Abstract
Interspecific polymorphisms of the 16S rRNA gene (rDNA) are widely used for species identification of mycobacteria. 16S rDNA sequences, however, do not vary greatly within a species, and they are either indistinguishable in some species, for example, in Mycobacterium kansasii and M. gastri, or highly similar, for example, in M. malmoense and M. szulgai. We determined 16S-23S rDNA internal transcribed spacer (ITS) sequences of 60 strains in the genus Mycobacterium representing 13 species (M. avium, M. conspicuum, M. gastri, M. genavense, M. kansasii, M. malmoense, M. marinum, M. shimoidei, M. simiae, M. szulgai, M. triplex, M. ulcerans, and M. xenopi). An alignment of these sequences together with additional sequences available in the EMBL database (for M. intracellulare, M. phlei, M. smegmatis, and M. tuberculosis) was established according to primary- and secondary-structure similarities. Comparative sequence analysis applying different treeing methods grouped the strains into species-specific clusters with low sequence divergence between strains belonging to the same species (0 to 2%). The ITS-based tree topology only partially correlated to that based on 16S rDNA, but the main branching orders were preserved, notably, the division of fast-growing from slowly growing mycobacteria, separate branching for M. simiae, M. genavense, and M. triplex, and distinct branches for M. xenopi and M. shimoidei. Comparisons of M. gastri with M. kansasii and M. malmoense with M. szulgai revealed ITS sequence similarities of 93 and 88%, respectively. M. marinum and M. ulcerans possessed identical ITS sequences. Our results show that ITS sequencing represents a supplement to 16S rRNA gene sequences for the differentiation of closely related species. Slowly growing mycobacteria show a high sequence variation in the ITS; this variation has the potential to be used for the development of probes as a rapid approach to mycobacterial identification.
The increase of infections caused by Mycobacterium tuberculosis and nontuberculous mycobacteria is receiving increasing attention worldwide. Nontuberculous mycobacteria are encountered with increasing frequency in clinical laboratories, and phenotypic features for their identification are well documented (6, 12, 39, 40). Numerical taxonomic matrices and 16S rRNA-based phylogenetic analyses have contributed to the systematics of mycobacteria (26, 29, 38). 16S rRNA gene (rDNA) sequence analysis, either by direct sequencing (17) or by using probes (18, 22, 25), is now widely used for rapid and accurate identification of mycobacteria. Unfortunately, the number of polymorphic sites in the 16S rDNA in the genus Mycobacterium is rather low, inasmuch as some species have the same sequence (M. kansasii and M. gastri or M. senegalense and M. farcinogenes) and others possess a very high degree of sequence similarity (e.g., M. malmoense and M. szulgai or M. marinum and M. ulcerans). This may lead to problems related to cross-reactivity when oligonucleotide probes are used (5) and makes the design of probes directed towards a broad panel of all clinically relevant species difficult (18).
In view of this, a study of more-variable sequences in the RNA operon of phylogenetically closely related mycobacterial species is needed. The genes coding for the rRNA are arranged in the order 5′-16S-23S-5S-3′, and they are separated by two noncoding spacer regions. The 16S-23S rDNA internal transcribed spacer (ITS) has been suggested to represent a potential target within the bacterial genome to find suitable sites for probes and from which to derive additional phylogenetic information. This genetic locus is flanked by well-conserved regions of the rRNA operon, contains both conserved and highly variable signatures, and is rather small (2, 13, 19). Previous work, particularly with respect to mycobacteria, has shown that both the high level of spacer sequence variation and the good reproducibility of ITS sequencing suggest the applicability of this approach (4, 9, 10, 35). Distinct ITS sequences found in the M. avium complex have been used to define infrasubspecific taxons, and such subspecies defined by a sequence were called a sequevar (4, 10). Thus, ITS is suitable for differentiating strains within some mycobacterial species and has the potential to be used as a marker for clinically relevant subspecies in these cases (1, 11, 24).
The aim of the present study was to further investigate the suitability of the ITS for the reliable molecular identification of mycobacteria. The ITS sequences of a total of 60 strains comprising 13 species of slowly growing mycobacteria were determined (i) to discover whether spacer sequences can differentiate slowly growing mycobacterial species which are identical or closely related on the basis of their 16S rDNA sequences, (ii) to evaluate the degree of interspecies divergence and intraspecies conservation of ITS sequences, and (iii) to compare the ITS-based clustering of the organisms with the tree obtained from 16S rDNA sequence analysis.
MATERIALS AND METHODS
Bacterial strains.
The sources, nucleotide sequence accession numbers, and sequevar assignments of 17 mycobacterial species investigated in this study are listed in Table 1. With the exception of M. conspicuum and M. triplex, more than one strain was included within each species to explore intraspecies variability. The following numbers of clinical isolates obtained from the strain collection held at our institute were included: M. avium, 12; M. gastri, M. simiae, and M. xenopi, 7 each; M. marinum, 5; M. kansasii, 4; M. szulgai, 3; M. genavense, 2; and M. malmoense, M. shimoidei, M. triplex, and M. ulcerans, 1 each. The M. shimoidei strain was isolated from the respiratory tract of a patient with chronic lung disease, and the M. triplex isolate was recovered from pleural effusion from a patient with empyema. All M. avium and M. genavense strains were isolated from the blood of patients with AIDS. Strain S134 (M. marinum) was kindly provided by P. Buchholz, Berlin, Germany, and strain S219 (M. ulcerans), isolated from an African patient with Buruli ulcera, was donated by G. Márquez de Bär, Cottbus, Germany. All strains used were identified to the species level by standard biochemical procedures (16). The species identity of all strains was confirmed by 16S rDNA sequencing (see below). Bacteria grown on Löwenstein-Jensen slants or in 7H12 broth were suspended in 1 ml Tris-HCl buffer (10 mM, pH 7.5). The cells were inactivated at 80°C for 10 min, washed twice with Tris-HCl buffer, and kept at −20°C until needed.
TABLE 1.
