025 Jan 1]. Available from: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer
- 2.The Lancet. Colorectal cancer screening: FIT for purpose? Lancet. 2025;405(10484):0. 10.1016/S0140-6736(25)00619-1. [DOI] [PubMed] [Google Scholar]
- 3.Patricia P-P, Hooi CG-AE, Asieh T, Talita G, Weinberger D-SA, Antonella R, Yi DW, Edward M, Daniel B, Danielle P-A, Newby. Incidence and survival of colorectal cancer in the united Kingdom from 2000–2021: a population-based cohort study. Am J Gastroenterol. 2025;0(0):0. 10.14309/ajg.0000000000003460. [Google Scholar]
- 4.Rebecca L, Siegel N, Sandeep W, Andrea C, Robert A, Smith., Ahmedin J. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):0. 10.3322/caac.21772. [Google Scholar]
- 5.Jiajie Z, Qizhi Y, Shuai Z, Longhe S, Ruiqi L, Jie W, Liuhua W, Daorong W. Evolving landscape of colorectal cancer: global and regional burden, risk factor dynamics, and future scenarios (the global burden of disease 1990–2050). Ageing Res Rev. 2025;104(0):0. 10.1016/j.arr.2025.102666. [DOI] [PubMed] [Google Scholar]
- 6.Md Sanower H, Hidayah K, Abdulrahman A, Zannat J, Jiun UD, Akbar O, Chee JY, Kaderi LKM, Mohiuddin KAKM, Chiau L, Wen MK, Abdul GM, Hadi. Colorectal cancer: A review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers (Basel). 2022;14(7):0. 10.3390/cancers14071732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qing L, Shan G, Hao L, Wei W, Ya-Qi M, Qing L, Lu W, Guan-Bin S, Jian-Peng S, Bo X. Signaling pathways involved in colorectal cancer: pathogenesis and targeted therapy. Signal Transduct Target Ther. 2024;9(1):0. 10.1038/s41392-024-01953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cynthia S, Bonhof., Floortje M, Jos W, Widdershoven., Dounya S. Colorectal cancer and cardiovascular disease: double the burden when it comes to your health-related quality of life? Acta Oncol. 2023;62(7):0. 10.1080/0284186X.2023.2245131. [DOI] [PubMed] [Google Scholar]
- 9.Eileen M, Melina A, Gini. A, Lorenzoni. V, Cabasag CJ, Mathieu L, Jerome V, Jacques F, Neil M, Freddie B. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72(2):0. 10.1136/gutjnl-2022-327736. [DOI] [PubMed] [Google Scholar]
- 10.Agustina T. A triple threat in colorectal cancer. Nat Rev Microbiol. 2025;23(5):0. 10.1038/s41579-025-01173-y. [DOI] [PubMed] [Google Scholar]
- 11.Kathryn A, Robb., Ben Y, Marie K, Murphy., Patrycja D, Alex MC, Gareth J, Hollands C, Sara MC, Ronan M E, O’Carroll, Rory C, O’Connor., Robert J C, Steele. (2025). Behavioural interventions to increase uptake of FIT colorectal screening in Scotland (TEMPO): a nationwide, eight-arm, factorial, randomised controlled trial. Lancet, 405(10484), 0. 10.1016/S0140-6736(24)02813-7 [DOI] [PubMed]
- 12.Nauzer F. Yields and completion rates of colorectal cancer screening modalities in patients aged 45–50. Gastroenterology. 2025;0(0):0. 10.1053/j.gastro.2025.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Juha KA, Rinne., Heikki H, Tarja P, Arto T, Anne M, Kyösti T, Olli H, Pasi O, Tero R. Jyrki, Kössi.(2025). Indocyanine green fluorescence imaging in prevention of colorectal anastomotic leakage: A randomized clinical trial. JAMA Surg, 0(0), 0. 10.1001/jamasurg.2025.0006 [DOI] [PMC free article] [PubMed]
