Abstract
Acute infectious diarrhea is common in children. Control requires knowledge of causes. Few comprehensive long-term studies of etiology have been undertaken in developed countries. This report is of a 13-year survey of 4,637 children from 0 to 14 years of age, admitted to a large children’s hospital for treatment of gastroenteritis, in which viruses, bacteria, and parasites were sought. A recognized enteric pathogen was identified in 56.6% of children. Group A rotaviruses occurred in 39.6% of children overall and in 55% of children 12 to 23 months of age. They were a frequent cause (18.7%) of acute gastroenteritis in children under 6 months and in those aged 5 to 13 years (16%). Rotaviruses were almost entirely responsible for winter admission peaks. Enteric adenovirus types 40 and 41 (6% overall) were more frequent in children under 12 months (9.4%). Salmonella spp. (5.8%) and Campylobacter jejuni (3.4%) were more common in children over 5 years (13.1% and 6.7%, respectively). The 43.5% of cases (60% in children under 6 months) where no enteric pathogen was identified are cause for concern. The involvement of small viruses (including caliciviruses and astroviruses) may be clarified when molecular biology techniques are utilized to address this gap in our knowledge. This comprehensive 13-year study of the cause of acute infectious diarrhea in children in developed countries reinforces the importance of rotavirus and highlights a large group for whom the etiology remains unknown, an issue of particular concern with babies under 6 months of age. New techniques have the potential to identify old and new pathogens causing disease in these vulnerable infants.
Acute infectious diarrhea is a common disease in young children throughout the world. Estimated incidence rates in developing countries range from 3.5 to 7.0 episodes per child per year during the first 2 years of life and from 2 to 5 episodes per child per year for the first 5 years (4). Attack rates in children less than 5 years old in developed countries range from 1.0 to 2.5 episodes of diarrhea per child per year, with 0.1 to 0.4 episodes resulting in attendance at a general-practice clinic and 0.001 to 0.003 episodes resulting in hospitalization (13–15, 18, 21, 27). It has been estimated that there are more than 20 million cases of diarrhea annually among children less than 5 years old in the United States, resulting in approximately 2.5 million physician visits, 220,000 hospitalizations, and up to 40 deaths.
Pediatric diarrhea is a costly disease in terms of direct (and indirect) monetary costs to each community, and it is a cause of emotional trauma for the child and the parents. The costs of treating rotavirus infection alone have been estimated to be in excess of £6.3 million annually in the United Kingdom (25) and $352 million in the United States (24). Control of pediatric diarrheal disease would lead to considerable health-care cost savings in all countries (8). Effective control of diarrheal disease in any community depends upon an accurate understanding of the relative importance of etiological agents, particularly in relation to the disease burden in various age groups. It is likely that the most cost-effective control measures will be those aimed at causes of disease at the severe end of the spectrum, i.e., disease requiring admission to hospital.
There are relatively few comprehensive studies of the viral, bacterial, and parasitic etiology of severe acute diarrhea in children admitted to hospital in developed countries (7, 12, 30, 31) and only one long-term study (extending over 6 years) describing annual changes in the occurrence of pathogens (11). We have conducted a survey of etiological agents in the stools of children aged 0 to 14 years with acute diarrhea who were admitted to the infectious-disease ward of one hospital (Royal Children’s Hospital [RCH], Melbourne, Australia) between April 1980 and March 1993. The results document the relative importance of bacterial, viral, and parasitic pathogens (identified predominantly by available routine diagnostic techniques) in causation of severe acute diarrhea in Melbourne children during a period of 13 consecutive years.
MATERIALS AND METHODS
Patients and geographical setting.
The study population included 4,637 patients, comprising all infants and children (0 to 14 years old) admitted directly to the gastroenteritis ward of the RCH, Melbourne, from April 1980 through March 1993 with a diagnosis of acute gastroenteritis (defined as diarrhea and/or vomiting of 4 days’ duration or less, where no alternative cause was identified).
The RCH, Melbourne, is a large tertiary pediatric center serving the state of Victoria, Australia. This state has a population of approximately 4 million and had a stable annual birth rate of 60,000 to 70,000 during the period of the survey. The climate is temperate, with seasons showing average minimum and maximum temperature ranges of 13.7 to 25.2°C in summer (December through February) and 6.3 to 14.0°C in winter (June through August). The RCH receives 20 to 25% of statewide pediatric admissions for gastroenteritis. Families are representative of all socioeconomic levels. Approximately 5,000 patients present to the hospital each year for treatment of acute gastroenteritis, many parents regarding the hospital as a primary-care facility. More than 90% of these patients are treated as outpatients. Those admitted are the subjects of this report.
