Abstract
Background and aims
Lipoprotein particle (p) size and count beyond lipoprotein lipid content [triglycerides (tg) and cholesterol (c)] are critical for their atherogenicity. This study characterized lipoprotein profiles in metabolically healthy individuals with overweight or obesity and assessed the impact of sex, obesity, and lipid background.
Methods
Proton-nuclear magnetic resonance (¹H-NMR) was used to assess the composition of very low-, low-, intermediate-, and high-density lipoproteins (VLDL, LDL, IDL, HDL), and particle number and size of VLDL, LDL, and HDL in 101 healthy subjects with overweight and obesity.
Results
Men showed significantly higher VLDLc and VLDLtg levels, counts of VLDLp (all subfractions) and LDLp (total and small), and smaller LDLp size, compared to women. Men had lower HDLc and HDLp (total and medium). In Obesity (Ob) compared to overweight (Ov), VLDLp number, VLDLtg and remnant cholesterol (RC) levels were significantly increased [Fold changes (FC) Ob.vs.Ov: 1.45, 1.39, and 1.26, respectively]. When stratified by sex, obesity-related VLDL and IDL profile deterioration was evident only in women. Individuals with LDLc ≥ 130 mg/dL showed increased RC compared to those with LDLc < 130 mg/dL (FC:1.26). The median 10-year cardiovascular disease (CVD) risk REGICOR was low (2%), but higher in men and in obesity. Individuals with higher CVD risk showed increased VLDLc, VLDLtg, VLDLp, and RC levels.
Conclusion
Men had a higher 10-year CVD risk and a less favorable triglyceride-rich lipoprotein and RC profile, while obesity aggravated these patterns, particularly in women. These findings support considering high-risk lipoprotein patterns in targeted CVD prevention for overweight and obese populations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-025-02723-2.
Keywords: Lipoproteins, Sex, Obesity, Remnant-cholesterol, Nuclear magnetic resonance, Cardiovascular diseases
Introduction
Lipoproteins are essential molecules that carry lipids like cholesterol and triglycerides (TG) through the bloodstream to tissues based on their metabolic needs. They play a vital role in lipid balance and cardiovascular health, with significant clinical relevance. Classified by size, lipid content, and apolipoproteins, they include chylomicrons, very low-, intermediate-, low-, and high-density lipoproteins (VLDL, IDL, LDL, HDL), each serving specific functions. The lipoproteins may undergo alterations that affect their structure, functionality, composition, and plasma concentrations and favor their accumulation in the arterial wall triggering pro-atherogenic processes [1–3].
While LDL cholesterol (LDLc) is the primary target of lipid-lowering therapies to reduce cardiovascular disease (CVD) risk [4]. Growing evidence suggests that additional variables, such as number and size of lipoprotein particles, may provide valuable information and perform better than LDLc in predicting CVD risk [1, 5]. This is the case of small and dense LDL particles (LDLp), which are strongly associated with atherosclerotic CVD (ASCVD) [3], and when present in high concentrations increase CVD risk even when LDLc is considered optimal [3]. Indeed, a significant proportion of cardiovascular events occur in subjects whose LDLc levels are well controlled, according to guidelines [6, 7].
The residual CVD risk has been related to alterations in the lipid metabolism, particularly in the triglyceride-rich lipoproteins (TRL) (VLDL, IDL) and the cholesterol they contain, known as remnant cholesterol (RC), which represents a major contributor [8, 9].
In the clinic, remnant cholesterol, has been suggested as the causal factor of the TRL-associated risk for ischemic heart disease, rather than TGs themselves [10].
HDLc is considered protective for CVD risk [11]. However, HDL particles are heterogeneous in size and composition [1] and their contribution to atherosclerosis and CVD risk is directly related to their structural characteristics, molecular composition and functional properties [11, 12].
In this respect, the Women’s Health Study (WHS), with 27,909 women, demostrated that only large HDL particles (HDLp), assessed using nuclear magnetic resonance (NMR), were associated with lower CVD risk [1]. Similarly, a greater number of large and medium size HDLp, along with lower concentration of small HDLp, has been associated to a better cardiometabolic risk profile [13, 14]. Furthermore, a prospective study conducted on a large cohort of healthy women found that an association between reduced cardiovascular risk and adherence to Mediterranean diet through the assessment of HDL and VLDL measures [15] Smaller VLDL are more strongly associated with ASCVD due to their propensity to be retained in the endothelium [16] (An exception is the large triglyceride-rich, buoyant VLDL, which are precursors of remnant particles with high atherogenic capacity [17, 18].
The particle (p) number, size and, composition [triglycerides (tg) and cholesterol (c)] of lipoproteins are relevant determinants of their atherogenic potential. NMR has emerged as an advanced tool to accurately assess these variables, providing deeper insights into their contribution to vascular risk [5].
Several factors are known to influence the lipoprotein profile, including sex [19–21], body mass index (BMI) [19, 20], age [19] and diet [21]. Differences in lipoprotein subclasses have been observed between type 2 diabetes mellitus (T2DM) and normoglycemic individuals [19, 22], and between subjects with obesity and normoweight [23]. However, little is known about the differences that exist within healthy individuals. In a large cohort of apparently healthy men and women, LDLp were closely associated with the occurrence of future coronary artery disease (CAD), however, the VLDL and HDL profile was not evaluated in this study [24]. To address the existing gap, the current study aims to characterize the lipid content, size, and number of lipoproteins using proton nuclear magnetic resonance (1H-NMR) in a cohort of metabolically healthy subjects with overweight and obesity, who had no known CVD risk factors and were not under regular pharmacological treatment. Additionally, the study aimed to evaluate the interaction between the lipoprotein subclass patterns and variables such as sex, BMI, background LDLc, and calculated CVD risk.
or obesity, without known cardiovascular risk factors or regular pharmacological treatment.
Materials and methods
Subjects and study design
The study population comprises 101 apparently metabolically healthy men and women with overweight or obesity [BMI 25–37 kg/m2] and age aged from 25 to 60 years. None of the subjects in the study population were under pharmacological treatment for any chronic condition, including lipid-lowering or other therapeutic agents. Individuals were excluded if they reported eating disorders, cardiovascular risk factors, a history of cardiovascular disease or cancer among other. Pregnancy was also a reason for exclusion. Additional exclusion criteria included consuming more than 60 g/day of alcohol, or being on a hypocaloric diet or weight loss program within 2 months prior to enrollment. Of 101 subjects, 14 (13.86%) were regular smokers (men: 16% and women: 12%).
Participants underwent a comprehensive physical examination by a physician at the time of biological sample collection to confirm their health status. All included participants were classified as metabolically healthy according to the Adult treatment panel III (ATP-III) guidelines [25], which define metabolic health as the presence of fewer than three of the following criteria for metabolic syndrome: abdominal obesity (waist circumference > 102 cm in men or > 88 cm in women), elevated triglycerides (≥ 150 mg/dL), low HDL cholesterol (< 40 mg/dL in men or < 50 mg/dL in women), high blood pressure (≥ 130/85 mm Hg), and elevated fasting glucose (≥ 100 mg/dL). In this study, participants with overweight or obesity were considered metabolically healthy since they did not meet the threshold for metabolic syndrome. As shown in Table S1, clinical and biochemical parameters for the study population were within normal physiological ranges. These included a median systolic blood pressure of 123.5 mmHg (IQR: 114–129) and a median diastolic pressure of 70 mmHg (IQR: 62–75), a median fasting glucose level of 4.7 mmol/L (IQR: 4.5–5.0) and a median fasting triglycerides 94.8 mg/dL (IQR: 62.8–134.5).
Overweight and obesity were defined according to the World Health Organization (WHO) criteria [26], with overweight classified as a body mass index (BMI) between 25.0 and 29.9 kg/m², and obesity as a BMI of 30.0 kg/m² or higher [27]. For comparisons, participants were grouped by sex (51 women and 50 men) and by BMI category, distinguishing individuals with overweight (N = 55) and those with obesity (N = 46), based on the BMI cut-off of 30.0 kg/m². Anthropometric, hemodinamic and biochemical data, including those of liver and kidney function markers are provided in Table S1.
This study utilized retrospective baseline samples from participants enrolled in previous nutritional trials conducted between 2015 and 2021 at the Research Institute Sant Pau (IR-HSCSP), Barcelona, Spain. All participants had provided written informed consent, explicitly allowing the retrospective use of their samples for future cardiovascular research. The study protocols, including the retrospective use of these samples, were reviewed and approved by the Human Ethics Review Committee of the Hospital de la Santa Creu i Sant Pau in Barcelona [28–31].
Biological samples
Twelve-hour fasting blood samples were collected between 8:00 and 11:00 am in tubes without anticoagulant for serum preparation. After collection, samples were allowed to coagulate for 30 min at 37 °C, followed by a further 30 min at 4 °C. Serum was then recovered by centrifugation at 1816 × g for 30 min. Serum samples were aliquoted, immediately frozen, and stored at −80 °C without thawing until analysis.
Anthropometric data, blood pressure, biochemical measurements and serum lipid profile
Anthropometric measurements (height, weight, waist, and blood pressure) were taken by trained personnel at the time of the biological sample collection. Body mass index (BMI) was calculated as weight (kg)/height (m2). Serum biochemical measurements were performed at the centralized laboratory for analysis of the Hospital de la Santa Creu I Sant Pau (Barcelona, Spain) using routine commercially available assays for glucose, hepatic and renal markers, standard serum levels of triglycerides (TG), total cholesterol (TC), and HDLc (Roche Diagnostics, Basel, Switzerland) to characterize the metabolically healthy population used in the study, as previously reported.