Strains analyzed and sequences used for aligning 16S-23S spacer sequences
| Species | Strain(s) and sourcea | Spacer sequevarb | Nucleotide sequence accession no. (EMBL) | Reference |
|---|---|---|---|---|
| M. avium | ATCC 25291T | Mav-A | L07855 | 10 |
| S127, S128, S130, S131, S133, S150 | Mav-A | This study | ||
| ATCC 35765 | Mav-B | L07856 | 10 | |
| S115, S116, S117, S118, S119, S120 | Mav-B | This study | ||
| M. conspicuum | DSM 44146 | X92668 | This study | |
| M. gastri | ATCC 15754T, S227, S228, S229 | Mga-A | X97633 | This study |
| S230, S231, S232, S233 | Mga-B | Y14182 | This study | |
| M. genavense | S80, S84 | Y14183 | This study | |
| M. intracellulare | ATCC 13950T | Min-A | L07859 | 10 |
| M. kansasii | ATCC 12478T, S236, S237, S238, S239 | Mka-Ac | L42262 and X97632 | Unpublished data and this study |
| M. malmoense | ATCC 29571T, S216 | Z35225 and Y14184 | Unpublished data and this study | |
| M. marinum | S101, S134, S240, S241, S242 | Y14185 | This study | |
| M. phlei | R82 | X74493 | 33 | |
| M. shimoidei | ATCC 27962T, S234 | X99219 | This study | |
| M. simiae | ATCC 25275T, S137, S144, S146, S147, S149 | Msi-Ad | X74056 and Y14186 | Reference 15 and this study |
| C13383 | Msi-Bd | Z46426 | 4 | |
| S142 | Msi-C | Y14187 | This study | |
| S136 | Msi-D | Y14188 | This study | |
| M. smegmatis | ATCC 19420T | X76257 | 14 | |
| M. szulgai | ATCC 35799T, S95, S96, S97 | X99220 | This study | |
| M. triplex | S139 | Y14189 | This study | |
| M. tuberculosis | ATCC 27294T | L15623 | 9 | |
| M. ulcerans | ATCC 19423T, S219 | X99217 | This study | |
| M. xenopi | ATCC 19250T, S90, S93, S94 | Mxe-A | L15624 and Y14190 | Reference 11 and this study |
| S88, S89, S92 | Mxe-B | Y14191 | This study | |
| S91 | Mxe-C | Y14192 | This study |
ATCC, American Type Culture Collection, Rockville, Md.; DSM, Deutsche Sammlung für Mikroorganismen und Zellkulturen, Braunschweig, Germany; S, strain collection, Institut für Mikrobiologie, Krankenhaus Zehlendorf-Heckeshorn, Berlin, Germany.
Sequevar designations in cases of intraspecies heterogeneity according to the nomenclature proposed by Frothingham and Wilson (10) and De Smet et al. (4).
Other sequences available in the EMBL database not included here are available.
Msi-A originally assigned to strain C13383 (clinical isolate) was now related to the sequence of the type strain.
Sequence analysis.
The cell pellet from a 100-μl aliquot of the slightly turbid bacterial suspensions mentioned above was sonicated with 50 μl of glass beads (100-μm diameter; Sigma, Diesenhofen, Germany) for 10 min in a water bath sonicator (Sonorex RK 156; Bandelin, Berlin, Germany). The lysate obtained after settlement of the glass beads containing the genomic DNA was then heated at 94°C for 10 min. Two independent DNA extractions were performed for each strain to confirm the results. Amplification of the 16S rDNA and ITS sequences was performed with the following primers. (i) For the 16S rDNA sequences, primers modified according to recently published sequences (33) were used, namely, Seq1 (identical to KY18), which is biotin-5′-CAC ATG CAA GTC GAA CGG AAA GG-3′, and Seq2 (which corresponds to the modified primer KY75), which is 5′-GCC CGT ATC GCC CGC ACG CT-3′. (ii) For the ITS sequences, primers Ec16S.1390p, biotin-5′-TTG TAC ACA CCG CCC GTC A-3′, and Mb23S.44n, 5′-TCT CGA TGC CAA GGC ATC CAC C-3′ (10), were used. The amplification was done with a 50-μl reaction mix containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.1% Triton X-100, 200 μM each deoxynucleoside triphosphate, i.e., dATP, dGTP, dCTP, and dUTP, 20 pmol of the unbiotinylated primer, 10 pmol of the biotinylated primer, 1 U of Thermus aquaticus DNA polymerase (all reagents from Pharmacia Biotech, Freiburg, Germany), and 5 μl of DNA. The thermal profile for both 16S rDNA and ITS amplification involved 38 cycles with the following steps: initial denaturation for 5 min at 95°C followed by 1 min of denaturation at 94°C, annealing at 62°C, and extension at 72°C. Amplified products were analyzed by 1.8% agarose gel electrophoresis.
The amplified PCR products were captured and purified with streptavidin-coated Dynabeads as described in the instructions of the manufacturer (Dynabeads M-280 streptavidin; Dynal AS, Oslo, Norway). Sequencing reactions were done by standard dideoxy sequencing methods with a DNA sequencing kit, and the procedure was done as described in the instructions of the manufacturer (Pharmacia). The sequencing procedure was performed with the A.L.F. DNA sequencer (Pharmacia). The primers used for sequencing were as follows: (i) for the 16S rDNA (including helix 10 and helix 18), 5′-fluorescein-labelled primers 244 and 259 (17); (ii) for the ITS, 5′-fluorescein-labelled primers complementary to the PCR primers for sequencing of both the sense and the antisense strands.
Data analysis.
New and additional database ITS sequences were aligned by using the respective tools of the ARB software package (30) according to primary-structure, as well as predicted secondary-structure, similarity. Secondary-structure prediction was in analogy to that proposed elsewhere (14, 15). Comparative analyses of ITS sequences were performed with distance matrix, maximum-parsimony, and maximum-likelihood methods (32) as implemented in the ARB package. The significance of the resulting tree topologies was tested by performing bootstrap analyses and varying the composition of the data sets by successively removing or including alignment positions according to their degree of evolutionary conservation. Conservation profiles were established by using the respective ARB subroutines. For comparison, we included a 16S rRNA-based tree. This tree was reconstructed by performing a distance matrix analysis of all available, at-least-90%-complete (in comparison with Escherichia coli 16S rRNA sequences), homologous primary structures from gram-positive bacteria with a high DNA G+C content as well as a selection of reference strains from the other major bacterial lines of descent. Evaluation and correction of the tree topology were done as described for the ITS-based tree.