Specimen collection.
Samples of feces from 3,785 of the total number of 4,637 patients (81.7%) were obtained within 48 h of admission to the hospital. Feces samples were obtained from ≥80% of children 0 to 23 months, 74 to 78% of children 24 to 35 months, and 56 to 69% of children ≥36 months old (see Table 1). The most common reason for failure to collect a fecal specimen was discharge within 24 h of admission. Fecal specimens were divided into three aliquots and were transported the same day to hospital laboratories of the departments of Bacteriology, Gastroenterology, and Virology, where they were stored at 4°C until processing. Specimens for bacteriological and virological culture were inoculated into appropriate media on the day of collection. Specimens for examination for viruses, by methods such as enzyme immunoassay (EIA) and negative-stain electron microscopy (EM), were prepared as 10% homogenates in phosphate-buffered saline (PBS), pH 7.0, and were clarified by low-speed centrifugation at 2,000 × g for 10 min. PBS extracts were stored at 4°C for 1 to 2 weeks until they were tested. Surplus PBS extracts and unextracted feces were stored at −70°C until further tests were required.
TABLE 1.
Pathogens isolated from patients hospitalized with gastroenteritis from April 1980 to March 1993, according to age group
| Age (mo) | No. admitted | No. examined | No. of patients from whom indicated pathogen isolated
|
|||||
|---|---|---|---|---|---|---|---|---|
| Rotavirus | Enteric adenovirusa | Salmonella | C. jejuni | Other | NPI | |||
| 60+ | 413 | 282 | 45 | 5 | 37 | 19 | 6 | 165 |
| 48–59 | 149 | 83 | 28 | 3 | 7 | 3 | 2 | 36 |
| 36–47 | 296 | 205 | 68 | 12 | 14 | 15 | 5 | 84 |
| 30–35 | 254 | 189 | 93 | 5 | 18 | 9 | 4 | 58 |
| 24–29 | 439 | 345 | 147 | 7 | 22 | 11 | 6 | 106 |
| 18–23 | 613 | 499 | 278 | 22 | 23 | 17 | 14 | 158 |
| 12–17 | 890 | 784 | 423 | 42 | 33 | 24 | 11 | 251 |
| 6–11 | 783 | 687 | 284 | 62 | 32 | 17 | 8 | 263 |
| 0–5 | 800 | 711 | 133 | 70 | 34 | 14 | 6 | 425 |
| Total (%) | 4,637 | 3,785 (100.0) | 1,499 (39.6) | 228 (6) | 220 (5.8) | 129 (3.4) | 62 (1.6) | 1,546 (43.5) |
Enteric adenoviruses were serotypes 40 and 41.
Bacteriology.
Specimens were examined for Salmonella spp., Shigella spp., Yersinia enterocolitica, Aeromonas, and Plesiomonas by using xylose-lysine deoxycholate agar (XLD), deoxycholate-citrate agar (DCA), and selenite F enrichment broth, incubated in air at 35°C for 18 to 24 h. DCA was incubated at room temperature for an additional 24 h to enhance recovery of Y. enterocolitica. Selenite F broth was subcultured onto XLD incubated in air at 35°C for 18 to 24 h. Isolates were identified by using standard biochemical and serological techniques. Isolates of Salmonella were serotyped and phage typed by the Microbiological Diagnostic Unit at the University of Melbourne. All specimens were cultured for Campylobacter jejuni. Initially this was performed by using Skirrows medium incubated at 42°C in an atmosphere of 7% CO2 and 85% N2. From 1988, the membrane filtration technique was used with horse blood agar incubated at 35°C for 48 h in 7% CO2 and 85% N2 (28). Enteropathogenic E. coli (EPEC) strains were isolated on MacConkey agar and identified by using polyvalent antisera. This practice was discontinued in 1985.
Microscopy.
All specimens were examined microscopically for the presence of leukocytes, erythrocytes, and parasites (amoebae, cysts, or ova) by using a saline-and-iodine wet preparation. Modified acid-fast smears for the detection of cryptosporidia were performed for all specimens during the warmer months of the year (November through April) from 1984 onward (29).
Virology.