1H-NMR for lipoprotein characterization
Lipoprotein (VLDL, LDL, HDL) pattern for particle size distribution, quantity and diameter and lipid composition (TG, cholesterol) of VLDL, IDL, LDL and HDL was determined in serum samples by high-resolution 1H-NMR spectroscopy and the Liposcale® test in vitro diagnostic medical device with Conformité Européenne marking (IVD-CE) [22]. Analysis was performed with a BrukerAvance III 600 Nuclear Magnetic Resonance (NMR) spectrometer (Bruker Biospin, Rheinstetten, Germany), set at proton frequency of 600.20 MHz (14.1 T) and at 310 K. The Liposcale® test (IVD-CE) utilizes 2-dimension (2D) diffusion-ordered ¹H NMR spectroscopy to directly quantify lipoprotein subclasses by measuring particle diffusion coefficients. This method decomposes NMR signals into nine distinct Lorentzian functions (F1–F9) corresponding to large, medium, and small subclasses of VLDL, LDL, and HDL [22]. Briefly, the methyl signal from a longitudinal eddy-current delay (LED) pulse spectra was surface-fitted with 9 Lorentzian functions associated with each lipoprotein subtype: large, medium, and small VLDL, LDL, and HDL. The area of each Lorentzian function was related to the lipid concentration of TG and cholesterol in lipoprotein subclasses that were estimated using partial least squares (PLS) regression models based on the total NMR signal intensity. The regression models have been previously calibrated with reference samples purified by ultracentrifugation and enzymatically analyzed.
Lipid concentration units were converted to lipid volume units using common conversion factors. The size of VLDL, LDL and HDL particles were calculated from its diffusion coefficient using the Stokes-Einstein equation. Specifically, the approximate particle size ranges defined for each subclass are as follows: VLDL (Small: 38.6–45.0 nm, Medium: 45.0–60.0 nm, Large: 60.0–81.9 nm); LDL (Small: 18.9–20.5 nm, Medium: 20.5–23.0 nm, Large: 23.0–26.5 nm); HDL (Small: 7.8–8.2 nm, Medium: 8.2–9.4 nm, Large: 9.4–11.5 nm) [22].
The particle number of each of the 9 lipoprotein subtypes was calculated by dividing the lipid volume by the mean volume of each lipoprotein subclass. Coefficients of variation for the particle sizes were less than 0.3% and for particle numbers between 2% and 4%.
Particle number and size for IDL are not reported by this method due to overlap in diffusion coefficients with small VLDL and large LDL particles, which limits resolution [22].
Cardiovascular risk assessment
Cardiovascular risk was estimated using the REGICOR (Registre Gironí del Cor) score, a validated adaptation of the Framingham equation to adjust it to the epidemiological and risk characteristics of the Spanish population [32]. The variables included in the REGICOR equation age, sex, total cholesterol, HDLc, systolic blood pressure, smoking status, and the presence of diabetes mellitus [33].
Statistical analysis
Statistical analyses were performed using STATA 17.0 (College Station, TX, USA) and StatView 5.0.1 software (SAS Institute, Cary, NC, USA). Normality of the data distribution was assessed using the Shapiro-Wilk test. Statistical differences between comparison groups were calculated using the Wilcoxon-Mann-Whitney test. Chi-square tests were used for categorical variable distribution. Correlations between continuous variables were assessed by the Spearman coefficient. The data are expressed as the median and the interquartile range [IQR]. Statistical significance was assumed when P-values were < 0.05.
Results
Study population
Both groups had similar age and sex distribution (P > 0.050). Individuals with obesity had higher serum concentrations of TC, TG and TG/HDLc compared to individuals with overweight (P = 0.029, P = 0.029 and P = 0.024, respectively). No differences were found in blood pressure, glucose levels (Table S1), or in smoking habits (overweight: 42.86% vs. obesity 57.14%, P > 0.050).
Cholesterol and triglyceride content in lipoproteins: studies by 1H-NMR
The triglyceride and cholesterol content of lipoproteins, assessed by 1H-NMR in the healthy study group is shown in Table S2. When discriminated by sex (Table 1.), men had significantly higher levels of VLDL cholesterol (VLDLc) and VLDL triglyceride (VLDLtg) than women with a similar BMI (men vs. women: 29.9 [28.3–31.6] vs. 29.4 [27.4–31.6] kg/m2, P = 0.525) (Table S1). HDLc levels were > 50 mg/dL in both sexes, although levels in women exceeded those in men (P < 0.001). No differences were found between the sexes in HDLtg, LDLc and LDLtg, or in the IDL fraction (IDLc, IDLtg) (Table 1.).
Table 1.
Lipoprotein lipid composition assessed by 1H-NMR
| (mg/dL) | Women | Men | P-Value |
| VLDLc | 12.5 [7.3–17.7] | 16.7 [11.4–24.4] | 0.002 |
| VLDLtg | 47.9 [34.9–75.1] | 72.6 [51.1–101.0] | 0.000 |
| IDLc | 8.5 [6.4–13.3] | 10.2 [7.6–11.7] | 0.352 |
| IDLtg | 9.3 [7.8–12.5] | 10.4 [9.0-11.4] | 0.443 |
| LDLc | 124.3 [116.2-149.8] | 135.4 [120.3-152.1] | 0.207 |
| LDLtg | 13.0 [11.1–16.4] | 13.9 [11.6–16.1] | 0.532 |
| HDLc | 58.9 [54.9–65.1] | 54.0 [49.6–57.8] | 0.000 |
| HDLtg | 14.7 [11.2–16.6] | 13.5 [10.9–16.0] | 0.254 |
| (mg/dL) | Overweight | Obese | P-Value |
| VLDLc | 12.7 [8.0–20.0] | 16.0 [11.8–22.3] | 0.052 |
| VLDLtg | 50.7 [37.0-88.6] | 70.3 [50.1–94.2] | 0.024 |
| IDLc | 8.7 [6.4–11.3] | 10.7 [7.1–12.8] | 0.080 |
| IDLtg | 9.7 [7.6–11.4] | 10.4 [8.7–12.2] | 0.052 |
| LDLc | 133.8 [116.2-147.2] | 132.3 [119.5-154.6] | 0.357 |
| LDLtg | 13.4 [11.3–16.3] | 13.9 [11.4–17.3] | 0.198 |
| HDLc | 56.4 [51.6–64.4] | 55.9 [53.2–60.9] | 0.623 |
| HDLtg | 13.5 [10.7–16.1] | 14.9 [12.1–16.5] | 0.193 |
Values are shown as median [IQR]. Lipoprotein cholesterol and triglycerides are expressed as mg/dL. P-Value: Wilcoxon Mann Whitney-test. Boldface indicates statistical significance (P < 0.05). 1H-NMR: Proton nuclear magnetic resonance; Very low-, low-, intermediate- and high- density lipoprotein (VLDL, LDL, IDL and HDL, respectively). c: Cholesterol; tg: Triglycerides
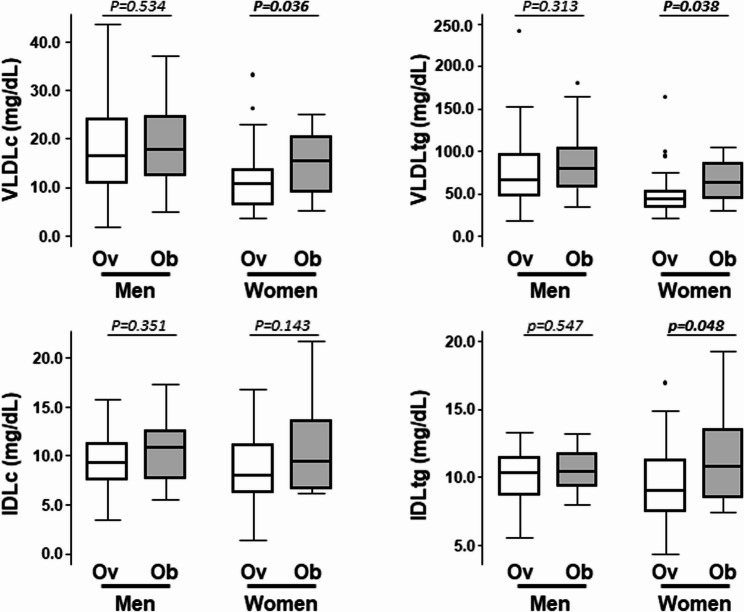
When the study population was analyzed according to the BMI (overweight vs. obesity, Table 1.), subjects with obesity had significantly higher serum VLDLtg levels than subjects with overweight (P = 0.024). A trend towards higher levels of VLDLc was also found in obesity (P = 0.052). Statistically significant differences between obesity and overweight were observed in women but no in men (Fig. 1). Similarly, IDLtg and IDLc tended to be higher in obesity although differences did not achieve statistical significance (P = 0.052 and P = 0.080, respectively) (Table 1.).
Fig. 1.
Triglyceride-rich Lipoproteins in relation to sex and obesity. Box plot of remnant cholesterol assessed by 1H-NMR levels in the total cohort (N = 101) and according sex and overweight/obesity. P-Value: Wilcoxon Mann Whitney-test. Boldface indicates statistical significance (P < 0.05). Ov: with Overweight; Ob: with Obesity; 1H-NMR: Proton nuclear magnetic resonance
Triglyceride levels in LDL and HDL did not differ between healthy subjects with overweight and obesity, nor did cholesterol levels in the 4 types of lipoproteins (VLDL, IDL, LDL, HDL) (Table 1.).