- 14.Roy H, Manon O, Gabriela F, Samuel A, Amine AA, Hervé A, Annie VR, Thibault Calvé, Claire C, Sophie G, Nassima Thérien, François T, Herawaty D, Rasmy S, Frank L, Richard S, Ramses R, Manuela M. Wassef., Eric, De Broux., Carole, Richard., Santos.(). Modulating gut microbiota prevents anastomotic leak to reduce local implantation and dissemination of colorectal cancer cells after surgery. Clin Cancer Res, 30(3), 0. [DOI] [PubMed]
- 15.Thea Helene D, Rask I, Benzon M-HS, Kristina LT, Anne Kjær, Krüger TjønnelandS, Christoffer Kjær, Ismail J, Oksbjerg GögenurS, Dalton. Cardiovascular events after elective colorectal cancer surgery in patients with stage I-III disease with no previous cardiovascular disease. Acta Oncol. 2023;62(7):0. 10.1080/0284186X.2023.2212844. [DOI] [PubMed] [Google Scholar]
- 16.Adrian G, Ulla G, Adrianna M, Helena L-Q, Peter L, Pablo P. The economic burden of cardiovascular disease and hypertension in low- and middle-income countries: a systematic review. BMC Public Health. 2018;18(1):0. 10.1186/s12889-018-5806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ian F, Walker., Fredrike G, Judy W, Ian N, Naveen A, Nida K, Helen E. The economic costs of cardiovascular disease, diabetes mellitus, and associated complications in South asia: A systematic review. Value Health Reg Issues. 2018;15(0):0. 10.1016/j.vhri.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Sheng-Shou H. Epidemiology and current management of cardiovascular disease in China. J Geriatr Cardiol. 2024;21(4):0. 10.26599/1671-5411.2024.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kershaw V, Patel., Zainali C, Subodh V, Matthew W, Segar., Katelyn R, Garcia., Chiadi E, Ndumele., Thomas J, Wang. JL Jr, Antoni J, Javed B-G, Carolyn B, Lam SP, Christie M, Ballantyne., James A, de Lemos., Alain G. Intensive lifestyle intervention, cardiac biomarkers, and cardiovascular outcomes in diabetes: look AHEAD cardiac biomarker ancillary study. J Am Coll Cardiol. 2024;85(5):0. 10.1016/j.jacc.2024.11.004. Bertoni., Mark, Espeland., Ambarish, Pandey.(. [Google Scholar]
- 20.Richard FD, Richard H, McManus. J, Clare J, Taylor., Nicholas R, Jones., Joy K, Rahman J, Sungwook W, Joseph K, Louise K, Jennifer J, Hirst A, Ly-Mee Y, Sam M. Low-dose spironolactone and cardiovascular outcomes in moderate stage chronic kidney disease: a randomized controlled trial. Nat Med. 2024;30(12):0. 10.1038/s41591-024-03263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heidi N, Vera J, Stefan J, Schunk., Raymond V, Sahir K, Joachim J. Post-translational modifications in kidney diseases and associated cardiovascular risk. Nat Rev Nephrol. 2024;20(8):0. 10.1038/s41581-024-00837-x. [DOI] [PubMed] [Google Scholar]
- 22.Dan-Qing L, Hong-Min L, Hao-Jie C, Shu-Min L, Xu-Lian T, Cheng-Shen Q, Li-Ying D, Hong-Xuan H, Zhi-Yuan X, Ling K, Bing-Yun Z, Pei-Dong Z, Jian G, Wen-Fang Z, Pei-Liang C, Dan L, Jin Y, Qing-Mei. Huang., Chen, Mao., Zhi-Hao, Li.(2025). Association of Accelerometer-Derived physical activity pattern with the risks of All-Cause, cardiovascular disease, and cancer death. J Am Heart Assoc, 14(8), 0. 10.1161/JAHA.124.039225 [DOI] [PMC free article] [PubMed]