All specimens were examined by EM to detect viruses shed in feces (rotaviruses, adenoviruses, and small viruses). The method incorporated the concentration of a clarified fecal homogenate (10%) with polyacrylamide before negative staining with 10% ammonium molybdate (pH 7.0). Samples were also tested for adenoviruses and rotaviruses by EIA. Specimens that were found to be rotavirus positive by EM but negative by EIA were examined by gel electrophoresis of extracted double-stranded RNA to identify electropherotypes resembling group B or C rotaviruses. Organisms in adenovirus-positive specimens were typed by EIA with monoclonal antibodies (MAbs) MA5-8 and MA5-15, specific for adenovirus types 40 and 41, respectively. These adenovirus-specific MAbs were kindly donated by J. C. de Jong of Rijkinstituut Voor Volkesgezondheid en Milieuhygiene, Bilthoven, The Netherlands.
Specimens obtained during 1981 through 1983 were inoculated onto human diploid fibroblast, primary cynomologus monkey kidney, and HeLa cell monolayer cultures. Primary isolates were subpassaged into fresh cultures and identified with appropriate specific antisera.
RESULTS
Admissions.
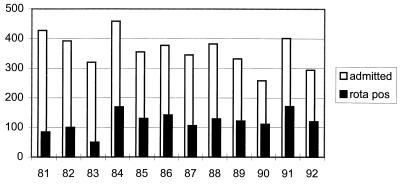
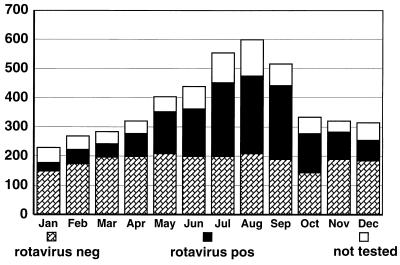
The total number of children admitted during the 13 years of the survey was 4,637 (58% male), ranging from a minimum annual total of 258 in 1990 to a maximum of 458 in 1984 (mean, 362; standard deviation, 54). Figure 1 shows the total number of admissions each year and the number associated with rotavirus infection. The overall monthly distribution of the number of children admitted during the 13-year period ranged from a minimum of 251 in January to a maximum of 602 in August (Fig. 2). Figure 3 shows the age distribution. The majority of children (67%) were aged <24 months, and of those children, 17.3% (800 children) were <6 months old. A total of 413 children (8.9%) were >5 years old.
FIG. 1.
Admissions to hospital for treatment of acute gastroenteritis (1981 to 1992). The number of children with rotavirus diarrhea is shown.
FIG. 2.
Cumulative monthly admissions of children with acute gastroenteritis during April 1980 to March 1993. Results of testing for rotavirus are shown. The seasonal swing is largely determined by rotavirus. neg, negative; pos, positive.
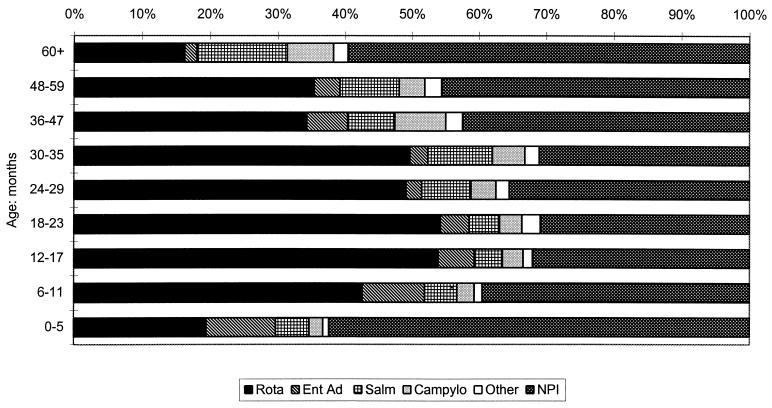
FIG. 3.
Etiology of gastroenteritis in 3,785 hospitalized children in relation to age. Rota, rotavirus; Ent Ad, enteric adenovirus serotypes 40 and 41; Salm, Salmonella spp.; Campylo, C. jejuni; Other, other pathogens and mixtures.
Prevalence of enteric pathogens.
Our analysis excludes those children from whom no specimen was available. Table 1 shows the total results for the 13-year period, listing pathogens isolated in relation to patient age group. Results are displayed graphically in terms of percentages of the various pathogens in children of each age group (Fig. 3). A recognized enteric pathogen was identified for 2,138 of 3,785 children (56.5%) from whom a specimen was obtained. Pathogens classified as “other” enteric pathogens in Table 1 included Shigella spp. (n = 11); Y. enterocolitica (n = 8); EPEC, including O44, O55, O86, O111, O127, and O128 (n = 12); cryptosporidia (n = 18); Giardia lamblia (n = 12); and Aeromonas hydrophila (n = 1). No attempt to identify phenotypic correlates of the EPEC strains identified was made.