Serum remnant cholesterol levels: differences by sex, BMI and LDLc background
Median level of remnant cholesterol (RC), cholesterol contained in triglyceride-rich lipoproteins (calculated as the sum of cholesterol carried in VLDL and IDL, as obtained by 1H-NMR), in the healthy study population was 24.7 [16.4–32.2] mg/dL, with values significantly higher in men than in women (Men: 27.0 [21.1–35.0] mg/dL, women: 21.4 [14.8–29.7] mg/dL; P = 0.001), matched by age and BMI. Moreover, plasma levels of RC were significantly higher in the group of people with obesity than in the group of people with overweight (27.4 [19.5–35.0] mg/dL vs. 21.7 [15.3–30.3] mg/dL, respectively; P = 0.031). When groups were stratified by sex, remnant cholesterol levels were 1.5fold higher in women with obesity than with overweight P but no differences were observed between men with obesity and with overweight (Fig. 1).
40% of the participants had background LDLc levels classified as borderline-high range (130–159 mg/dL) or in the high range (160–189 mg/dL), according the ATPIII guidelines [34], and were defined as High-LDLc group, in contrast to those subjects with background LDLc < 130 mg/dL, who were defined as the Low-LDLc group. The two subgroups, with mean serum LDLc levels of 143.9 [138.8–160.1] mg/dL and 101.6 [91.6–114.9] mg/dL, respectively, significantly differed in levels of remnant cholesterol (Table S3. P = 0.041), with the differences being associated to the female sex (Low- vs. High-LDLc background groups: RC-levels 18.4 [12.9–26.1] mg/dL vs. 28.7 [16.4–35.2] mg/dL; P = 0.011), whereas no differences were found in males with Low- and High-LDLc background (RC-levels: 26.9 [20.6–34.9] mg/dL vs. 27.0 [21.7–35.0] mg/dL). Among subjects with LDLc < 130 mg/dL, women had lower levels of remnant cholesterol than men P.
The subgroup with a ratio TG/HDLc above the median level of the study population (median ratio, 1.95 [1.17–3.02]) showed 2-fold higher RC levels (32.1 [26.8–38.5]) than the subgroup with TG/HDLc ratio below the median value (16.4 [13.9–21.7], P < 0.001 for differences between groups).
Levels of plasma RC were not correlated with age (Spearman correlation analysis, (rho = 0.052, P = 0.604) in the total healthy cohort, nor when men and women were analyzed separately (men: rho= −0.043, P = 0.768; women: rho = 0.076, P = 0.594).
Particle number and size of VLDL, LDL and HDL
Size and number of particles for each lipoprotein subfraction were measured by 1H-NMR spectroscopy. Values for the total study population (N = 101) are given in Table S2. The diameter and particle number of VLDL, LDL, and HDL lipoproteins were analyzed based on sex and BMI. Table 2. shows the size and total number of VLDLp, LDLp, and HDLp, and Figs. 2 and 3 show the distribution of small, medium, and large subclasses for each lipoprotein type, also in relation to sex and BMI, respectively.
Table 2.
Number particles and diameter of lipoproteins assed by 1H-NMR according to sex and BMI
| A | Women | Men | p-Value |
| VLDLp (nmol/L) | 34.6 [26.0–53.0 | 51.9 [38.6–72.5] | 0.001 |
| VLDL⌀ (nm) | 42.1 [42.0–42.3] | 42.2 [42.0–42.3] | 0.423 |
| LDLp (nmol/L) | 1222.6 [1114.6–1466.2] | 1368.8 [1212.2–1526.0] | 0.042 |
| LDL⌀ (nm) | 21.2 [21.0–21.3] | 21.0 [20.8–21.1] | 0.000 |
| HDLp (µmol/L) | 29.6 [27.4–32.7] | 27.4 [25.2–29.5] | 0.001 |
| HDL⌀ (nm) | 8.3 [8.2–8.3] | 8.3 [8.2–8.3] | 0.557 |
| B | Overweight | Obese | p-Value |
| VLDLp (nmol/L) | 36.1 [27.3–61.6] | 52.3 [36.6–67.0] | 0.028 |
| VLDL⌀ (nm) | 42.2 [42.0–42.3] | 42.2 [42.0–42.3] | 0.616 |
| LDLp (nmol/L) | 1305.3 [1126.1–1439.9] | 1319.9 [1176.7–1536.5] | 0.233 |
| LDL⌀ (nm) | 21.0 [20.8–21.2] | 21.0 [20.9–21.2] | 0.900 |
| HDLp (µmol/L) | 28.8 [26.1–31.5] | 28.3 [25.6–30.4] | 0.790 |
| HDL⌀ (nm) | 8.3 [8.2–8.3] | 8.3 [8.2–8.3] | 0.592 |
Values are shown as median [IQR]. P-Value: Wilcoxon Mann Whitney-test. Boldface indicates statistical significance (P < 0.05). 1H-NMR: Proton nuclear magnetic resonance; VLDL: Very low-density lipoprotein; LDL: Low-density lipoprotein; HDL: High-density lipoprotein; p: Particles; ⌀: Diameter
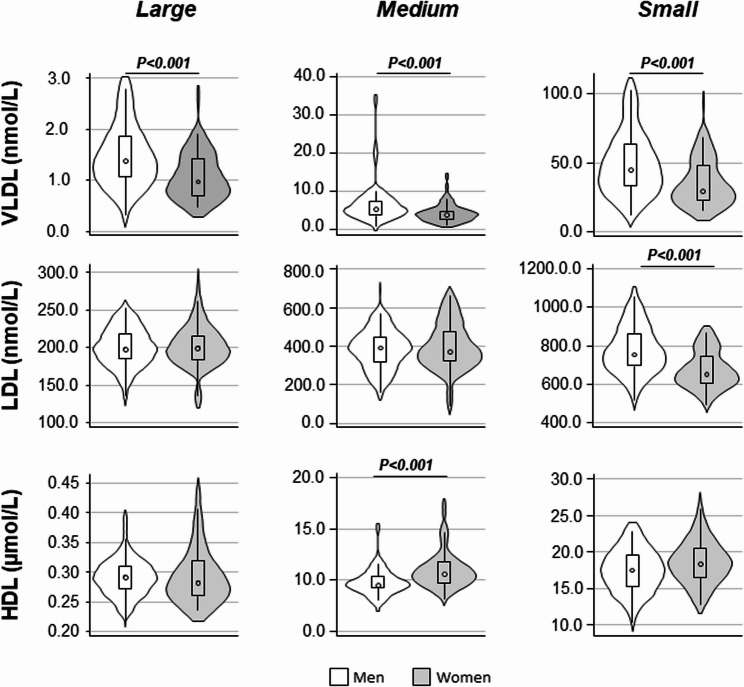
Fig. 2.
Number of lipoprotein particles by sex. Violin plot representing differences of the lipoprotein subclasses between men and women. P-Value: Wilcoxon Mann Whitney-test. Boldface indicates statistical significance P. Very low-density lipoprotein; LDL: Low-density lipoprotein; HDL: High-density lipoprotein. p: Particles. Specifically, the approximate particle size ranges defined for each subclass are as follows: VLDL (Small: 38.6–45.0 nm, Medium: 45.0–60.0 nm, Large: 60.0–81.9 nm); LDL (Small: 18.9–20.5 nm, Medium: 20.5–23.0 nm, Large: 23.0–26.5 nm); HDL (Small: 7.8–8.2 nm, Medium: 8.2–9.4 nm, Large: 9.4–11.5 nm)
Fig. 3.
Number of lipoprotein particles by body mass index. Violin plot representing differences of the lipoprotein subclasses between subjects with overweight and obesity. P-Value: Wilcoxon Mann Whitney-test. Boldface indicates statistical significance P. Very low-density lipoprotein; LDL: Low-density lipoprotein; HDL: High-density lipoprotein; p: Particles. Specifically, the approximate particle size ranges defined for each subclass are as follows: VLDL (Small: 38.6–45.0 nm, Medium: 45.0–60.0 nm, Large: 60.0–81.9 nm); LDL (Small: 18.9–20.5 nm, Medium: 20.5–23.0 nm, Large: 23.0–26.5 nm); HDL (Small: 7.8–8.2 nm, Medium: 8.2–9.4 nm, Large: 9.4–11.5 nm)
Compared with men, women had lower numbers of total VLDLp P and LDLp (P along with higher concentrations of HDLp P and a larger median LDLp size P Table 2.A. As shown in Fig. 2, the lower number of VLDLp in women was observed across all lipoprotein particle size. In contrast, differences in LDLp and HDLp between women and men were size-dependent. Women had significantly fewer small LDLp particles than men P , whereas no differences were found for medium and large LDLp between sexes. For HDLp, women showed a trend toward higher numbers across all size subfractions; however, the difference was significant only for the medium-size subfraction P.
When participants were stratified by BMI, subjects with obesity had a significantly higher concentration of VLDLp (Table 2.B), which statistically significant differences observed in both the large and small subfractions (Fig.3) compared to subjects with overweight. A similar, though not significant, trend was found for the medium VLDLp subfraction. The statistically significant difference in VLDLp pattern between individuals with overweight and obesity was only observed in women when the analysis was performed separately by sex (Table3).
Table 3.