- 23.Zhang C, Cheng Y, Luo D, et al. Association between cardiovascular risk factors and colorectal cancer: a systematic review and meta-analysis of prospective cohort studies. EClinicalMedicine. 2021;34:100794. 10.1016/j.eclinm.2021.100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsin-Yin H, Yih-Jong C, Cheng-Tzu H, Tzu-Lin Y, Ming-Chieh T, Chia-Chun W, Bo-Yu H, Jing-Rong J, Chun-Ju C, Wen-Chung L, Kuo-Liong C. Increased standardised incidence ratio of cardiovascular diseases among colorectal cancer patients. Int J Colorectal Dis. 2022;37(4):0. 10.1007/s00384-022-04129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jawad Ahmad Z, Mikail Gögenur, Sarah E, Ismail Gögenur(. Major adverse cardiovascular events after colorectal cancer surgery, oncological outcomes, and Long-term mortality: A nationwide retrospective propensity Score-Matched cohort study. Ann Surg Open. 2025;6(1):0. 10.1097/AS9.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taolan Z, Hongxia Z, Hongjuan H, Haihong H, Wendi Z, Lingxiang J, Ming T, Wei DE, Yaoguang H, Junlin F, Mingxiang Z, Zou. Cardiovascular-specific mortality and risk factors in colorectal cancer patients: A cohort study based on registry data of over 500,000 individuals in the US. Prev Med. 2023;179(0):0. 10.1016/j.ypmed.2023.107796. [DOI] [PubMed] [Google Scholar]
- 27.Ji Hyun S, Su-Yeon C, Sun Y, Young KS, Kyung-Do Y. Increased risk of colorectal cancer in young males with higher cardiovascular risk: a nationwide population-based cohort study. World J Gastrointest Oncol. 2025;17(3):0. 10.4251/wjgo.v17.i3.101260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai T, Wu C. Association of cardiovascular disease on cancer: observational and Mendelian randomization analyses. Sci Rep. 2024. 10.1038/s41598-024-78787-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basso C, Gennaro N, Dotto M, Ferroni E, Noale M, Avossa F, Corti MC. Congestive heart failure and comorbidity as determinants of colorectal cancer perioperative outcomes. Updates Surg. 2022;74(2):609–17. 10.1007/s13304-021-01086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan AOO, Jim MH, Lam KF, Morris JS, Siu DCW, Tong T, Lam SK. Prevalence of colorectal neoplasm among patients with newly diagnosed coronary artery disease. Jama-Journal Am Med Association. 2007;298(12):1412–9. 10.1001/jama.298.12.1412. [DOI] [PubMed] [Google Scholar]
- 31.Chan AOO, Lam KF, Tong T, Siu DCW, Jim MH, Hui WM, Wong BCY. Coexistence between colorectal cancer/adenoma and coronary artery disease: results from 1382 patients. Aliment Pharmacol Ther. 2006;24(3):535–9. 10.1111/j.1365-2036.2006.02958.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen Q-F, Katsouras CS, Liu C, Shi J, Luan X, Ni C, Zhou X-D. Gender-specific risks for cancer incidents in patients with different heart failure phenotypes. ESC Heart Fail. 2025;12(1):497–507. 10.1002/ehf2.15097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chou S-H, Lin C-P, Lin Y-S, Lee T-H, Yang C-K, Lin Y-S, Chu P-H. Sex disparities in the association between acute myocardial infarction and colon cancer risk. Cancer Med. 2023;12(3):2958–69. 10.1002/cam4.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flynn DE, Mao D, Yerkovich ST, Franz R, Iswariah H, Hughes A, Chandrasegaram MD. The impact of comorbidities on post-operative complications following colorectal cancer surgery. PLoS One. 2020. 10.1371/journal.pone.0243995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobo O, Moledina SM, Raisi-Estabragh Z, Shanmuganathan JWD, Chieffo A, Ayoubi A, Mamas F, M. A. Emergency department cardiovascular disease encounters and associated mortality in patients with cancer: A study of 20.6 million records from the USA. Int J Cardiol. 2022;363:210–7. 10.1016/j.ijcard.2022.06.053. [DOI] [PubMed] [Google Scholar]