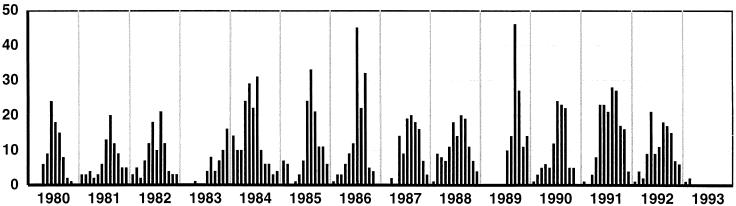
Group A rotaviruses were the single most common pathogen. The overall prevalence of rotavirus infection among children admitted who provided a fecal specimen was 39.6%, ranging from an annual minimum of 20.2% in 1983 (when a prolonged drought appeared to have disrupted the usual seasonal pattern of rotavirus occurrence) to a maximum of 55.7% in 1991 (Fig. 4). Rotaviruses were predominant in children of the 6- to 35-month age group (49% of patients in this age range). Peak incidence occurred in the 12- to 23-month age group, where 55% of patients exhibited group A rotavirus infection. Rotaviruses also occurred in a smaller but significant percentage of very young infants, being identified in 133 of 711 (18.7%) infants less than 6 months of age. They were identified as the sole etiological agent in older children aged 5 to 13 years (median, 7 years) in 45 of 282 patients (16%). Serotype G1 rotaviruses predominated throughout the 13 years, with epidemics of serotype G2 and G4 and sporadic occurrence of G3 strains. These results have been presented in detail elsewhere (3).
FIG. 4.
Monthly rotavirus isolations over the 13-year study period. Seasonal fluctuations are shown. Note the unusual summer pattern in 1983 to 1984.
Two non-group A rotaviruses (belonging to group C) were identified during the survey.
Enteric adenovirus types 40 and 41 were present in 228 of the total number of 3,785 (6%) patients. They were noticeably more common in younger children, occurring in 9.4% (132 of 1,398) of children under 12 months of age, compared with 5.0% (64 of 1,283) of children aged 12 to 24 months and only 2.9% (32 of 1,104) of children aged >2 years (P < 0.001, X22).
Small viruses (approximately 30 nm) lacking identifiable morphology were seen by EM in 13 of 3,785 (0.3%) specimens. None were cultivable in the cell lines available. Recent examination of these specimens by using molecular biological techniques has allowed identification of the majority of these small viruses as astroviruses (26).
Cultivable viruses were identified in diarrheal feces obtained from 117 of 827 (14.1%) children during the period 1981 to 1983. Viruses isolated included adenoviruses (n = 42) of types 1, 2, 5, 6, 7, and 31; echoviruses (n = 41) of types 6, 9, 11, 14, 15, 22, and 30; coxsackieviruses (n = 12) of types A4, A9, B2, B3, and B5; poliovirus vaccine strains (n = 21); and reovirus (n = 1). During the period when EM and cell culture were performed in parallel, only 10 of 42 (24%) adenoviruses adaptable to cell culture were visualized by EM. None of the other cultivable viruses were seen by EM. Cultivable viruses were present as mixed infections with enteric pathogens in 33 of 117 (28.2%) children, organisms identified including rotaviruses (n = 17), enteric adenoviruses 40 and 41, (n = 6), Salmonella spp. (n = 5), Shigella spp. (n = 2), C. jejuni (n = 1), and G. lamblia (n = 2).
Salmonella spp. were isolated from 220 of 3,785 (5.8%) patients and caused a higher proportion of diarrhea (13%) in older children, those aged from 5 to 14 years (median 8 years), than in young children <5 years old (5.2%). Salmonella typhimurium was the most common species identified.
C. jejuni strains were isolated from 129 of 3,785 (3.4%) patients and were more common (6.7%) in children aged 5 to 14 years (median, 9 years) than in younger children (3.1%). Methods of culture for C. jejuni were not optimal early in the study. However, there was no obvious change in its occurrence after 1988, when new isolation techniques were introduced.
Shigella spp. were uncommon and were identified in only 11 (0.3%) children.
Parasites, including G. lamblia and Cryptosporidia, were identified in 12 (0.3%) and 18 (0.5%) children, respectively.
Mixtures of two or more pathogens were identified in 62 of 3,785 (1.6%) children. These mixtures included rotaviruses with Salmonella spp. (n = 18), C. jejuni (n = 14), enteric adenoviruses (n = 9), or Shigella spp. (n = 5) and enteric adenoviruses with Salmonella spp. (n = 10), C. jejuni (n = 2), G. lamblia (n = 2), Shigella spp. (n = 1), or Cryptosporidia (n = 1). Ages of children with mixed infections ranged from 1 month to 7.6 years, the median age being 25 months.