Comparison of VLDL profile between subjects with overweight and obesity in men and women groups
| Men. Ov (N = 26) |
Men. Ob (N = 24) |
P-Value | Women. Ov (N = 29) |
Women. Ob (N = 22) |
P-Value | |
|---|---|---|---|---|---|---|
| VLDLc (mg/dL) | 16.6 [11.1–24.2] | 17.8 [12.6–24.5] | 0.534 | 10.6 [6.6–13.5] | 15.5 [9.1–20.3] | 0.036 |
| VLDLtg (mg/dL) | 65.8 [47.7–96.5] | 79.2 [57.9–103.9] | 0.313 | 44.1 [34.0–52.6] | 63.0 [44.7–84.9] | 0.038 |
| VLDLp (nmol/L) | 47.1 [34.3–72.5] | 57.0 [42.0–73.3] | 0.382 | 33.0 [24.4–38.1] | 47.3 [31.0–61.0] | 0.035 |
| Large | 1.2 [1.0–1.9] | 1.5 [1.1–1.8] | 0.351 | 0.9 [0.7–1.1] | 1.3 [0.9–1.5] | 0.023 |
| Medium | 5.1 [3.7–7.1] | 6.0 [3.8–8.6] | 0.426 | 3.5 [2.2–4.6] | 4.2 [3.3–5.5] | 0.287 |
| Small | 40.7 [29.2–65.0] | 50.4 [36.9–63.4] | 0.404 | 27.7 [21.3–32.9] | 42.4 [26.5–51.7] | 0.029 |
| VLDL ⌀ (nm) | 42.2 [42.0–42.4] | 42.2 [42.1–42.3] | 0.756 | 42.2 [42.1–42.3] | 42.1 [41.9–42.3] | 0.258 |
| LDLc (mg/dL) | 134.1 [125.2–140.0] | 143.9 [119.9–161.1] | 0.252 | 123.7 [116.2–149.8] | 127.5 [118.7–147.4] | 0.747 |
| LDLtg (mg/dL) | 13.5 [11.4–15.2] | 15.2 [12.1–16.7] | 0.260 | 12.5 [10.8–16.3] | 13.1 [11.2–17.7] | 0.518 |
| LDLp (nmol/L) | 1326.0 [1228.7–1410.2] | 1444.4 [1202.1–1579.0] | 0.341 | 1222.6 [1097.1–1461.5] | 1248.2 [1166.6–1517.8] | 0.494 |
| Large | 197.2 [183.0–211.1] | 202.1 [185.3–232.6] | 0.294 | 206.5 [179.3–219.8] | 194.8 [187.9–213.2] | 0.924 |
| Medium | 390.2 [289.0–409.6] | 419.6 [321.0–511.7] | 0.174 | 372.1 [313.6–474.6] | 378.0 [326.3–475.9] | 0.849 |
| Small | 755.3 [688.1–842.7] | 771.1 [711.0–872.8] | 0.449 | 641.7 [617.8–705.9] | 660.5 [606.5–772.3] | 0.361 |
| LDL ⌀ (nm) | 21.0 [20.7–21.1] | 21.0 [20.8–21.1] | 0.691 | 21.1 [21.0–21.4] | 21.2 [21.0–21.2] | 0.690 |
Values are shown as median [IQR]. P-Value: Wilcoxon Mann Whitney-test. Boldface indicates statistical significance (P < 0.05). VLDL: Very low-density lipoprotein; LDL: Low-density lipoprotein; OV: with overweight; OB: with obesity C: Cholesterol; Tg: triglycerides; p: Particles. ⌀: diameter
In contrast, the number and size of circulating LDLp and HDLp did not differ between groups with overweight and obesity when the total study population was considered (Table 2.B), nor when group comparisons were made across size subfractions (small, medium, large) of LDLp and HDLp.
Additional lipoprotein-related variables, including non-HDLp and the non-HDLp/HDLp ratio were calculated (Fig. 4). The non-HDLp/HDLp ratio was statistically higher in men compared to women (P = 0.001), whereas no significant sex-related differences were observed in levels of non-HDLp (P = 0.052). These variables did not differ between subjects with overweight and with obesity (P > 0.050, data not shown).
Fig. 4.
Comparison of other atherogenic variables between men and women. P-Value: Wilcoxon Mann Whitney-test. Statistical significance: P < 0.05. Data between subjects with overweight and obesity are not presented because no statistically significant differences were observed for these variables
A comparison of HDL composition, number, and diameter (⌀) between subjects with a TG/HDLc ratio below or above the median is given in Table S4. Subjects with a TG/HDLc ratio above the median value had a lower number of total HDLp, and HDLp of medium-size, and higher concentration of large HDLp (all P < 0.050) compared to those with the TG/HDLc ratio below the median value.
Impact of age on sex differences in the lipoprotein profile
Participants were stratified according to the median age of the study population, which was 45 years. The younger group (≤ 45 years) included 24 women (median age: 37 [34–40] years) and 27 men (38 [32–43] years), while the older group (> 45 years) comprised 26 women (55 [49–57] years) and 24 men (51 [48–54] years).
As shown in Table S5, sex differences in RC levels between sexes were evident in participants younger than 45 years, with men showing significantly higher levels than women (median RC: 26.5 [22.9–36.0] mg/dL vs. 19.7 [11.9–29.3] mg/dL; P = 0.017). However, these differences were not observed in the group older than 45 years (27.5 [20.4–32.4] mg/dL in men vs. 23.9 [15.4–31.3] mg/dL in women; P = 0.275). Sex differences in other lipoprotein traits, including VLDLp, small LDLp, LDL particle size, and HDLp, remained significant across both age groups. When comparing participants below and above 45 years within each sex, no statistically significant differences were observed in RC, VLDLp, LDLp, small LDLp, LDL size, or HDLp levels. However, in women, RC levels showed a non-significant trend toward higher values with age, whereas no such trend was observed in men.
VLDL profile in relation to cardiovascular disease risk
REGICOR (the Registre Gironí del cor) risk equation was applied to estimate the 10-year CVD risk for the entire cohort and separately by sex and BMI. Nine subjects were excluded from the REGICOR analysis as they did not meet the minimum age requirement of 37 years or had cholesterol levels, HDLc, or blood pressure values outside the range considered for the risk calculation.
Among the remaining 92 participants, the median 10-year CVD risk was 2.0 [1.0–3.0] %, classifying this population as the low risk (< 5.0%). When analyzed by sex, women had a significantly lower 10-year CVD risk than men (Men: 3.0 [2.0–3.0] %, Women: 2.0 [1.0–3.0] %, P = 0.001). Although not statistically significant, 10-year CVD risk tended to be higher in individuals with obesity compared to those with overweight (P = 0.097).
Since remnant cholesterol levels and VLDL were the variables showing the greatest differences related to sex and BMI, changes in remnant cholesterol and the VLDL profile (including TG and cholesterol content, size, and particle number) were further analyzed in association with the REGICOR-estimated CVD risk. The total population was divided into two groups based on the median 10-year CVD risk of 2.0%. As shown in Table S6, subjects with a 10-year CVD risk above the median (REGICOR risk 2.0%) had significantly higher levels of VLDLc and VLDLtg, and larger number of VLDLp. RC levels were 1.5 times higher (P < 0.001) in the group with a REGICOR value > 2% compared to those with a risk below the median value.
Differences in the VLDL profile and RC content were compared according to 10-year CVD risk, separately analyzing men and women (Table S7A) as well as subjects with obesity and with overweight (Table S7B). Regardless of sex or degree of obesity, subjects with a 10-year CVD risk > 2% had higher levels of VLDLc, VLDLtg, VLDLp, and RC compared to those with lower CVD risk (REGICOR value < 2%). While this pattern was consistent across all subgroups, the difference in VLDLc levels between women with lower and higher CVD risk did not reach statistical significance (P = 0.124).
Using a non-parametric Spearman rank test, small, medium, and large VLDL particle subfractions were correlated with the REGICOR score, as well as with BMI and waist circumference (Table S8). In the case of LDL particles, the correlation with REGICOR was observed only for the small subfraction, whereas for HDL particles, it was found in the large subfraction.
DISCUSSION
In this study, an in-depth characterization of the plasma lipoprotein profile was conducted using 1H-NMR in a cohort of metabolically healthy men and women with overweight and obesity, a group often underrepresented in research, yet crucial for understanding early lipoprotein changes in CVD risk.
The present study advances the field by reporting on particle size (small, medium and large) and count across lipoprotein subclasses including VLDL, LDL and HDL and assessing lipid content within each lipoprotein fraction. In addition, the association between these lipoprotein features, remnant cholesterol, and cardiovascular risk was investigated as estimated by the REGICOR score.
To the best of current knowledge, prior NMR-based studies [35, 36] have primarily focused on populations with established metabolic disorders (e.g., diabetes, hypertension, or dyslipidemia). In contrast, the present study provides novel insights by isolating the impact of overweight and obesity in individuals with no comorbidities, allowing us to detect subtle, early alterations in the lipoprotein profile.
Based on this healthy cohort, the number of VLDLp was identified as a differential factor between women and men of the same age and BMI range. The higher number of VLDLp together with their higher content of cholesterol and TG in men suggests a less favorable VLDL profile associated increased cardiovascular risk [35, 37]. Consistent with the obtained results, both the Framingham Offspring Study and the STRRIDE study reported higher VLDLp concentrations in men than in women [38, 39]. However, a key difference is that this Spanish cohort is composed exclusively of asymptomatic, untreated adults from a Mediterranean population, with generally lower baseline cardiovascular risk and distinct lifestyle patterns compared to the predominantly North American cohorts of Framingham and STRRIDE, which include a broader range of metabolic profiles and treatment exposures.
In addition, it was found that men exhibited a distinct lipoprotein profile characterized by higher concentrations of LDLp, particularly in the smaller size, resulting in a reduced median LDL particle diameter. This pattern is clinically significant given strong evidence that small, dense LDL particles are potent atherogenic risk indicators [40] and high LDLp counts are consistently associated with increased CVD risk [41]. Importantly, this investigation provides novel evidence that within a healthy Spanish cohort, a population with Mediterranean lifestyle influences, men display not only quantitative (higher LDLp) but also qualitative (smaller particle size) lipoprotein alterations, highlighting the value of advanced profiling for early atherogenic risk detection.