- 36.Koo CY, Tai B-C, Chan DKH, Tan LL, Tan KK, Lee C-H. Chemotherapy and adverse cardiovascular events in colorectal cancer patients undergoing surgical resection. World J Surg Oncol. 2021. 10.1186/s12957-021-02125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leedy DJ, Reding KW, Vasbinder AL, Anderson GL, Barac A, Wactawski-Wende J, Cheng RK. The association between heart failure and incident cancer in women: an analysis of the women’s health initiative. Eur J Heart Fail. 2021;23(10):1712–21. 10.1002/ejhf.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wassertheil-Smoller S, McGinn AP, Martin L, Rodriguez BL, Stefanick ML, Perez M. The associations of atrial fibrillation with the risks of incident invasive breast and colorectal cancers. Am J Epidemiol. 2017;185(5):372–84. 10.1093/aje/kww185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sicheng Z, Zheng W, Haitao Z, et al. Analysis of the impact of cardiovascular disease on the perioperative period of elderly patients with colorectal cancer aged over 80 years old[J]. Chin J Clin Oncol. 2019;46(05):233–8. [Google Scholar]
- 40.Li Yiming C, Kuilong L. Analysis of postoperative complications in elderly patients with colorectal cancer and cardiovascular disease[J]. Knowl Prev Treat Cardiovasc Dis. 2023;13(10):49–52. [Google Scholar]
- 41.Zhang X. Effect of cardiovascular disease on surgical outcome and cardiovascular complications in elderly patients with colorectal cancer[J]. Chin J Anorectal Dis. 2018;38(12):17–9. [Google Scholar]
- 42.Watanabe Y, Ozasa K, Ito Y, Suzuki K, Kojima M, Suzuki S, Tokudome S, Tamakoshi K, Toyoshima H, Kawado M, Hashimoto S, Hayakawa N, Wakai K, Tamakoshi A, JACC Study Group. Medical history of circulatory diseases and colorectal cancer death in the JACC study. J Epidemiol. 2005;15:S168–72. PMID: 16127229; PMCID: PMC8639038. [DOI] [PMC free article] [PubMed]
- 43.Reicher-Reiss H, Jonas M, Goldbourt U, Boyko V, Modan B. Selectively increased risk of cancer in men with coronary heart disease. Am J Cardiol. 2001;87(4):459–62. 10.1016/s0002-9149(00)01405-3. [DOI] [PubMed] [Google Scholar]
- 44.Banke A, Schou M, Videbaek L, Møller JE, Torp-Pedersen C, Gustafsson F, Dahl JS, Køber L, Hildebrandt PR, Gislason GH. Incidence of cancer in patients with chronic heart failure: a long-term follow-up study. Eur J Heart Fail. 2016;18(3):260–6. 10.1002/ejhf.472. Epub 2016 Jan 10. PMID: 26751260. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz B, Schou M, Gislason GH, Køber L, Torp-Pedersen C, Andersson C. Prevalence and incidence of various cancer subtypes in patients with heart failure vs matched controls. Int J Cardiol. 2020;316:209–13. 10.1016/j.ijcard.2020.05.035. [DOI] [PubMed] [Google Scholar]
- 46.Selvaraj S, Bhatt DL, Claggett B, Djoussé L, Shah SJ, Chen J, Imran TF, Qazi S, Sesso HD, Gaziano JM, Schrag D. Lack of association between heart failure and incident cancer. J Am Coll Cardiol. 2018;71(14):1501–10. 10.1016/j.jacc.2018.01.069. [DOI] [PubMed] [Google Scholar]