No acknowledged enteric pathogens (NPI) were identified in 43.5% of patients. This situation was more frequent in infants under 6 months of age (58.5%) and children of 5 years or more (60%) than in children aged 6 to 49 months (34.2%).
Seasonal occurrence of enteric pathogens.
Overall hospital admissions for acute diarrhea showed a seasonal pattern (Fig. 2), with the number of patients admitted in the cooler months of the year (May to September) being approximately double the number of those admitted during the warmer months (October to April). The seasonal patterns for all gastroenteritis admissions reflected the patterns of admissions recorded for rotavirus infections (Fig. 2). Rotaviruses were detected in children admitted to the hospital in 135 of the 156 months studied. The numbers fluctuated monthly (Fig. 2 and 4), with the annual peak prevalence occurring in colder months (May to September) in 12 of the 13 years. There was no winter peak of rotavirus infection in 1983; this coincided with a severe, prolonged drought. The rotavirus season began late in July 1983, peaked in December, and continued through the southern-hemisphere summer of 1983 to 1984, merging with the 1984 winter peak. Rotaviruses were not detected for 1 to 3 months during most summers.
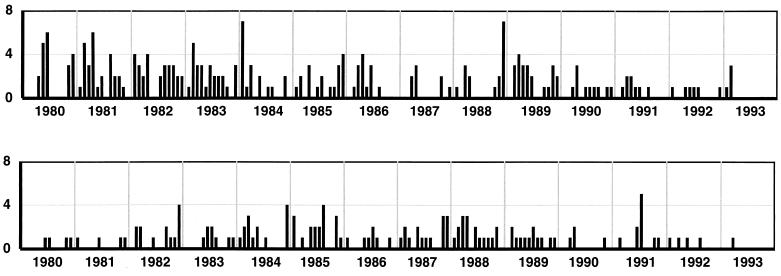
Unlike the situation for rotaviruses, no consistent seasonal patterns of occurrence of enteric adenovirus infection were observed, and clusters of adenovirus infections occurred in any month. There was no evidence of seasonal patterns of Salmonella and Campylobacter infections (Fig. 5). No seasonal periodicity was observed for gastroenteritis for which no cause could be assigned (NPI).
FIG. 5.
Monthly isolations of Salmonella spp. (top) and C. jejuni (bottom) over the 13-year study period. No seasonal pattern is apparent.
DISCUSSION
This survey confirms worldwide studies implicating group A rotaviruses as the single most important cause of severe diarrhea in young children (0 to 3 years) admitted to hospitals in developed and developing countries (2, 14, 27). Over the 13 years of this study, a recognized enteric pathogen was identified in 56.5% of patients; these pathogens included group A rotaviruses (39.6%), enteric adenovirus types 40 and 41 (6%), Salmonella spp. (5.8%), and C. jejuni (3.4%).
Our results show remarkable agreement with the results of an 8-year U.S. survey of viral etiology, in which 34.5% of children admitted to hospital with severe diarrhea excreted rotaviruses (5). The burden of rotavirus disease was greatest in young children aged less than 5 years. Nevertheless, rotaviruses were identified as the sole etiologic agent of severe acute diarrhea in 16% of children over 5 years of age, including children aged 10 years or more, none of whom had preexisting disease likely to predispose them to or exacerbate symptoms of enteric infection. Clinical symptoms in these older children on presentation to hospital were severe enough to require admission for treatment. This implies that age does not necessarily result in physiological resistance to disease in humans, contrary to observations for experimental animals, where clinically apparent diarrhea is rare after the first few weeks of life (1). During the 13 years of this survey, rotaviruses were identified by postmortem analysis of intestinal contents as the cause of death for two patients who were dead on arrival at the hospital (unpublished data).
Non-group A rotaviruses were identified to be present in only two children during the period of this survey, emphasizing the rarity of their involvement in etiology of severe acute diarrhea in children. This confirms more recent studies of children in the United States which used molecular biological techniques of diagnosis (19). Although cultivable viruses, including enteroviruses, were identified in 14.1% of patients, it is unlikely that many of these were etiologically related to diarrhea. Most were excreted in small numbers, below the level of detectability by EM (<106 particles/ml), and 30% were present as mixed infections with known enteric pathogens. While no control group of children was examined during this survey, the range of cultivable viruses identified resembles that of viruses previously cultured from healthy infants examined during a controlled study conducted in the United States in 1970 (32).