RESULTS
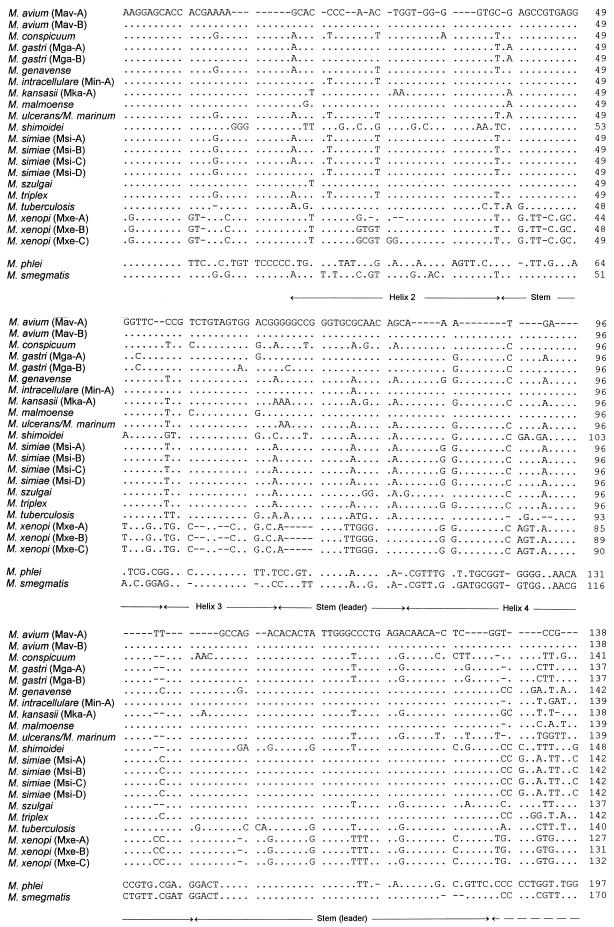
PCR amplification with primers Ec16S.1390p and Mb23S.44n resulted in the detection of a single band of approximately 480 bp in all 60 strains investigated. The variation in product length was not considerable between the different species of slow growers, but a smaller product of approximately 430 bp was noted for M. xenopi. The sequence alignment and the ITS sizes for all species studied are shown in Fig. 1. The sizes of the spacers of slow growers ranged from 235 nucleotides (nt) for M. xenopi to 285 nt for M. gastri, showing that the spacer sequences of slow growers are approximately 75 nt shorter than those of rapid growers. This is due to features that clearly differentiate slow growers from fast growers (14, 15), notably, a missing expansion of helix 4 and a shorter helix 5 in slowly growing mycobacteria, a finding which was confirmed here for all strains studied (Fig. 1). Longer stretches of conserved noncoding regions which could be characteristic of the genus were limited to only a few sites, for example, positions 1 to 10 and parts of the two stem-loop sequences (Fig. 1). In contrast, a high degree of sequence variability was found dispersed over the whole spacer sequence, with the highest degree of diversity found in the antitermination elements (referred to as boxes [Fig. 1]) and helices 5 and 6. As a result of this variability, we found an intraspecies sequence polymorphism in 4 of 11 species for which multiple strains within one species were analyzed. M. gastri and M. avium each split into two distinct sequevars, designated Mga-A and Mga-B and Mav-A and Mav-B, respectively, based on the nomenclature proposed by Frothingham and Wilson (10) and De Smet et al. (4). The base difference between Mga-A and Mga-B was found to be 5 nt, and that between Mav-A and Mav-B was 1 nt; the latter finding was described previously (10). Members of M. simiae exhibited a bigger variation, with the formation of four distinct sequevars. M. xenopi sequevars distinguishable from the type strain sequevar exhibited a 4- or 5-base-long insertion at position 28. M. triplex and M. genavense were the two species with the highest ITS similarity values (Table 2). Hence, the lowest level of ITS sequence divergence between any two species in this setting of strains was at least 13 nt (4%). M. marinum and M. ulcerans had the same ITS sequence. Much lower levels of similarity were obtained for ITS than for 16S rDNA sequences (Table 2). ITS base changes between species closely related on the basis of their 16S rDNA sequences (similarity greater than 99%) accounted for the 7 to 8% difference between M. gastri and M. kansasii and for the 12% difference between M. malmoense and M. szulgai. ITS similarity values as low as 63% could be found when the sequences of slow-growing mycobacteria were compared with those of two representatives of fast-growing mycobacteria. M. xenopi occupied an intermediate position in its sequence similarity with rapid growers, but its short ITS sequence and the missing expanded helix 4 indicate that this species belongs to the group of slow growers.
FIG. 1.
Alignment of 16S-23S rDNA ITS sequences including those of 13 mycobacterial species investigated in this study together with sequences of 4 other species published elsewhere (9, 10, 14, 34) (Table 1). Sequevar designations are shown in parentheses. The sequence of M. simiae (Msi-B) was published by De Smet et al. (4). The length of the ITS is indicated at the end of the sequences in nucleotides. The complete ITS sequence between the end of the 16S rRNA gene and the beginning of the 23S rRNA gene is shown. With the exception of M. conspicuum, whose ITS ends with GG, the 3′ end of the ITS was inferred to be GTGT. Dots indicate identity, and hyphens represent alignment gaps. Possible features of secondary structures predicted in analogy to those previously proposed for pre-rRNA transcripts of mycobacteria are indicated by labelled arrows, whereas the stem-loop structures designated leader and spacer 2 interact with leader and 23S-5S rRNA spacer regions, respectively (14, 15).
TABLE 2.