- 47.Cary P, Gross., Zhenchao G, Gail J, McAvay., Heather G, Allore., Mary Y, Mary E, Tinetti. Multimorbidity and survival in older persons with colorectal cancer. J Am Geriatr Soc. 2007;54(12):0. 10.1111/j.1532-5415.2006.00973.x. [DOI] [PubMed] [Google Scholar]
- 48.Ciaran ON, Donnelly DW, Mark H, Therese K, Colin R, Fox., Gerard W, Anna G. Survival of cancer patients with pre-existing heart disease. BMC Cancer. 2022;22(1):0. 10.1186/s12885-022-09944-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.V E P P, Lemmens. MLG, Janssen-Heijnen C, D G W, Verheij S, Houterman. OJ, Repelaer, van Driel., J W, W. Coebergh.(2005). Co-morbidity leads to altered treatment and worse survival of elderly patients with colorectal cancer. Br J Surg, 92(5), 0. 10.1002/bjs.4913 [DOI] [PubMed]
- 50.Colleen A, Cuthbert., Brenda R, Yuan H, Winson X, Cheung Y. The effect of comorbidities on outcomes in colorectal cancer survivors: a population-based cohort study. J Cancer Surviv. 2018;12(6):0. 10.1007/s11764-018-0710-z. [DOI] [PubMed] [Google Scholar]
- 51.Justin C, Brown., Bette J, Caan., Carla M, Prado., Erin W, Jingjie X, Elizabeth M, Cespedes Feliciano., Candyce H, Kroenke., Jeffrey A, Meyerhardt. Body composition and cardiovascular events in patients with colorectal cancer: A Population-Based retrospective cohort study. JAMA Oncol. 2019;5(7):0. 10.1001/jamaoncol.2019.0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Desai R, Mondal A, Patel V, Singh S, Chauhan S, Jain A. Elevated cardiovascular risk and acute events in hospitalized colon cancer survivors: a decade-apart study of two nationwide cohorts. World J Clin Oncol. 2024;15(4):548–53. 10.5306/wjco.v15.i4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mehdi N, Vandana K, Cassius B, Mohammad G, Tahmineh H, Anahita S, Hassan B, Elsayed Z. Soliman., Hassan, Ashktorab.(2015). Atrial fibrillation and colonic neoplasia in African Americans. PLoS ONE, 10(8), 0. 10.1371/journal.pone.0135609 [DOI] [PMC free article] [PubMed]
- 54.Wesley T, O’Neal., Susan G, Lakoski., Waqas Q, Suzanne E, Judd., George H, Virginia J, Howard., Mary C, Elsayed Z, Soliman. Relation between cancer and atrial fibrillation (from the reasons for geographic and Racial differences in stroke Study). Am J Cardiol. 2015;115(8):0. 10.1016/j.amjcard.2015.01.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cilie C, van ‘t Klooster., Paul M, Ridker., Nancy R, Cook., Joachim GJV, Aerts J, Folkert W, Asselbergs W, van der Graaf Y, Frank LJ, Visseren. Prediction of lifetime and 10-Year risk of cancer in individual patients with established cardiovascular disease. JACC CardioOncol. 2021;2(3):0. 10.1016/j.jaccao.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tal C, Alexander F, Yariv G, Yonatan M, Shmuel T, Alon K, Maia K, Gabriella B, Tomer I, Abraham A, Nili N-S, Jonathan L, Elad M. Atherosclerotic cardiovascular diseases are associated with incident metastatic and nonmetastatic cancer. JACC CardioOncol. 2025;6(6):0. 10.1016/j.jaccao.2024.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Florido R, Daya NR, Ndumele CE, Koton S, Russell SD, Prizment A, Blumenthal RS, Matsushita K, Mok Y, Felix AS, Coresh J, Joshu CE, Platz EA, Selvin E. Cardiovascular disease risk among cancer survivors: the atherosclerosis risk in communities (ARIC) study. J Am Coll Cardiol. 2022;80(1):22–32. 10.1016/j.jacc.2022.