HDL cholesterol (HDLc) levels are typically regarded as inversely related to cardiovascular disease (CVD) risk, although recent observational studies have reported a U-shaped association, with very high HDLc levels also linked to increased all-cause mortality [42]. Despite this complexity, there is a broad consensus that higher concentrations of total HDLp are generally associated with reduced risk of CVD [43]. In the current healthy cohort both men and women had HDLc levels predominantly in the range from 50 to 60 mg/dL, with women typically exhibiting higher levels than men. Interestingly, while no differences were observed in the triglyceride content of HDL between sexes, women had a higher number of HDLp than men, a difference that was particularly evident in the medium-sized HDL subclass. Medium-sized HDLp are believed to play a key role in the functional properties of HDL that contribute to their overall cardioprotective effects [44]. Small HDL particles are generally considered the most functional [45], and are associated with a lower CVD risk profile [46]. However, the literature remains mixed, as some studies have also linked small HDL particles with greater severity of coronary artery disease (CAD) [47] and an adverse cardiometabolic risk profile [14], while larger HDL particle size have been associated with both with higher [48] and, paradoxically lower CAD risk [13, 47] in different reports [48].
It is important to note that apparent inconsistencies among studies may be due to differences in the methods used to identify HDL subclasses. Discrepancies arise because techniques like NMR spectroscopy, density gradient ultracentrifugation (classifying HDL2/HDL3), and non-denaturing gradient gel electrophoresis (defining HDL2a/2b, HDL3a/3b/3c) apply distinct particle size thresholds and compositional criteria for subclass definitions.
Several factors may explain the higher levels of VLDLp and LDLp, and lower levels of HDLp observed in men compared to women. Premenopausal women typically produce less VLDL and clear it more efficiently, partly due to higher estrogen levels, which reduce hepatic VLDL production and upregulate LDL receptor expression, enhancing LDL clearance [49–51]. Women also tend to have greater insulin sensitivity, which lowers hepatic fatty acid accumulation and reduces the formation of VLDL, IDL and LDL [52]. The higher insulin sensitivity in women is often associated with a pear-shaped distribution of subcutaneous fat in the lower body, which, in turn, is linked to reduced TG and VLDL production [53, 54].
Thus, in healthy subjects, obesity was associated with an increase in serum VLDLp (total, large and small), and VLDL-associated triglycerides. This reflects the known link between obesity and VLDLp overproduction, as VLDLp carry the highest triglyceride content among lipoproteins [55]. Obesity is associated with elevated cholesteryl ester transfer protein (CETP) activity, which promotes a lipid exchange that shifts HDL and LDL toward smaller, denser particles. HDL and LDL size decreases due to triglyceride enrichment and hepatic lipase action [56, 57].
Interestingly, in this study in healthy men and women matched for BMI and age, obesity significantly affected the VLDL profile in women, while no differences were observed between overweight and obese men. Remnant cholesterol (RC), measured by 1H-NMR under fasting conditions, also showed a sex-specific and obesity-specific pattern, with larger increases in men and subjects with obesity. Notably, the obesity effect on RC persisted only in women.
While RC is known to increase ASCVD and mortality risk similarly in both sexes [58, 59], there is no clear evidence that it is a better predictor in women. However, growing data suggest that TRLs may play a key role in premature coronary disease in women [60] and that sex differences in RC vary with age [61]. RC levels are significantly higher in men than in women at early ages but differences between sexes narrowed in the group above 45 years, with women showing a more male-like lipid phenotype. In contrast, the ongoing research found no correlation between RC and age, possibly due to the low overall CVD risk in the cohort. Differing from RC levels, sex differences in VLDLp, LDL size, and HDLp persisted in both age groups.
Sex-related effect of ageing on RC levels may be due to hormonal or metabolic changes in women since menopause is associated with changes in lipid metabolism towards a more atherogenic lipid profile including increased levels of TC, LDLc, and TG levels, while HDLc decreases [62]. Unfortunately, menopausal status was not clinically verified in this study. However, the reported findings suggest the need for a more specific age-related study on RC levels in women, investigating how these levels evolve during the postmenopausal period.
RC levels exhibit a complex relationship with LDLc, with emerging evidence highlighting RC as an independent cardiovascular risk factor, particularly when LDLc is low [63]. In this investigation, although all subjects showed LDLc levels below the pathological range and none of them were under statin treatment, higher RC concentration was evidenced in the group with higher LDLc background (plasma levels above the median of the study group), particularly in women, where levels were notably higher compared to those with low LDLc. This suggests that the increase in LDLc levels may have a more pronounced impact on women, with implications for cardiovascular risk assessment in both sexes [64].
The ratio TG/HDLc, has been proposed as a strong predictor of CVD comparable to LDLc [65]. In the present study, men and individuals with obesity had higher TG/HDLc ratios than women and subjects with overweight. Further analysis showed that a higher TG/HDLc ratio was also associated with a HDL profile characterized by low HDLc and HDLp concentrations and more elevated levels of HDLtg. In a study in young individuals with obesity, an elevated TG/HDLc ratio has been associated with higher levels of proatherogenic lipoprotein subclasses, such as VLDL, IDL, and LDL [66].
VLDL is known to contribute to atherosclerotic CVD [37]. Therefore, these results suggest that metabolically healthy individuals with obesity may have a less favorable CVD risk profile. A recent, cross-sectional study of 5,301 participants found that increased RC levels were associated with increased visceral adipose tissue [67]. As VLDL showed the most significant changes by BMI and sex in this study, the combined impact of these factors on VLDL profiles was examined. These findings strongly support the view that obesity affects women more than men, as women with obesity had a worse VLDL profile than women with overweight, a pattern not observed in men. This is consistent with the Framingham Heart Study, where obesity increased CVD risk by 64% in women compared with 46% in men [68].
The cardiovascular risk score REGICOR, specifically adapted and validated for the Spanish population [32], evidences that all subjects included in the study were at low or very low risk. To notice those with a risk above 2% were the subjects with higher concentrations of VLDLp and remnant cholesterol, aligning with previous findings in Spanish healthcare workers showing strong associations between REGICOR scores and lipid profiles, age, smoking, and adherence to a Mediterranean diet [69].
Strengths and Limitations
This study has strengths and weaknesses. A key strength is the use of 1H-NMR to accurately analyze lipoprotein particles and their lipid content, crucial for assessing CVD risk. Including both subjects with overweight and obesity, men and women, also allows for direct comparison of sex- differences in lipid profiles. However, the study has some limitations, such as the cross-sectional design, which precludes establishing causal relationships, and the sample size, which may have reduced the ability to detect significant differences in certain subgroups and might affect the generalizability of these findings. Nevertheless, the study was designed as an exploratory investigation to generate hypotheses and provide initial insights into lipoprotein profiles and cardiometabolic risk markers in relation to sex, age, and menopausal status in a metabolically healthy cohort. In addition, data on lifestyle, diet and physical activity, and individual menopause age in women were unavailable for the study.
Conclusion
In conclusion, the results of this study provide evidence that in a metabolically healthy population with overweight and obesity, men and individuals with obesity exhibit a more pro-atherogenic lipoprotein profile than women and individuals with overweight, respectively. Specifically, the more unfavorable CVD risk profile in men and individuals with obesity is primarily characterized by an elevated content of cholesterol and TG carried by VLDL (large, medium and small particles), along with higher levels of RC. Interestingly, obesity-related differences in the VLDL profile and RC levels are particularly evident in women but not in men. This suggests that an increase in BMI above 30 kg/m2 has a greater impact on women, independently of the age, leading to a higher CVD risk profile.
These findings highlight the importance of identifying VLDL and remnant particles as indicators of cardiovascular risk in otherwise healthy individuals who are overweight or obese. Such profiling could facilitate more personalised risk stratification and contribute to preventive strategies before clinical disease develops. This approach could lead to targeted lifestyle recommendations and monitoring for individuals who are overweight or have early-stage obesity.
Supplementary Information
Acknowledgements
The technical assistance of Montse Gomez-Pardo is acknowledged.
Authors’ contributions
Author Contributions: Conceptualization, T.P. and L.B.; methodology, V.S., N.M.-G., T.P., and L.B.; formal analysis, V.S., A.L.-Y., N.M.-G., T.P. and L.B.; investigation, T.P. and L.B.; data curation, V.S., N.M.-G., A.L.-Y., T.P. and L.B.; writing—original draft preparation, V.S., A.L.-Y., T.P. and L.B.; writing—review and editing, V.S., N.M.-G., A.L.-Y., T.P., G.V. and L.B.; visualization, V.S., A.L.-Y., T.P. and L.B.; supervision, V.S., L.B. and TP.; project administration, T.P. and L.B.; funding acquisition, T.P., L.B., and G.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Institute of Health Carlos III (ISCIII): PMP22/00108 from the ISCIII with Next Generation EU funds from the Recovery and Resilience Mechanism (RRM) Program to T.P and L.B; FIS PI22/01930 to T.P, and by the Spanish Ministry of Economy and Competitiveness of Science “Agencia Estatal de Investigación (AEI)” Proj PID2021-128891OB-I00 to GV and PID2019-107160RB-I00 to L.B, all co-funded by FEDER “Una Manera de Hacer Europa. We thank the Generalitat of Catalunya (Secretaria d’Universitats i Recerca, Departament d’Economia i Coneixement, 2021 SGR 01006). A.L.-Y. received financial support through the “Juan de la Cierva-Formación” program, funded by MCIN (MCIN/AEI/10.13039/501100011033) and by the European Union (NextGenerationEU/PRTR). V.S. recieved a predoctoral fellowship funded by the IR-HSCSP, and is currently recipient of a research contract funded by PMP22/00108 from the ISCIII, supported by Next Generation EU funds from the Recovery and Resilience Mechanism (RRM) Programme. N.M.-G. received a predoctoral fellowship funded by the IR-HSCSP.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
LB declares to have acted as SAB member of Sanofi, Ionnis and NovoNordisk; to have received speaker fees from Sanofi and NovoNordisk and to have founded the Spin-off Ivastatin Therapeutics S (all unrelated to this work).T.P. discloses to have received speaker fees from AB-BIOTICS S.A. and to be a co-founder of Spin-off Ivastatin Therapeutics S.L. (both unrelated to this study).G.V. discloses to be a co-founder of Spin-off Ivastatin Therapeutics S.L. (unrelated to this study).The remaining authors have no competing interests to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lina Badimon and Teresa Padro contributed equally.