Similarity values for 16S-23S rDNA spacer (ITS) sequences (lower left) and 16S rRNA sequences (upper right) for representatives of the genus Mycobacterium
| Species (sequevar) | % Sequence similaritya
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | |
| 1. M. gastri (Mga-A) | — | 99.0 | 100 | 98.6 | 98.8 | 98.7 | — | 98.7 | 99.2 | 97.1 | — | — | — | 96.3 | 96.7 | 98.3 | 97.0 | 94.8 | — | — | 95.4 | 95.6 | |
| 2. M. gastri (Mga-B) | 98 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | |
| 3. M. szulgai | 92 | 91 | 99.0 | 98.5 | 98.6 | 98.9 | — | 99.2 | 99.6 | 97.5 | — | — | — | 97.1 | 97.1 | 98.8 | 97.5 | 95.4 | — | — | 95.8 | 96.0 | |
| 4. M. kansasii (Mka-A) | 93 | 92 | 92 | 98.6 | 98.8 | 98.7 | — | 98.7 | 99.2 | 97.1 | — | — | — | 96.7 | 96.7 | 98.3 | 97.0 | 94.8 | — | — | 95.4 | 95.6 | |
| 5. M. ulcerans-M. marinum | 91 | 91 | 90 | 92 | 99.2 | 98.6 | — | 98.1 | 98.5 | 96.6 | — | — | — | 96.7 | 96.3 | 97.9 | 97.2 | 95.3 | — | — | 95.6 | 96.0 | |
| 6. M. tuberculosis | 90 | 89 | 89 | 88 | 89 | 98.4 | — | 98.1 | 98.8 | 96.5 | — | — | — | 96.2 | 96.3 | 97.8 | 97.0 | 95.3 | — | — | 96.0 | 96.1 | |
| 7. M. avium (Mav-A) | 84 | 84 | 84 | 82 | 81 | 79 | — | 99.2 | 98.8 | 97.1 | — | — | — | 96.9 | 97.3 | 98.3 | 97.3 | 95.0 | — | — | 95.5 | 95.6 | |
| 8. M. avium (Mav-B) | 84 | 84 | 83 | 82 | 91 | 79 | 99 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | |
| 9. M. intracellulare (Min-A) | 85 | 84 | 83 | 85 | 84 | 79 | 94 | 94 | 98.8 | 97.1 | — | — | — | 96.2 | 96.7 | 98.4 | 97.1 | 95.2 | — | — | 95.4 | 95.8 | |
| 10. M. malmoense | 90 | 89 | 88 | 88 | 85 | 86 | 89 | 89 | 89 | 97.2 | — | — | — | 97.0 | 96.6 | 98.5 | 97.0 | 94.9 | — | — | 95.3 | 95.7 | |
| 11. M. simiae (Msi-A) | 89 | 88 | 86 | 87 | 89 | 84 | 84 | 84 | 88 | 86 | — | — | — | 99.4 | 99.1 | 98.0 | 96.4 | 95.1 | — | — | 96.6 | 96.7 | |
| 12. M. simiae (Msi-B) | 87 | 86 | 85 | 86 | 88 | 82 | 83 | 83 | 87 | 85 | 98 | — | — | — | — | — | — | — | — | — | — | — | |
| 13. M. simiae (Msi-C) | 89 | 88 | 87 | 88 | 89 | 83 | 84 | 84 | 89 | 87 | 99 | 98 | — | — | — | — | — | — | — | — | — | — | |
| 14. M. simiae (Msi-D) | 89 | 88 | 87 | 88 | 89 | 83 | 84 | 84 | 89 | 87 | 99 | 97 | 99 | — | — | — | — | — | — | — | — | — | |
| 15. M. genavense | 88 | 87 | 86 | 87 | 86 | 81 | 84 | 84 | 86 | 86 | 92 | 90 | 92 | 92 | 99.5 | 97.0 | 95.9 | 94.5 | — | — | 96.2 | 96.1 | |
| 16. M. triplex | 88 | 87 | 86 | 87 | 87 | 81 | 85 | 85 | 86 | 88 | 93 | 91 | 93 | 93 | 96 | 97.3 | 96.2 | 94.8 | — | — | 96.4 | 96.5 | |
| 17. M. conspicuum | 87 | 86 | 87 | 87 | 87 | 85 | 82 | 82 | 84 | 86 | 86 | 85 | 86 | 85 | 86 | 86 | 97.2 | 95.4 | — | — | 96.0 | 96.2 | |
| 18. M. shimoidei | 79 | 78 | 79 | 78 | 78 | 77 | 77 | 77 | 78 | 76 | 79 | 77 | 79 | 79 | 79 | 78 | 76 | 96.9 | — | — | 95.7 | 96.2 | |
| 19. M. xenopi (Mxe-A) | 70 | 69 | 70 | 71 | 69 | 69 | 67 | 67 | 67 | 69 | 69 | 69 | 69 | 69 | 67 | 67 | 68 | 70 | — | — | 94.9 | 95.0 | |
| 20. M. xenopi (Mxe-B) | 70 | 69 | 69 | 70 | 70 | 68 | 67 | 67 | 68 | 69 | 69 | 69 | 70 | 69 | 68 | 68 | 68 | 71 | 99 | — | — | — | |
| 21. M. xenopi (Mxe-C) | 69 | 69 | 69 | 70 | 69 | 68 | 66 | 66 | 67 | 69 | 69 | 69 | 69 | 69 | 67 | 67 | 68 | 70 | 99 | 99 | — | — | |
| 22. M. phlei | 66 | 67 | 70 | 68 | 70 | 69 | 68 | 68 | 69 | 71 | 69 | 68 | 69 | 69 | 67 | 68 | 67 | 71 | 64 | 64 | 64 | 98.2 | |
| 23. M. smegmatis | 75 | 74 | 78 | 75 | 80 | 75 | 75 | 75 | 77 | 76 | 81 | 80 | 81 | 81 | 77 | 78 | 75 | 80 | 63 | 64 | 63 | 72 | |
The fraction of identical residues was determined only for sequence pairs with positions occupied by nucleotides in both of the particular sequence pairs. —, not determinable.
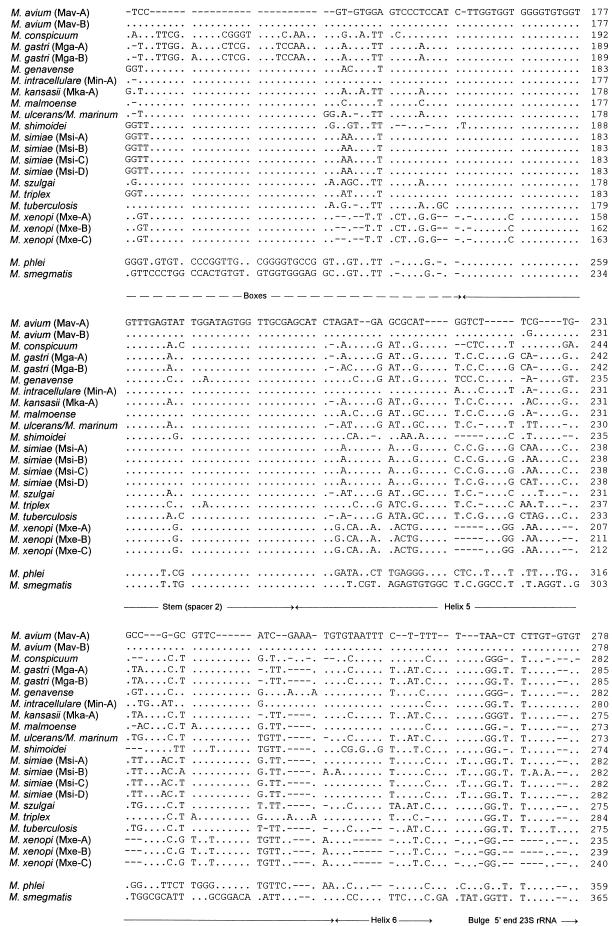
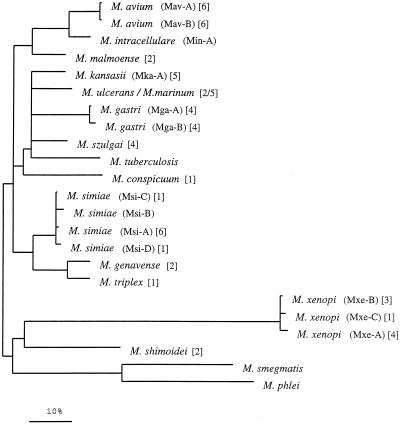
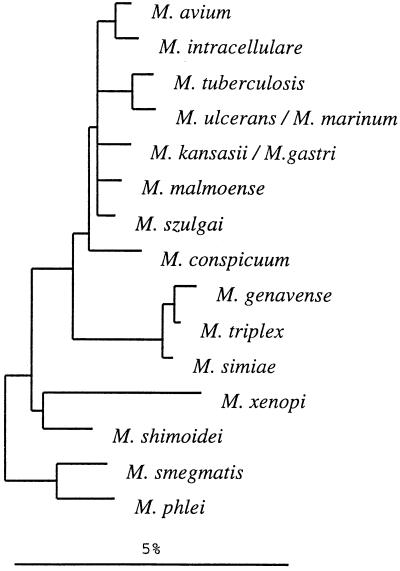