Over the 13 years of this study, patterns of diarrhea-related admissions fluctuated from month to month and year to year. Winter epidemics of rotavirus infection usually extended over a range of 3 to 6 months. The number of admissions in any one winter was not directly related to the presence of more than one rotavirus serotype (3). Episodes unrelated to rotavirus, which included those associated with other pathogens, together with NPI episodes, were remarkably consistent in occurrence from year to year and throughout the seasons. The periodicity of gastroenteritis admissions appears to have been caused primarily by the periodicity of rotavirus-associated episodes, confirming a survey of hospitalized children in the United States, based on interpretation of national hospital discharge data records (20). The seasonality of rotavirus epidemics remains unexplained. An interesting phenomenon associated with a prolonged drought that began during the spring (September to November) of 1982 and extended throughout the summer and autumn of 1983 occurred in this study. Rotaviruses were seldom identified in children admitted to the hospital during a 9-month period (October 1982 to June 1983). Following their reappearance in July 1983, the epidemic carried on through the unusually wet summer (December to February) of 1983 to 84 and merged with the 1984 winter epidemic. This appears to have been a “catch-up” phenomenon, with rotaviruses infecting a larger-than-usual population of susceptible infants.
The importance of enteric adenoviruses belonging to serotypes 40 and 41 in the etiology of severe acute diarrhea in young children is confirmed in this study, in which 6% of diarrhea episodes were caused by this virus (6, 9). Most adenovirus infections occurred in children <24 months of age. Our fecal cell culture results confirmed that the majority of adenoviruses visualized in stool specimens by EM represent noncultivable enteric serotypes 40 and 41 (6, 17). No consistent seasonal pattern of occurrence of enteric adenovirus infection was observed, and peaks of adenovirus identification appeared to be random.
Small viruses were occasionally detected in 0.3% of patients by EM, and all were later identified as astroviruses (26). The prevalence of astroviruses was underestimated in this EM survey. A year-long survey conducted during 1995 using an astrovirus-specific cDNA probe (26) identified astrovirus infection in 3 to 4% of children admitted to hospital with severe diarrhea. Recently developed tests to detect human caliciviruses were not incorporated in this survey (10, 16, 23). The development of molecular biological techniques to detect astroviruses and caliciviruses, once applied to similar surveys, may identify these viruses as common etiological agents of diarrhea in young children.
The overall results show Salmonella and Campylobacter to be the major bacterial pathogens in the community studied. Parasitic and mixed infections are rare. The low prevalence of bacterial and parasitic pathogens compared with rates observed in similar surveys in developing countries has been recorded in many similar surveys in developed countries (2).
The large percentage of cases (43.5%) where no recognized pathogen was identified raises a number of issues. Firstly, techniques for identifying known pathogens may not have been optimal during the course of this study. Many different organisms may cause diarrhea, and it is not possible in a clinical survey such as this to screen specimens for the entire range of potential enteropathogens. For example, techniques to detect diarrheagenic E. coli were not routinely included. However, previous surveys indicate that these enteric pathogens are rare in children in developed countries (2, 22). In addition, Clostridium difficile can cause severe diarrhea in children but was sought only when requested by clinicians. Secondly, even when specifically sought, some pathogens may not be detected because of excretion in insufficient numbers. In particular, intermittent excretion or collection of feces only during the recovery phase may result in failure to isolate the causative pathogen. The inability to identify a pathogen in many infants <6 months of age (60%) may have been influenced by passively acquired maternal antibody that could rapidly reduce microbial shedding to undetectable levels. Thirdly, and of most importance, there may be other (some as yet unknown) pathogens causing severe diarrhea in young children for which diagnostic tests were not available during the course of this study. Our results imply that these elusive pathogens could be of particular importance in diarrhea affecting infants less than 6 months of age.
The results of this study emphasize the importance of viral infections in causation of severe diarrhea in children. They give strong support for resistance to routine prescription of antibiotics. Measures to control childhood diarrheal diseases in Australia and other developed countries should be directed at prevention of infection with Salmonella spp. C. jejuni, and enteric adenoviruses and at development of an effective rotavirus vaccine.
ACKNOWLEDGMENTS
We thank J. C. de Jong, Rijksmintienit voor Volksgengondheid en Mileiuhygiene, The Netherlands, for providing MAbs specific for adenoviruses 40 and 41. Over the 13 years of this study, many staff contributed to this survey, including P. Masendycz, L. Adams, L. Vaelioja, S. Lawrance, and staff of the RCH Virology and Bacteriology Departments. John Carlin, Clinical Epidemiology and Biostatistics Unit, RCH, gave valuable assistance with presentation of data. We are grateful to A. Peace for typing the manuscript.