References
- 1.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931–9. 10.1161/CIRCULATIONAHA.108.816181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidemann BE, Koopal C, Bots ML, Asselbergs FW, Westerink J, Visseren FLJ. The relation between VLDL-cholesterol and risk of cardiovascular events in patients with manifest cardiovascular disease. Int J Cardiol. 2021;322:251–7. 10.1016/j.ijcard.2020.08.030. [DOI] [PubMed] [Google Scholar]
- 3.Vekic J, Zeljkovic A, Cicero AFG, Janez A, Stoian AP, Sonmez A, et al. Atherosclerosis development and progression: the role of atherogenic small, dense LDL. Medicina (B Aires). 2022;58:299. 10.3390/medicina58020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida J-M, Capodanno D. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With. Eur Heart J 2021;42:3227–337. 10.1093/eurheartj/ehab484
- 5.Fernández-cidón B, Candás‐estébanez B, Gil‐serret M, Amigó N, Corbella E, Ángeles Rodríguez‐Sánchez M, Padró‐miquel A, Brotons C, Hernández‐mijares A, Calmarza P, Jarauta E, Brea AJ, Mauri M, Guijarro C, Vila À, Valdivielso P, Corbella X, Pintó X. Physicochemical properties of lipoproteins assessed by nuclear magnetic resonance as a predictor of premature cardiovascular disease. PRESARV-SEA study. J Clin Med. 2021;10:1379. 10.3390/JCM10071379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotseva K, De Backer G, De Bacquer D, Rydén L, Hoes A, Grobbee D, et al. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: results from the European society of cardiology ESC-EORP EUROASPIRE V registry. Eur J Prev Cardiol. 2019;26:824–35. 10.1177/2047487318825350. [DOI] [PubMed] [Google Scholar]
- 7.Muñoz-García N, Cordero A, Padro T, Mendieta G, Vilahur G, Flores E, et al. First time ACS in patients with on-target lipid levels: inflammation at admission and re-event rate at follow-up. Eur J Clin Invest. 2024;54:e14305. 10.1111/ECI.14305. [DOI] [PubMed] [Google Scholar]
- 8.Ginsberg HN, Packard CJ, Chapman MJ, Borén J, Aguilar-Salinas CA, Averna M, et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies—a consensus statement from the European atherosclerosis society. Eur Heart J. 2021;42:4791–806. 10.1093/eurheartj/ehab551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin A, Nerlekar N, Rajagopalan A, Yuvaraj J, Modi R, Mirzaee S, Munnur RK, Seckington M, Doery JC, Seneviratne S, Nicholls SJ, Wong DT. Remnant cholesterol and coronary atherosclerotic plaque burden assessed by computed tomography coronary angiography. Atherosclerosis. 2019;284:24–30. 10.1016/J.ATHEROSCLEROSIS.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Balling M, Afzal S, Varbo A, Nordestgaard BG, Langsted A. Remnant cholesterol: quantification, concentrations by sex and age, and risk of ischemic heart disease. Clin Chem. 2024;217. 10.1093/CLINCHEM/HVAE217. [DOI] [PubMed] [Google Scholar]
- 11.Santos-Gallego CG, Requena-Ibáñez JA, Badimón JJ. High-density lipoprotein cholesterol: a new marker in heart failure. Revista Española de Cardiología (English Edition). 2022;75:855–7. 10.1016/J.REC.2022.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Aicha S, Casaní L, Muñoz-García N, Joan-Babot O, Peña E, Aržanauskaitė M, et al. HDL (high-density lipoprotein) remodeling and magnetic resonance imaging-assessed atherosclerotic plaque burden: study in a preclinical experimental model. Arterioscler Thromb Vasc Biol. 2020;40:2481–93. 10.1161/ATVBAHA.120.314956. [DOI] [PubMed] [Google Scholar]
- 13.El Harchaoui K, Arsenault BJ, Franssen R, Després J-P, Hovingh GK, Stroes ESG, Otvos JD, Wareham NJ, Kastelein JJP, Khaw K-T. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med. 2009;150:84–93. 10.7326/0003-4819-150-2-200901200-00006. [DOI] [PubMed] [Google Scholar]
- 14.Arsenault BJ, Lemieux I, Després J-P, Gagnon P, Wareham NJ, Stroes ESG, Kastelein JJP, Khaw K-T, Boekholdt SM. HDL particle size and the risk of coronary heart disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. Atherosclerosis. 2009;206:276–81. 10.1016/j.atherosclerosis.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad S, Moorthy MV, Demler OV, Hu FB, Ridker PM, Chasman DI, Mora S. Assessment of risk factors and biomarkers associated with risk of cardiovascular disease among women consuming a mediterranean diet. JAMA Netw Open. 2018;1:e185708. 10.1001/JAMANETWORKOPEN.2018.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmes MV, Millwood IY, Kartsonaki C, Hill MR, Bennett DA, Boxall R, et al. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J Am Coll Cardiol. 2018;71:620–32. 10.1016/J.JACC.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gugliucci A. Triglyceride-rich lipoprotein metabolism: key regulators of their flux. J Clin Med. 2023;12:4399. 10.3390/JCM12134399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castañer O, Pintó X, Subirana I, Amor AJ, Ros E, Hernáez Á, et al. Remnant cholesterol, not LDL cholesterol, is associated with incident cardiovascular disease. J Am Coll Cardiol. 2020;76:2712–24. 10.1016/J.JACC.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Puig-Jové C, Castelblanco E, Falguera M, Hernández M, Soldevila B, Julián MT, Teis A, Julve J, Barranco-Altirriba M, Franch-Nadal J. Advanced lipoprotein profile in individuals with normal and impaired glucose metabolism. Revista Española De Cardiología (English Edition). 2022;75:22–30. 10.1016/j.rec.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Magkos F, Mohammed BS, Mittendorfer B. Effect of obesity on the plasma lipoprotein subclass profile in normoglycemic and normolipidemic men and women. Int J Obes. 2008;32:1655–64. 10.1038/ijo.2008.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bédard A, Corneau L, Lamarche B, Dodin S, Lemieux S. Sex differences in the impact of the Mediterranean diet on LDL particle size distribution and oxidation. Nutrients. 2015;7:3705–23. 10.3390/nu7053705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallol R, Amigó N, Rodríguez MA, Heras M, Vinaixa M, Plana N, Rock E, Ribalta J, Yanes O, Masana L, Liposcale. A novel advanced lipoprotein test based on 2D diffusion-ordered 1H NMR spectroscopy [S]. J Lipid Res. 2015;56:737–46. 10.1194/jlr.D050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siurana JM, Sabaté-Rotés A, Amigó N, Martínez-Micaelo N, Arciniegas L, Riaza L, Mogas E, Rosés-Noguer F, Ventura PS, Yeste D. Different profiles of lipoprotein particles associate various degrees of cardiac involvement in adolescents with morbid obesity. Front Pediatr. 2022;10:887771. 10.3389/FPED.2022.887771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Harchaoui K, van der Steeg WA, Stroes ESG, Kuivenhoven JA, Otvos JD, Wareham NJ, Hutten BA, Kastelein JJP, Khaw K-T, Boekholdt SM. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. J Am Coll Cardiol. 2007;49:547–53. 10.1016/j.jacc.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 25.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American heart association/national heart, lung, and blood Institute scientific statement. Circulation. 2005;112:2735–52. 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 26.Body mass index (BMI). n.d. https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/body-mass-index (accessed July 21, 2025).