A dendrogram reflecting the ITS sequence-based clustering of all test strains is shown in Fig. 2. Within the consensus tree, five clusters among the slowly growing mycobacteria could be defined; of these clusters, M. shimoidei and M. xenopi showed sufficient sequence variation to emerge as distinct branches. The biggest cluster was composed of more than one closely related species, namely, M. conspicuum, M. gastri, M. kansasii, M. marinum, M. szulgai, M. tuberculosis, and M. ulcerans. The somewhat-separated position of M. conspicuum was supported by the majority of the different analyses; however, its significance was less than that of the other branchings. Two additional clusters comprised M. simiae, M. genavense, and M. triplex and M. avium, M. intracellulare, and M. malmoense. The significance of the branching orders was estimated by applying alternative treeing methods to various data sets which differed in sequence as well as in alignment position and by performing bootstrap analyses. The multifurcations indicate that a significant relative branching order could not be determined or that a common branching order was not supported by the results obtained by applying different treeing methods. Depending on the treeing method used, the corresponding bootstrap values were 25% and lower. A 16S rRNA-based tree is given in Fig. 3 for comparison. The overall pictures of both trees are similar with regard to the separation of fast and slow growers, the positions of M. shimoidei and M. xenopi, the clustering of M. avium together with M. intracellulare, and the grouping of M. genavense, M. triplex, and M. simiae. Interestingly, both the ITS and the 16S rRNA sequences of M. xenopi contain a number of unusual residues in comparison to those of the other organisms (as evidenced by the longer branches in both trees), which indicates a higher rate of substitution. However, there can also be seen remarkable differences in the substructures of the two trees. No further subgrouping was supported by the 16S rRNA data for the group comprising M. avium, M. intracellulare, M. tuberculosis, M. ulcerans, M. marinum, M. kansasii, M. gastri, M. malmoense, M. szulgai, and the slightly deeper branching M. conspicuum. In contrast, the ITS analyses allowed the definition of two subclusters, one containing M. avium, M. intracellulare, and M. malmoense and one containing M. kansasii, M. ulcerans-M. marinum, M. gastri, M. szulgai, M. tuberculosis, and the somewhat more distant M. conspicuum. A closer relationship between M. tuberculosis and M. ulcerans-M. mannum such as that shown in the 16S rRNA-based tree was not supported by the ITS data. M. malmoense and M. szulgai are members of different ITS clusters, while a closer relationship of these two species had been reported on the basis of 16S rRNA data (7, 26, 28). The latter finding could be determined with only low significance when distance methods were applied; however, this was not supported by maximum-parsimony or maximum-likelihood analyses (Fig. 3).
FIG. 2.
Distance matrix tree showing the divergence of ITS sequences of the investigated mycobacteria. All alignment positions which are occupied by residues were used for the calculation of binary distance values. The topology of the tree was evaluated and corrected according to the results of maximum-parsimony and maximum-likelihood analyses. Multifurcations indicate that a relative branching order could not be unambiguously determined or that a common branching order was not supported by the different treeing methods. The corresponding sequences of the fast-growing mycobacteria M. phlei and M. smegmatis were used as outgroup references. Numbers in brackets indicate the numbers of strains sequenced. The bar represents 10% estimated sequence divergence.
FIG. 3.
16S rRNA-based distance matrix tree for a selection of mycobacterial species. The tree was reconstructed with all available, at-least-90%-complete (with respect to the homologous E. coli molecule), 16S rRNA primary structures of gram-positive bacteria with a high DNA G+C content as well as from a selection of reference organisms of the other major bacterial phyla. The topology of the tree was evaluated and corrected by the methods applied to ITS sequences (Fig. 2). The bar indicates 5% estimated sequence divergence.
DISCUSSION
Sequence-specific differentiation of mycobacteria by using the ITS.