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sangeeta C, Ahluwalia., Cary P, Gross., Sarwat I, Chaudhry., Yuming M, Ning., Linda L-S, Peter H, Van Ness., Terri R, Fried. Impact of comorbidity on mortality among older persons with advanced heart failure. J Gen Intern Med. 2011;27(5):0. 10.1007/s11606-011-1930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.David N, Andreas S, Ursula H-S, Christian MPlöderl, Dieter S, Wolfgang L, Elmar P, Christian A, Datz. Cardiovascular risk and known coronary artery disease are associated with colorectal adenoma and advanced neoplasia. J Am Coll Cardiol. 2017;69(18):0. 10.1016/j.jacc.2017.02.065. [DOI] [PubMed] [Google Scholar]
- 60.Wojciech K, Thomas H. Marwick.(). Reply: effect of aldosterone antagonism on exercise tolerance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2017;69(18):2352–3. 10.1016/j.jacc.2017.01.070. [DOI] [PubMed]
- 61.Chen Z, Yunjiu C, Dongling L, Jinghua W, Jianhua L, Yujun L, Weijie Z, Zewei Z, Kehang G, Ruijie Z, Jun Y, Weihong S, Hao C. Association between cardiovascular risk factors and colorectal cancer: A systematic review and meta-analysis of prospective cohort studies. EClinicalMedicine. 2021;34(0):0. 10.1016/j.eclinm.2021.100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryan J, Koene., Anna E, Prizment., Anne B, Suma H, Konety. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):0. 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shahzeb I, Sridhar M, Hamza RM, Aditya S, El GA, Subham G, Sakshi R, Rishab RH, Andrew BW, Awuah. Evolving perspectives in reverse cardio-oncology: A review of current status, pathophysiological insights, and future directives. Curr Probl Cardiol. 2024;49(3):0. 10.1016/j.cpcardiol.2024.102389. [DOI] [PubMed] [Google Scholar]
- 64.Ming Y, Tiepeng L, Shujin G, Kangping S, Chuhui G, Ning H, Dejiang P, Hengyi X. CVD phenotyping in oncologic disorders: cardio-miRNAs as a potential target to improve individual outcomes in revers cardio-oncology. J Transl Med. 2024;22(1):0. 10.1186/s12967-023-04680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiahui L, Xiunan W, Tong W, Miaomiao Z, Ying G, Lili YC, Chi. Intestinal mucosal barrier: a potential target for traditional Chinese medicine in the treatment of cardiovascular diseases. Front Pharmacol. 2024;15(0):0. 10.3389/fphar.2024.1372766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muzhou H, Hao W, Shuyue Y, Siying Z, Guiping Z, Haiyun S, Peng L. Triglyceride glucose index and atherogenic index of plasma for predicting colorectal neoplasms in patients without cardiovascular diseases. Front Oncol. 2022;12(0):0. 10.3389/fonc.2022.1031259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sandra V, Marija T, Nemanja P, Jelena M, de Miron D, Sopic. The converging roles of MicroRNAs and lipid metabolism in atherosclerotic cardiovascular disease and cancer. Semin Cancer Biol. 2025;114(0):0. 10.1016/j.semcancer.2025.06.005. [DOI] [PubMed] [Google Scholar]
- 68.Elisa A, Barbara B, Marzia C, Ak K, Paola M, Antonino M, Gaia B, Paolo S, Madeddu. Shared molecular, cellular, and environmental hallmarks in cardiovascular disease and cancer: any place for drug repurposing? Pharmacol Rev. 2025;77(2):0. 10.1016/j.pharmr.2024.100033. [DOI] [PubMed] [Google Scholar]