- 27.Di Cesare M, Bentham J, Stevens GA, Zhou B, Danaei G, Lu Y, Bixby H, Cowan MJ, Riley LM, Hajifathalian K, Fortunato L, Taddei C, Bennett JE, Ikeda N, Khang YH, Kyobutungi C, Laxmaiah A, Li Y, Lin HH, Miranda JJ, Mostafa A, Turley ML, Paciorek CJ, Gunter M, Ezzati M, Abdeen ZA, Hamid ZA, Abu-Rmeileh NM, Acosta-Cazares B, Adams R, Aekplakorn W, Aguilar-Salinas CA, Ahmadvand A, Ahrens W, Ali MM, Alkerwi A, Alvarez-Pedrerol M, Aly E, Amouyel P, Amuzu A, Andersen LB, Anderssen SA, Andrade DS, Anjana RM, Aounallah-Skhiri H, Ariansen I, Aris T, Arlappa N, Arveiler D, Assah FK, Avdicová M, Azizi F, Babu B V., Balakrishna N, Bandosz P, Banegas JR, Barbagallo CM, Barceló A, Barkat A, Barros M V., Bata I, Batieha AM, Batista RL, Baur LA, Beaglehole R, Romdhane H Ben, Benet M, Bernabe-Ortiz A, Bernotiene G, Bettiol H, Bhagyalaxmi A, Bharadwaj S, Bhargava SK, Bhatti Z, Bhutta ZA, Bi HS, Bi Y, Bjerregaard P, Bjertness E, Bjertness MB, Björkelund C, Blake M, Blokstra A, Bo S, Bobak M, Boddy LM, Boehm BO, Boeing H, Boissonnet CP,Bongard V, Bovet P, Braeckman L, Bragt MCE, Brajkovich I, Branca F, Breckenkamp J,Brenner H, Brewster LM, Brian GR, Bruno G, Bueno-De-Mesquita HB, Bugge A, Burns C,De León AC, Cacciottolo J, Cama T, Cameron C, Camolas J, Can G, Cândido APC, Capuano V, Cardoso VC, Carvalho MJ, Casanueva FF, Casas JP, Caserta CA, Castetbon K, Chamukuttan S, Chan AW, Chan Q, Chaturvedi HK, Chaturvedi N, Chen CJ, Chen F, Chen H, Chen S,Chen Z, Cheng CY, Chetrit A, Chiolero A, Chiou ST, Chirita-Emandi A, Cho Y, Christensen K, Chudek J, Cifkova R, Claessens F, Clays E, Concin H, Cooper C, Cooper R, Coppinger TC, Costanzo S, Cottel D, Cowell C, Craig CL, Crujeiras AB, D’Arrigo G, D’Orsi E,Dallongeville J, Damasceno A, Damsgaard CT, Dankner R, Dauchet L, De Backer G, De Bacquer D, De Gaetano G, De Henauw S, De Smedt D, Deepa M, Deev AD, Dehghan A, Delisle H, Delpeuch F, Dhana K, Di Castelnuovo AF, Dias-Da-Costa JS, Diaz A, Djalalinia S,Do HTP, Dobson AJ, Donfrancesco C, Döring A, Doua K, Drygas W, Egbagbe EE, Eggertsen R, Ekelund U, El Ati J, Elliott P, Engle-Stone R, Erasmus RT, Erem C, Eriksen L, De La Peña JE, Evans A, Faeh D, Fall CH, Farzadfar F, Felix-Redondo FJ, Ferguson TS,Fernández-Bergés D, Ferrante D, Ferrari M, Ferreccio C, Ferrieres J, Finn JD, Fischer K, Flores EM, Föger B, Foo LH, Forslund AS, Fortmann SP, Fouad HM, Francis DK, Do Carmo Franco M, Franco OH, Frontera G, Fuchs FD, Fuchs SC, Fujita Y, Furusawa T, Gaciong Z, Gafencu M, Gareta D, Garnett SP, Gaspoz JM, Gasull M, Gates L, Geleijnse JM, Ghasemian A, Giampaoli S, Gianfagna F, Giovannelli J, Giwercman A, Goldsmith RA, Gross MG, Rivas JPG, Gorbea MB, Gottrand F, Graff-Iversen S, Grafnetter D, Grajda A, Grammatikopoulou MG, Gregor RD, Grodzicki T, Grøntved A, Gruden G, Grujic V, Gu D, Guan OP, Gudnason V, Guerrero R, Guessous I, Guimaraes AL, Gulliford MC, Gunnlaugsdottir J, Guo XH,Guo Y, Gupta PC, Gureje O, Gurzkowska B, Gutierrez L, Gutzwiller F, Halkjær J, Hardy R, Kumar RH, Hayes AJ, He J, Hendriks ME, Cadena LH, Heshmat R, Hihtaniemi IT, Ho SY, Ho SC, Hobbs M, Hofman A, Hormiga CM, Horta BL, Houti L, Htay TT, Htet AS, Htike MMT, Hu Y, Hussieni AS, Huu CN, Huybrechts I, Hwalla N, Iacoviello L, Iannone AG,Ibrahim MM, Ikram MA, Irazola VE, Islam M, Iwasaki M, Jackson RT, Jacobs JM, Jafar T, Jamil KM, Jamrozik K, Jasienska G, Jiang CQ, Joffres M, Johansson M, Jonas JB,Jørgensen T, Joshi P, Juolevi A, Jurak G, Jureša V, Kaaks R, Kafatos A, Kalter-Leibovici O, Kapantais E, Kasaeian A, Katz J, Kaur P, Kavousi M, Keil U, Boker LK, Kelishadi R, Kemper HHCG, Kengne AP, Kersting M, Key T, Khader YS, Khalili D, Khaw KTH, Khouw IMSL, Kiechl S, Killewo J, Kim J, Kiyohara Y, Klimont J, Kolle E, Kolsteren P, Korrovits P, Koskinen S, Kouda K, Koziel S, Kratzer W, Krokstad S, Kromhout D, Kruger HS, Kula K, Kulaga Z, Kumar RK, Kusuma YS, Kuulasmaa K, Laamiri FZ, Laatikainen T, Lachat C,Laid Y, Lam TH, Landrove O, Lanska V, Lappas G, Laugsand LE, Le Nguyen Bao K, Le TD,Leclercq C, Lee J, Lee J, Lehtimäki T, Rampal L, León-Munoz LM, Lim WY, Lima-Costa MF, Lin X, Linneberg A, Lissner L, Litwin M, Liu J, Lorbeer R, Lotufo PA, Lozano JE,Luksiene D, Lundqvist A, Lunet N, Lytsy P, Ma G, Machi S, Maggi S, Magliano DJ, Makdisse M, Malekzadeh R, Malhotra R, Rao KM, Manios Y, Mann JI, Manzato E, Margozzini P, Markey O, Marques-Vidal P, Marrugat J, Martin-Prevel Y, Martorell R, Masoodi SR, Matsha TE,Mazur A, Mbanya JCN, McFarlane SR, McGarvey ST, McKee M, McLachlan S, McLean RM, McNulty BA, Md Yusof S, Mediene-Benchekor S, Meirhaeghe A, Meisinger C, Mendes LL, Menezes AMB, Mensink GBM, Meshram II, Metspalu A, Mi J, Michaelsen KF, Mikkel K, Miller JC,Miquel JF, Mišigoj-Duraković M, Mohamed MK, Mohammad K, Mohammadifard N, Mohan V,Yusoff MFM, Molbo D, Møller NC, Molnár D, Mondo CK, Monterrubio EA, Monyeki KDK, Moreira LB, Morejon A, Moreno LA, Morgan K, Mortensen EL, Moschonis G, Mossakowska M, Mota J, Motlagh ME, Motta J, Mu TT, Muiesan ML, Müller-Nurasyid M, Murphy N, Mursu J, Murtagh EM, Musa KI, Musil V, Nagel G, Nakamura H, Námešná J, Nang EEK, Nangia VB, Nankap M, Narake S, Navarrete-Muñoz EM, Nenko I, Neovius M, Nervi F, Neuhauser HK, Nguyen ND, Nguyen QN, Nieto-Martínez RE, Ning G, Ninomiya T, Nishtar S, Noale M, Norat T,Noto D, Al Nsour M, O’Reilly D, Ochoa-Avilés AM, Oh K, Olayan IH, Olinto MTA, Oltarzewski M, Omar MA, Onat A, Ordunez P, Ortiz AP, Osler M, Osmond C, Ostojic SM, Otero JA,Overvad K, Paccaud FM, Padez C, Pajak A, Palli D, Palloni A, Palmieri L, Panda-Jonas S, Panza F, Parnell WR, Parsaeian M, Pednekar MS, Peeters PH, Peixoto SV, Pereira AC, Pérez CM, Peters A, Peykari N, Pham ST, Pigeot I, Pikhart H, Pilav A, Pilotto L, Pistelli F, Pitakaka F, Piwonska A, Piwonski J, Plans-Rubió P, Poh BK, Porta M,Portegies MLP, Poulimeneas D, Pradeepa R, Prashant M, Price JF, Puiu M, Punab M, Qasrawi RF, Qorbani M, Bao TQ, Radic I, Radisauskas R, Rahman M, Raitakari O, Raj M, Rao SR,Ramachandran A, Ramke J, Ramos R, Rampal S, Rasmussen F, Redon J, Reganit PFM, Ribeiro R, Riboli E, Rigo F, De Wit TFR, Ritti-Dias RM, Rivera JA, Robinson SM, Robitaille C, Rodríguez-Artalejo F, Del Cristo Rodriguez-Perez M, Rodríguez-Villamizar LA, Rojas-Martinez R, Rojroongwasinkul N, Romaguera D, Ronkainen K, Rosengren A, Rouse I, Rubinstein A, Rühli FJ, Rui O, Ruiz-Betancourt BS, Horimoto ARVR, Rutkowski M, Sabanayagam C,Sachdev HS, Saidi O, Salanave B, Martinez ES, Salomaa V, Salonen JT, Salvetti M, Sánchez-Abanto J, Sandjaja, Sans S, Santos DA, Santos O, Dos Santos RN, Santos R, Sardinha LB, Sarrafzadegan N, Saum KU, Savva SC, Scazufca M, Rosario AS, Schargrodsky H, Schienkiewitz A, Schmidt IM, Schneider IJ, Schultsz C, Schutte AE, Sein AA, Sen A, Senbanjo IO, Sepanlou SG,Shalnova SA, Shaw JE, Shibuya K, Shin Y, Shiri R, Siantar R, Sibai AM, Silva AM, Silva DAS, Simon M, Simons J, Simons LA, Sjostrom M, Slowikowska-Hilczer J, Slusarczyk P,Smeeth L, Smith MC, Snijder MB, So HK, Sobngwi E, Söderberg S, Soekatri MYE, Solfrizzi V, Sonestedt E, Sørensen TIA, Sorić M, Jérome CS, Soumare A, Staessen JA, Starc G,Stathopoulou MG, Staub K, Stavreski B, Steene-Johannessen J, Stehle P, Stein AD, Stergiou GS, Stessman J, Stieber J, Stöckl D, Stocks T, Stokwiszewski J, Stratton G, Strufaldi MW, Sun CA, Sundström J, Sung YT, Sunyer J, Suriyawongpaisal P, Swinburn BA, Sy RG,Szponar L, Tai ES, Tammesoo ML, Tamosiunas A, Tang L, Tang X, Tanser F, Tao Y, Tarawneh M, Tarp J, Tarqui-Mamani CB, Taylor A, Tchibindat F, Thijs L, Thuesen BH, Tjonneland A, Tolonen HK, Tolstrup JS, Topbas M, Topór-Madry R, Torrent M, Traissac P, Trichopoulou A, Trichopoulos D, Trinh OTH, Trivedi A, Tshepo L, Tulloch-Reid MK, Tuomainen TP,Tuomilehto J, Tynelius P, Tzotzas T, Tzourio C, Ueda P, Ukoli FAM, Ulmer H, Unal B,Valdivia G, Vale S, Valvi D, Van Der Schouw YT, Van Herck K, Van Minh H, Van Valkengoed IGM, Vanderschueren D, Vanuzzo D, Vatten L, Vega T, Velasquez-Melendez G, Veronesi G, Monique Verschuren WM, Viegi G, Viet L, Viikari-Juntura E, Vineis P, Vioque J,Virtanen JK, Visvikis-Siest S, Viswanathan B, Vollenweider P, Voutilainen S, Vrijheid M, Wade AN, Wagner A, Walton J, Mohamud WNW, Wang MD, Wang Q, Wang YX, Wannamethee SG, Wareham N, Weerasekera D, Whincup PH, Widhalm K, Widyahening IS, Wiecek A, Wilks RJ, Willeit J, Wojtyniak B, Wong JE, Wong TY, Woo J, Woodward M, Wu FC, Wu JF, Wu SL, Xu H, Xu L, Yamborisut U, Yan W, Yang X, Yardim N, Ye X, Yiallouros PK, Yoshihara A, You QS, Younger-Coleman NO, Yusoff AF, Zainuddin AA, Zambon S, Zdrojewski T, Zeng Y, Zhao D, Zhao W, Zheng Y, Zhou M, Zhu D, Zimmermann E, Cisneros JZ. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. The Lancet 2016;387:1377–96. 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed]