The conventional methods for identifying mycobacteria based on growth characteristics and biochemical tests are time-consuming and often not unambiguous in their interpretation. Currently, the widely accepted strategy formulated to improve methods of mycobacterial strain identification includes analysis of the gene encoding 16S rRNA (3, 17, 18, 22, 23, 25, 26, 33). Furthermore, the potential utility of alternative targets such as the gene encoding a 65-kDa heat shock protein (hsp65) has been described (31). Each technique has several advantages and disadvantages. The small number of polymorphic positions within the 16S rDNA obviates the need of nucleotide sequencing (17, 23). The limited availability of commercial probes is a reflection of this problem, and only a few studies have highlighted the potential applicability of a wider panel of probes directed to 16S rDNA sequences (3, 18, 22). In the clinical laboratory, easy and cost-effective identification of mycobacteria is of high priority. Undoubtedly, a target with a higher level of variability would considerably facilitate the wide introduction of hybridization methods or even simpler methods such as multiplex PCR into the routine laboratory. On the other side, an excessively high degree of variability such as that found in the hsp65 gene (31) may be undesirable because such a variety or instability of species-specific signatures will make development of reliable probes that cover all strains within a species very cumbersome. Finally, the phylogenetic information deducible from a gene with such a low selective pressure for its sequence conservation (as compared to coding and noncoding rDNA sequences) may result in inconsistent conclusions. The principal goal of our study was to further investigate the level of ITS polymorphism and thereby assess the utility of this target for mycobacterial species identification. This study demonstrates that the ITS of the genus Mycobacterium exhibits variations in length and, importantly, shows a reasonable number of base substitutions and insertion or deletion sites. We have shown that this higher degree of variation is of value for discriminating closely related species such as M. gastri and M. kansasii. Unfortunately, ITS sequence analysis failed to discriminate between M. marinum and M. ulcerans. This disappointing, yet not surprising, finding stands in agreement with 16S rDNA data, according to which these two species are very closely related (22, 23). Single base-pair variations related to three residues at the 3′ end of the 16S rDNA distinguish M. marinum from M. ulcerans (23). The only mycobacterial species so far found to have the same ITS sequences are members of the M. tuberculosis complex (9). A high degree of genomic relatedness between M. marinum and M. ulcerans in DNA-DNA hybridizations and shared phenotypic characteristics strongly suggest that these two mycobacteria represent a single species (6, 23). It is even more apparent now from the high ITS sequence conservation found that clarification of this issue warrants additional studies. This study confirms and extends previous observations that the ITS sequence between the 16S rRNA and 23S rRNA contains sufficient interspecific polymorphisms and intraspecific conservation to serve as a valuable target for mycobacterial identification. Besides its greater variability, two further advantages of this target can be pointed out. Unlike a 16S rDNA-based PCR, where the genus-specific primers are separated by a long stretch of target sequence (more than 500 bp) (3, 17, 33), an ITS-based PCR would imply a smaller PCR product, resulting in a more efficient and sensitive target amplification. In addition, the ITS has the potential to be used for clinically significant strain differentiation (1, 4, 11, 24). We can expect that more sequevars will be characterized when a bigger number of strains are analyzed. We did not find any intraspecific variation in the five M. kansasii strains studied here, but other reports give evidence that more than one sequevar exists (1, 41). Genetic identification of possibly more pathogenic strains of nontuberculous mycobacteria (whose clinical significance after isolation from patient specimens is often difficult to assess) could be of extreme diagnostic value. In view of this, seeking further studies on this issue, for example, on the epidemiological significance of the M. xenopi sequevars found here, is an imperative.
ITS sequence comparison as an adjunct to Mycobacterium phylogeny.
It is well documented that a wide range of phylogenetic relationships (domain to species) of bacteria can be substantiated by a sequence comparison of their rRNAs (20, 21, 27). This scientific rationale cannot be doubted, and comparative 16S rDNA sequencing has contributed largely to our understanding of mycobacterial taxonomy of well-resolved species (26, 29, 34). However, this approach provides a rather low resolution on the level of closely related, recently emerged species within the genus Mycobacterium, as demonstrated by high 16S rDNA similarities or even sequence identity of different species (8, 26). Despite considerable phenotypic diversity, slowly growing mycobacteria appear to have diverged over a short period. Hence, ITS sequences have been proposed to be a useful supplement when 16S rDNA shows insufficient diversity to differentiate recently diverged species (10, 15). The ITS sequence does not code for a final product, but it has an important processing function in forming pre-RNAs; as a consequence, there is presumably some functional selective pressure for its conservation (10, 15). This assumption is consistent with the stability of species-specific ITS signatures found with high reproducibility in different strains. For example, identical sequences distinct for two M. avium sequevars were found in three completely different geographical regions in clinical samples from patients with AIDS in this study and by two other working groups (4, 10).
As shown with other taxa, the evolutionary rate of the ITS is higher than that of 16S rRNA, and rearrangements in the central region are relatively recent. Consequently, phylogenetic information on the ancestry of only moderately related species may not be maintained (19). Therefore, we must expect that the two molecules provide different levels of phylogenetic resolution. High levels of relatedness and identical ITS- and 16S rRNA-based tree topologies were found for M. genavense and M. triplex, for M. simiae and both M. genavense and M. triplex, and for M. avium and M. intracellulare. Comparison of the sequences of M. gastri with M. kansasii can be regarded as an example of the higher resolution of ITS data at the species level. Our ITS data suggest a divergence between M. gastri and M. kansasii. Phenetic data supported the resolution of these two bacteria as distinct species (36). At the 16S rDNA sequence level, these two species are identical (26). The finding that the T-catalases of these two species are related is not useful in this context since divergence of T-catalase is not an accurate reflection of natural (evolutionary) relationships (37).
The ITS-based subclustering of M. avium, M. intracellulare, and M. malmoense compared with that of M. kansasii, M. ulcerans, M. marinum, M. gastri, M. szulgai, M. tuberculosis, and M. conspicuum, which is not significantly evident from rRNA data, is another example of different resolution. A closer relationship of M. tuberculosis and M. marinum-M. ulcerans is not supported by ITS sequence data but is rather stable in 16S rRNA-based trees. However, given the high sequence similarity (99.2%) of these organisms and the short distance to the next neighbors (1.2 to 2.2%), it cannot be excluded (and can hardly be tested) that the identities at the highly variable positions responsible for the clustering of M. tuberculosis and M. marinum-M. ulcerans may result from multiple base changes during the course of evolution and thus may represent false identities (20).
A closer relationship was described for M. malmoense and M. szulgai based on a 16S rRNA sequence comparison (26). As mentioned above, this grouping can be determined with low significance by distance methods analyzing the currently available data set but is not supported by maximum-parsimony nor maximum-likelihood analysis. The assignment of M. malmoense and M. szulgai to different subclusters according to their ITS sequence similarities is supported by other data. Numerical taxonomy characterizes M. szulgai as a species that emerges as a discrete cluster with the second highest matching score to M. kansasii (38). In contrast, a clear phenotypic resolution (with some overlaps with the M. avium complex) placing M. malmoense far from M. szulgai was found. Therefore, by taking into account that numerical taxonomy is relevant and complementary to semantide studies (34, 38), the position of M. malmoense in the ITS phylogeny appears reasonable and contributes to satisfying the claim that a phylogenetically based scheme should be accompanied by phenotypic consistency (34). The M. malmoense-M. szulgai case nicely illustrates the limitations of the rRNA approach and the demand for a polyphasic approach for taxonomic analyses at and above the species level (34).
In conclusion, comparative ITS sequencing represents a useful tool for species (strain) differentiation and identification if the primary structures contain polymorphic and diagnostic residues or stretches, respectively. The occurrence of conserved primary- and secondary-structure elements among mycobacterial ITS sequences indicates a potential for phylogenetic investigations. However, as demonstrated by the high divergence of the M. xenopi sequence from other ITS sequences of slowly growing mycobacteria, the rate of base changes in ITS sequences may vary to a large scale within different (phylogenetic) groups. Consequently, whether ITS sequences contain useful phylogenetic information for the particular group of interest must be carefully determined. In the case of rapidly growing mycobacteria which carry multiple rRNA operons, the analysis may be complicated by interoperon heterogeneities.