The work was supported by the RCH Research Foundation. R.F. Bishop is a Senior Principal Research Fellow of the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Bass D M, Greenberg H B. Pathogenesis of viral gastroenteritis. In: Field M, editor. Diarrheal diseases. New York, N.Y: Elsevier; 1991. pp. 139–157. [Google Scholar]
- 2.Bern C, Glass R I. Impact of diarrheal diseases worldwide. In: Kapikian A Z, editor. Viral infections of the gastrointestinal tract. 2nd ed. New York, N.Y: Marcel Dekker; 1994. pp. 1–26. [Google Scholar]
- 3.Bishop R F, Unicomb L E, Barnes G L. Epidemiology of rotavirus serotypes in Melbourne, Australia, from 1973 to 1989. J Clin Microbiol. 1991;29:862–868. doi: 10.1128/jcm.29.5.862-868.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black R E. Epidemiology of diarrheal disease: implications for control by vaccines. Vaccine. 1993;11:100–106. doi: 10.1016/0264-410x(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 5.Brandt C D, Kim H W, Rodriguez W J, Arrobio J O, Jeffries B C, Stallings E P, Lewis C, Miles A J, Chanock R M, Kapikian A Z, Parrott R H. Pediatric viral gastroenteritis during eight years of study. J Clin Microbiol. 1983;18:71–78. doi: 10.1128/jcm.18.1.71-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandt C D, Kun H W, Rodriguez W J, Arrobio J O, Jeffries B C, Stallings E P, Lewis C, Miles A J, Gardner M K, Parrott R H. Adenoviruses and pediatric gastroenteritis. J Infect Dis. 1985;151:437–443. doi: 10.1093/infdis/151.3.437. [DOI] [PubMed] [Google Scholar]
- 7.Caprioli A, Pizzella C, Morelli R, Giammanco A, Arista S, Crotti D, Faccini M, Guglielmetti P, Piersimoni C, Luzzi I the Italian Study Group on Gastrointestinal Infections. Enteropathogens associated with childhood diarrhea in Italy. Pediatr Infect Dis J. 1996;15:876–883. doi: 10.1097/00006454-199610000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Creese A L. Cost effectiveness of potential immunization interventions against diarrhea disease. Soc Sci Med. 1986;23:231–240. doi: 10.1016/0277-9536(86)90343-6. [DOI] [PubMed] [Google Scholar]
- 9.de Jong J C, Bijlsma K, Wermenbol A G, Verwij-Uijterwaal M W, van der Avoort H G A M, Wood D J, Bailey A S, Osterhaus A D M E. Detection, typing, and subtyping of enteric adenoviruses 40 and 41 from fecal samples and observation of changing incidences of infections with these types and subtypes. J Clin Microbiol. 1993;31:1562–1569. doi: 10.1128/jcm.31.6.1562-1569.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinulos M B, Matson D O. Recent developments with human caliciviruses. Pediatr Infect Dis J. 1994;13:998–1003. doi: 10.1097/00006454-199411000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Durepaire N, Pradie M P, Ploy M C, Mounier M, Ranger-Rogez S, Martin C, Denis F. Adenoviruses from stool samples in a university hospital. Comparison with other main enteropathogens (rotavirus, Campylobacter, Salmonella) Pathol Biol. 1995;43:601–610. [PubMed] [Google Scholar]
- 12.Ellis M E, Watson B, Mandal B K, Dunbar E M, Craske J, Curry A, Roberts J, Lomax J. Micro-organisms in gastroenteritis. Arch Dis Child. 1984;59:848–855. doi: 10.1136/adc.59.9.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fergusson D M, Dimond M E, Shannon F T. Morbidity during the pre-school years. Aust Paediatr J. 1985;21:139–145. doi: 10.1111/j.1440-1754.1984.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 14.Glass, R. I., P. E. Kilgore, R. C. Holman, S. Jin, J. C. Smith, P. A. Woods, M. J. Clarke, M. S. Ho, and J. R. Gentsch. 1996. The epidemiology of rotavirus diarrhea in the United States: surveillance and estimates of disease burden. J. Infect. Dis. 174(Suppl. 1):S5–S11. [DOI] [PubMed]
- 15.Glass R I, Lew J F, Gangarosa R E, Le Baron C W, Ho M S. Estimates of morbidity and mortality rates for diarrheal diseases in American children. J Pediatr. 1991;118:27–33. doi: 10.1016/s0022-3476(05)81422-2. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg H B, Matsui S M. Astroviruses and caliciviruses: emerging enteric pathogens. Infect Agents Dis. 1992;1:71–91. [PubMed] [Google Scholar]