- 69.Jenny N, Poynter., Stephen B, Gruber., Peter DR, Higgins., Ronit A, Joseph D, Bonner., Hedy S, Rennert., Marcelo L, Joel K. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352(21):0. 10.1056/NEJMoa043792. Greenson., Gad, Rennert.(. [DOI] [PubMed] [Google Scholar]
- 70.Elisa A, Barbara B, Marzia C, Ak K, Paola M, Antonino M, Gaia B, Paolo S, Madeddu. Shared molecular, cellular, and environmental hallmarks in cardiovascular disease and cancer: any place for drug repurposing? Pharmacol Rev. 2025;77(2):0. 10.1016/j.pharmr.2024.100033. [DOI] [PubMed] [Google Scholar]
- 71.Adam C, Fields., Beatrice D, Rebecca E, Scully., Matthias F, Stopfkuchen-Evans., Luisa M, Antonia H, Joel E, Goldberg., Ronald B. Reduction in cardiac arrhythmias within an enhanced recovery after surgery program in colorectal surgery. J Gastrointest Surg. 2019;24(5):0. 10.1007/s11605-019-04298-7. [DOI] [PubMed] [Google Scholar]
- 72.Rodrigues A, Georgios S. Kararigas.(). Menopause-Related Estrogen decrease and the pathogenesis of hfpef: JACC review topic of the week. J Am Coll Cardiol 2020;75(9):1074-82. 10.1016/j.jacc.2019.12.049. [DOI] [PubMed]
- 73.Janelle M, Guirguis-Blake., Corinne V, Evans., Leslie A, Perdue., Sarah I, Bean., Caitlyn A, Senger. Aspirin use to prevent cardiovascular disease and colorectal cancer: updated evidence report and systematic review for the US preventive services task force. JAMA. 2022;327(16):0. 10.1001/jama.2022.3337. [DOI] [PubMed] [Google Scholar]
- 74.Yen-Hsiang L, Ren-Jun H, Tzu-Hwei W, Chen-Ta W, Sheng-Yao H, Hsu C-Y, Wen-Lin H, Dai-Wei L. Aspirin and primary cancer risk reduction in ischemic cardiac or cerebrovascular disease survivors: A nationwide Population-Based Propensity-Matched cohort study. Cancers (Basel). 2023;15(1):0. 10.3390/cancers15010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ji-Xuan H, Zhi-Hang T, Ji-Lin W, Lu Z, Chen-Yang Y, Zi-Ran K, Yuanhong X, Jialu L, Shiyuan L, Yun C, Jia X, Enhao Z, Ming W, Jinxian C, Zheng W, Qiang L, Hui-Min C, Wenyu S, Tian-Hui Z, Cheng-Bei. Microbiota-derived Tryptophan catabolites mediate the chemopreventive effects of Statins on colorectal cancer. Nat Microbiol. 2023;8(5):0. 10.1038/s41564-023-01363-5. Zhou., Jie, Hong., Haoyan, Chen., Hua, Xiong., Ying-Xuan, Chen., Jing-Yuan, Fang.(. [DOI] [PubMed] [Google Scholar]
- 76.Wang. HM, Liao. ZX, Komaki. R, Welsh. JW, O’Reilly. MS, Chang. JY, Zhuang. Y, Levy LB, Lu. C, Gomez DR. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann Oncol. 2013;24(5):0. 10.1093/annonc/mds616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chengu N, Jing Z, Patrick I 3. rd, Okolo.(2025). Plant Terpenoids in Combination with Conventional Therapeutics in Colorectal Cancer: A Promising Option. Curr Oncol Rep, undefined(0), 0. 10.1007/s11912-025-01674-2 [DOI] [PubMed]
- 78.Chengu N, Jing Z, Jie L, Joshi U, Charoo I, SoonKhai L, Hassan S, Salman Z, Patrick I 3. Anatomical location, risk factors, and outcomes of lower gastrointestinal bleeding in colorectal cancer patients: a national inpatient sample analysis (2009–2019). Int J Colorectal Dis. 2023;38(1):0. 10.1007/s00384-023-04503-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data, from 23 articles involving 5,770,648 patients, were obtained from the published literature mentioned (feasible Doi attached) or websites (URLs attached) in the ‘References’ section.