ACKNOWLEDGMENT
M. E. Hamid was supported by a fellowship from the Alexander von Humboldt Foundation.
REFERENCES
- 1.Alcaide F, Richter I, Bernasconi C, Springer B, Hagenau C, Schulze-Röbbecke R, Tortoli E, Martín R, Böttger E, Telenti A. Heterogeneity and clonality among isolates of Mycobacterium kansasii: implications for epidemiological and pathogenicity studies. J Clin Microbiol. 1997;35:1959–1964. doi: 10.1128/jcm.35.8.1959-1964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry T, Colleran G, Glennon M, Dunican L K, Gannon F. The 16S/23S ribosomal spacer region as a target for DNA probes to identify eubacteria. PCR Methods Appl. 1991;1:51–56. doi: 10.1101/gr.1.1.51. [DOI] [PubMed] [Google Scholar]
- 3.De Beenhouwer H, Liang Z, de Rijk P, van Eekeren C, Portaels F. Detection and identification of mycobacteria by DNA amplification and oligonucleotide-specific capture plate hybridization. J Clin Microbiol. 1995;33:2994–2998. doi: 10.1128/jcm.33.11.2994-2998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Smet A L, Brown I N, Yates M, Ivanyi J. Ribosomal internal transcribed spacers are identical among Mycobacterium avium-intracellulare complex isolates from AIDS patients, but vary among isolates from elderly pulmonary disease patients. Microbiology. 1995;141:2739–2747. doi: 10.1099/13500872-141-10-2739. [DOI] [PubMed] [Google Scholar]
- 5.Emler S, Ninet B, Rohner P, Auckenthaler R, Jäger D, Hirschel B. Molecular basis for cross-reactivity between a strain of Mycobacterium terrae and DNA probes for Mycobacterium tuberculosis complex. Eur J Microbiol Infect Dis. 1995;14:627–629. doi: 10.1007/BF01690741. [DOI] [PubMed] [Google Scholar]
- 6.Falkinham J O. Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev. 1996;9:177–215. doi: 10.1128/cmr.9.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Floyd M M, Guthertz L S, Silcox V A, Duffey P S, Jang Y, Desmond E P, Crawford J T, Butler W R. Characterization of an SAV organism and proposal of Mycobacterium triplex sp. nov. J Clin Microbiol. 1996;34:2963–2967. doi: 10.1128/jcm.34.12.2963-2967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox G E, Wisotzkey J D, Jurtshuk P. How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int J Syst Bacteriol. 1992;42:166–170. doi: 10.1099/00207713-42-1-166. [DOI] [PubMed] [Google Scholar]
- 9.Frothingham R, Hills H G, Wilson K H. Extensive DNA sequence conservation throughout the Mycobacterium tuberculosis complex. J Clin Microbiol. 1994;32:1639–1643. doi: 10.1128/jcm.32.7.1639-1643.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frothingham R, Wilson K H. Sequence-based differentiation of strains in the Mycobacterium avium complex. J Bacteriol. 1993;175:2818–2825. doi: 10.1128/jb.175.10.2818-2825.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frothingham R, Wilson K H. Molecular phylogeny of the Mycobacterium avium complex demonstrates clinically meaningful divisions. J Infect Dis. 1994;169:305–312. doi: 10.1093/infdis/169.2.305. [DOI] [PubMed] [Google Scholar]
- 12.Good R C. Opportunistic pathogens in the genus Mycobacterium. Annu Rev Microbiol. 1985;39:347–369. doi: 10.1146/annurev.mi.39.100185.002023. [DOI] [PubMed] [Google Scholar]
- 13.Gürtler V, Stanisch V A. New approaches to typing and identification of bacteria using the 16S-23S rDNA spacer region. Microbiology. 1996;142:3–16. doi: 10.1099/13500872-142-1-3. [DOI] [PubMed] [Google Scholar]
- 14.Ji Y-E, Colston M J, Cox R A. The ribosomal RNA (rrn) operons of fast-growing mycobacteria: primary and secondary structures and their relation to rrn operons of pathogenic slow-growers. Microbiology. 1994;140:2829–2840. doi: 10.1099/00221287-140-10-2829. [DOI] [PubMed] [Google Scholar]
- 15.Ji Y-E, Kempsell K E, Colston M J, Cox R A. Nucleotide sequences of the spacer-1, spacer-2 and trailer regions of the rrn operons and secondary structures of precursor 23S rRNAs and precursor 5S rRNAs of slow-growing mycobacteria. Microbiology. 1994;140:1763–1773. doi: 10.1099/13500872-140-7-1763. [DOI] [PubMed] [Google Scholar]
- 16.Kent P T, Kubica G P. Public health mycobacteriology—a guide for the level III laboratory. U.S. Department of Health and Human Services publication (CDC) 86-8230. Centers for Disease Control, Atlanta, Ga. 1985. [Google Scholar]
- 17.Kirschner P, Springer B, Vogel U, Meier A, Wrede A, Kiekenbeck M, Bange F C, Böttger E C. Genotypic identification of mycobacteria by nucleic acid sequence determination: report of a 2-year experience in a clinical laboratory. J Clin Microbiol. 1993;31:2882–2889. doi: 10.1128/jcm.31.11.2882-2889.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kox L F F, van Leeuwen J, Knijper S, Jansen H M, Kolk A H. PCR assay based on DNA coding for 16S rRNA for detection and identification of mycobacteria in clinical samples. J Clin Microbiol. 1995;33:3225–3233. doi: 10.1128/jcm.33.12.3225-3233.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leblond-Bourget N, Philippe H, Mangin I, Decaris B. 16S rRNA and 16S to 23S internal transcribed spacer sequence analyses reveal inter- and intraspecific Bifidobacterium phylogeny. Int J Syst Bacteriol. 1996;46:102–111. doi: 10.1099/00207713-46-1-102. [DOI] [PubMed] [Google Scholar]
- 20.Ludwig W, Schleifer K H. Bacterial phylogeny based on 16S and 23S rRNA sequence analysis. FEMS Microbiol Rev. 1994;15:155–173. doi: 10.1111/j.1574-6976.1994.tb00132.x. [DOI] [PubMed] [Google Scholar]
- 21.Olsen G J. Phylogenetic analysis using ribosomal RNA. Methods Enzymol. 1988;164:793–812. doi: 10.1016/s0076-6879(88)64084-5. [DOI] [PubMed] [Google Scholar]
- 22.Portaeles F, Aguiar J, Fissette K, Fonteyne A, de Beenhouwer H, de Rijk P, Guédénon A, Lemans R, Steunou C, Zinsou C, Dumonceau J M, Meyers W M. Direct detection and identification of Mycobacterium ulcerans in clinical specimens by PCR and oligonucleotide-specific capture plate hybridization. J Clin Microbiol. 1997;35:1097–1100. doi: 10.1128/jcm.35.5.1097-1100.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Portaeles F, Fonteyne P-A, de Beenhouwer H, de Rijk P, Guédénon A, Hayman J, Meyers W M. Variability in 3′ end of 16S rRNA sequences of Mycobacterium ulcerans is related to geographic origin of isolates. J Clin Microbiol. 1996;34:962–965. doi: 10.1128/jcm.34.4.962-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Portaeles, F., P. de Rijk, G. Jannes, R. Lemans, W. Mijs, L. Riqouts, and R. Rossau. 1996. The 16S-23S rRNA spacer, a useful tool for taxonomical and epidemiological studies of the M. chelonae complex. Tubercle Lung Dis. 77(Suppl. 2):17–18.