- 17.Grimwood K, Carzino R, Barnes G L, Bishop R F. Patients with enteric adenovirus gastroenteritis admitted to an Australian pediatric teaching hospital from 1981 to 1992. J Clin Microbiol. 1995;33:131–135. doi: 10.1128/jcm.33.1.131-136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isaacs D, Day D, Crook S. Childhood gastroenteritis: a population study. Br Med J. 1986;293:545–546. doi: 10.1136/bmj.293.6546.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang B, Dennehy P H, Spangenberger S, Gentsch J R, Glass R I. First detection of group C rotavirus in fecal specimens of children with diarrhea in the United States. J Infect Dis. 1995;172:45–50. doi: 10.1093/infdis/172.1.45. [DOI] [PubMed] [Google Scholar]
- 20.Jin S, Kilgore P E, Holman R C, Clarke M J, Gangarosa E J, Glass R I. Trends in hospitalizations for diarrhea in United States children from 1979 through 1992: estimates of the morbidity associated with rotavirus. Pediatr Infect Dis J. 1996;15:397–404. doi: 10.1097/00006454-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Koopman J S, Turkish V J, Monto A S, Gouvea V, Srivastava S, Isaacson R E. Patterns and etiology of diarrhea in three clinical settings. Am J Epidemiol. 1984;119:114–123. doi: 10.1093/oxfordjournals.aje.a113712. [DOI] [PubMed] [Google Scholar]
- 22.Kotloff K L, Wassermann S S, Steciak J Y, Tall B D, Losonsky G A, Nair P, Morris J G, Levine M M. Acute diarrhea in Baltimore children attending an outpatient clinic. Pediatr Infect Dis J. 1988;7:753–759. doi: 10.1097/00006454-198811000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Levett P N, Gu M, Luan B, Fearson M, Stubberfield J, Jamieson F, Petrie M. Longitudinal study of molecular epidemiology of small round-structured viruses in a pediatric population. J Clin Microbiol. 1996;34:1497–1501. doi: 10.1128/jcm.34.6.1497-1501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matson D O, Estes M K. Impact of rotavirus infection at a large pediatric hospital. J Infect Dis. 1990;162:598–604. doi: 10.1093/infdis/162.3.598. [DOI] [PubMed] [Google Scholar]
- 25.Noel J S, Parker S P, Choules K, Phillips A D, Walker-Smith J, Cubitt W D. Impact of rotavirus infection on a pediatric hospital in the East End of London. J Clin Pathol. 1994;47:67–70. doi: 10.1136/jcp.47.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palombo E A, Bishop R F. Annual incidence, serotype distribution, and genetic diversity of human astrovirus isolates from hospitalized children in Melbourne, Australia. J Clin Microbiol. 1996;34:1750–1753. doi: 10.1128/jcm.34.7.1750-1753.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan, M. J., M. Ramsay, D. Brown, N. J. Gay, C. P. Farrington, and P. G. Wall. 1996. Hospital admissions attributable to rotavirus infection in England and Wales. J. Infect. Dis. 174(Suppl. 1):S12–S18. [DOI] [PubMed]
- 28.Steele T W, McDermott S N. Technical note: the use of membrane filters applied directly to the surface of agar plates for the isolation of Campylobacter jejuni from feces. Pathology. 1984;16:262–265. doi: 10.3109/00313028409068535. [DOI] [PubMed] [Google Scholar]
- 29.Tzipori S, Smith M, Birch C, Barnes G, Bishop R. Cryptosporidiosis in hospital patients with gastroenteritis. Am J Trop Med Hyg. 1983;32:931–934. doi: 10.4269/ajtmh.1983.32.931. [DOI] [PubMed] [Google Scholar]
- 30.Uhnoo I, Wadell G, Svensson L, Olding-Stenkvist E, Ekwall E, Molby R. Aetiology and epidemiology of acute gastroenteritis in Swedish children. J Infect. 1986;13:73–89. doi: 10.1016/s0163-4453(86)92348-0. [DOI] [PubMed] [Google Scholar]
- 31.Vesikari T, Maki M, Sarkkinen H K, Arstilla P P, Halonen P E. Rotavirus, adenovirus, and non-viral enteropathogens in diarrhoea. Arch Dis Child. 1981;56:264–270. doi: 10.1136/adc.56.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yow M D, Melnick J L, Blattner R J, Stephenson W B, Robinson N M, Burkhardt M A. The association of viruses and bacteria with infantile diarrhea. Am J Epidemiol. 1970;92:33–39. doi: 10.1093/oxfordjournals.aje.a121177. [DOI] [PubMed] [Google Scholar]