- 25.Reisner B S, Gatson A M, Woods G L. Use of Gen-Probe AccuProbes to identify Mycobacterium tuberculosis complex, Mycobacterium kansasii, and Mycobacterium gordonae directly from BACTEC TB broth cultures. J Clin Microbiol. 1994;32:2995–2998. doi: 10.1128/jcm.32.12.2995-2998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogall T, Wolters J, Floher T, Böttger E C. Towards a phylogeny and definition of the species at the molecular level within the genus Mycobacterium. Int J Syst Bacteriol. 1990;40:323–330. doi: 10.1099/00207713-40-4-323. [DOI] [PubMed] [Google Scholar]
- 27.Schleifer K H, Ludwig W. Phylogenetic relationships of bacteria. In: Fernholm B, Bremer K, Jörnvall H, editors. The hierarchy of life. Amsterdam, The Netherlands: Elsevier Science Publishers B. V.; 1989. pp. 103–117. [Google Scholar]
- 28.Schröder K-H, Naumann L, Kroppenstedt R M, Reischl U. Mycobacterium hassiacum sp. nov., a new rapidly growing thermophilic mycobacterium. Int J Syst Bacteriol. 1997;47:86–91. doi: 10.1099/00207713-47-1-86. [DOI] [PubMed] [Google Scholar]
- 29.Stahl D A, Urbance J W. The division between fast- and slowly growing species corresponds to natural relationships among the mycobacteria. J Bacteriol. 1990;172:116–124. doi: 10.1128/jb.172.1.116-124.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strunk, O., O. Gross, B. Reichel, M. May, S. Hermann, N. Stuckman, B. Nonhoff, M. Lenke, A. Ginhart, A. Vilbig, T. Ludwig, A. Bode, K.-H. Schleifer, and W. Ludwig. ARB: a software environment for sequence data. Nucleic Acids Res., submitted for publication. [DOI] [PMC free article] [PubMed]
- 31.Swanson D, Pan X, Musser J M. Identification and subspecific differentiation of Mycobacterium scrofulaceum by automated sequencing of a region of the gene (hsp65) encoding a 65-kilodalton heat shock protein. J Clin Microbiol. 1996;34:3151–3159. doi: 10.1128/jcm.34.12.3151-3159.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swoffold D L, Olsen G J. Phylogeny reconstruction. In: Hillis D M, Moritz C, editors. Molecular systematics. Sunderland, Mass: Sinauer Associates; 1990. pp. 411–501. [Google Scholar]
- 33.Tevere V J, Hewitt P L, Dare A, Hocknell P, Keen A, Spadoro J P, Young K K Y. Detection of Mycobacterium tuberculosis by PCR amplification with pan-Mycobacterium primers and hybridization to an M. tuberculosis-specific probe. J Clin Microbiol. 1996;34:918–923. doi: 10.1128/jcm.34.4.918-923.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandamme P, Pot B, Gillis M, De Vos P, Kersters K, Swings J. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol Rev. 1996;60:407–438. doi: 10.1128/mr.60.2.407-438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van der Giessen J W B, Haring R M, Zeijst B A M. Comparison of the 23S ribosomal RNA and the spacer region between 16S-23S rRNA genes of the closely related Mycobacterium avium and Mycobacterium paratuberculosis and the fast-growing Mycobacterium phlei. Microbiology. 1994;140:1103–1108. doi: 10.1099/13500872-140-5-1103. [DOI] [PubMed] [Google Scholar]
- 36.Wayne L G, Andrade L, Froman S, Käppler W, Kubala E, Meissner G, Tsukamura M. A co-operative numerical analysis of Mycobacterium gastri, Mycobacterium kansasii, and Mycobacterium marinum. J Gen Microbiol. 1979;109:319–327. doi: 10.1099/00221287-109-2-319. [DOI] [PubMed] [Google Scholar]
- 37.Wayne L G, Diaz G A. Serological, taxonomic, and kinetic studies of the T and M classes of mycobacterial catalase. Int J Syst Bacteriol. 1982;32:296–304. [Google Scholar]
- 38.Wayne L G, Good R C, Krichevsky M I, Blacklock Z, David H L, Dawson D, Gross W, Hawkins J, Jenkins P A, Juhlin I, Käppler W, Kleeberg H H, Levy-Frebault V, McDurmont C, Nel E E, Portaels F, Rüsch-Gerdes S, Schröder K H, Silcox V A, Szabo I, Tsukamura M, van Den Breen L, Vergmann B, Yakrus M A. Third report of the cooperative, open-ended study of slowly growing mycobacteria by the International Working Group on Mycobacterial Taxonomy. Int J Syst Microbiol. 1989;39:267–278. doi: 10.1099/00207713-41-4-463. [DOI] [PubMed] [Google Scholar]
- 39.Wayne L G, Sramek H A. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin Microbiol Rev. 1992;5:1–25. doi: 10.1128/cmr.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woods G L, Washington J A. Mycobacteria other than Mycobacterium tuberculosis: review of microbiological and clinical aspects. Rev Infect Dis. 1987;9:275–294. doi: 10.1093/clinids/9.2.275. [DOI] [PubMed] [Google Scholar]
- 41.Yang M, Ross B C, Dwyer B. Isolation of a DNA probe for identification of Mycobacterium kansasii, including the genetic subgroup. J Clin Microbiol. 1993;31:2769–2772. doi: 10.1128/jcm.31.10.2769-2772.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]