- 28.López-Yerena A, Muñoz-García N, de Santisteban Villaplana V, Padro T, Badimon L. Effect of moderate beer intake on the lipid composition of human red blood cell membranes. Nutrients. 2024;16:3541. 10.3390/nu16203541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santisteban V, Muñoz-Garcia N, López-Yerena A, Puntes M, Badimon L, Padro T. Efficacy of food industry by-product β-glucan/chitin–chitosan on lipid profile of overweight and obese individuals: sustainability and nutraceuticals. Nutrients. 2024;16:3420. 10.3390/nu16193420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Padro T, Santisteban V, Huedo P, Puntes M, Aguiló M, Espadaler-Mazo J, Badimon L. Lactiplantibacillus plantarum strains KABP011, KABP012, and KABP013 modulate bile acids and cholesterol metabolism in humans. Cardiovasc Res. 2024;120:708–22. 10.1093/cvr/cvae061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.López-Yerena A, Padro T, de Santisteban Villaplana V, Muñoz-García N, Pérez A, Vilahur G, et al. Vascular and platelet effects of tomato soffritto intake in overweight and obese subjects. Nutrients. 2023;15:5084. 10.3390/nu15245084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marrugat J, Solanas P, D’Agostino R, Sullivan L, Ordovas J, Cordón F, Ramos R, Sala J, Masià R, Rohlfs I, Elosua R, Kannel WB. Estimación Del Riesgo Coronario En España mediante La Ecuación de Framingham Calibrada. Rev Esp Cardiol. 2003;56:253–61. 10.1157/13043951. [DOI] [PubMed] [Google Scholar]
- 33.Marrugat J, Vila J, Baena-Díez JM, Grau M, Sala J, Ramos R, et al. Validez relativa de La estimación Del Riesgo cardiovascular a 10 Años En Una cohorte poblacional Del estudio REGICOR. Rev Esp Cardiol. 2011;64:385–94. 10.1016/j.recesp.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 34.National Cholesterol Education Program (NCEP). Expert panel on detection, evaluation and T of HBC in A (Adult TPI. Third report of the National cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 35.Wei D, Marrachelli VG, Melgarejo JD, Liao C-T, Janssens S, Verhamme P, et al. Lipoprotein profiles of fat distribution and its association with insulin sensitivity. Front Endocrinol (Lausanne). 2022;13:978745. 10.3389/fendo.2022.978745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Festa A, Williams K, Hanley AJG, Otvos JD, Goff DC, Wagenknecht LE, Haffner SM. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the insulin resistance atherosclerosis study. Circulation. 2005;111:3465–72. 10.1161/CIRCULATIONAHA.104.512079. [DOI] [PubMed] [Google Scholar]
- 37.Lee H-C, Akhmedov A, Chen C-H. Spotlight on very-low-density lipoprotein as a driver of cardiometabolic disorders: implications for disease progression and mechanistic insights. Front Cardiovasc Med. 2022;9:993633. 10.3389/fcvm.2022.993633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freedman DS, Otvos JD, Jeyarajah EJ, Shalaurova I, Cupples LA, Parise H, et al. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: the Framingham study. Clin Chem. 2004;50:1189–200. 10.1373/clinchem.2004.032763. [DOI] [PubMed] [Google Scholar]
- 39.Johnson JL, Slentz CA, Duscha BD, Samsa GP, McCartney JS, Houmard JA, et al. Gender and racial differences in lipoprotein subclass distributions: the STRRIDE study. Atherosclerosis. 2004;176:371–7. 10.1016/j.atherosclerosis.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 40.Kathiresan S, Otvos JD, Sullivan LM, Keyes MJ, Schaefer EJ, Wilson PWF, D’Agostino RB, Vasan RS, Robins SJ. Increased small low-density lipoprotein particle number: a prominent feature of the metabolic syndrome in the Framingham heart study. Circulation. 2006;113:20–9. 10.1161/CIRCULATIONAHA.105.567107. [DOI] [PubMed] [Google Scholar]
- 41.Chary A, Tohidi M, Hedayati M. Association of LDL-cholesterol subfractions with cardiovascular disorders: a systematic review. BMC Cardiovasc Disord. 2023;23:533. 10.1186/s12872-023-03578-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Y, Liu X, Lo K, Liu L, Yu Y, Chen C, et al. The u shaped relationship between high-density lipoprotein cholesterol and all-cause or cause-specific mortality in adult population. Clin Interv Aging. 2020. 10.2147/CIA.S271528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mackey RH, Greenland P, Goff DC, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol. 2012;60:508–16. 10.1016/j.jacc.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaisar T, Kanter JE, Wimberger J, Irwin AD, Gauthier J, Wolfson E, et al. High concentration of medium-sized HDL particles and enrichment in HDL paraoxonase 1 associate with protection from vascular complications in people with long-standing type 1 diabetes. Diabetes Care. 2020;43:178–86. 10.2337/DC19-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin Q, Lau ESH, Luk AO, Tam CHT, Ozaki R, Lim CKP, Wu H, Chow EYK, Kong APS, Lee HM, Fan B, Ng ACW, Jiang G, Lee KF, Siu SC, Hui G, Tsang CC, Lau KP, Leung JY, Tsang M wo, Cheung EYN, Kam G, Lau IT, Li JK, Yeung VT, Lau E, Lo S, Fung S, Cheng YL, Chow CC, Yu W, Tsui SKW, Huang Y, Lan H, yao, Szeto CC, So WY, Jenkins AJ, Chan JCN, Ma RCW. High-density lipoprotein subclasses and cardiovascular disease and mortality in type 2 diabetes: analysis from the Hong Kong Diabetes Biobank. Cardiovasc Diabetol. 2022;21:293. 10.1186/S12933-022-01726-Y [DOI] [PMC free article] [PubMed]
- 46.Liu S, Wu Y, Fan Z, Tian Y, Liu S. Relation between high density lipoprotein particles concentration and cardiovascular events: a meta-analysis. Lipids Health Dis. 2018;17:142. 10.1186/S12944-018-0732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu RX, Li S, Li XL, Zhang Y, Guo YL, Zhu CG, et al. High-density lipoprotein subfractions in relation with the severity of coronary artery disease: a Gensini score assessment. J Clin Lipidol. 2015;9:26–34. 10.1016/J.JACL.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Prats-Uribe A, Sayols-Baixeras S, Fernández-Sanlés A, Subirana I, Carreras-Torres R, Vilahur G, Civeira F, Marrugat J, Fitó M, Hernáez Á, Elosua R. High-density lipoprotein characteristics and coronary artery disease: a Mendelian randomization study. Metabolism. 2020;112:154351. 10.1016/J.METABOL.2020.154351. [DOI] [PubMed] [Google Scholar]
- 49.Magkos F, Patterson BW, Mohammed BS, Klein S, Mittendorfer B. Women produce fewer but triglyceride-richer very low-density lipoproteins than men. J Clin Endocrinol Metab. 2007;92:1311–8. 10.1210/jc.2006-2215. [DOI] [PubMed] [Google Scholar]
- 50.Palmisano BT, Zhu L, Stafford JM. Role of estrogens in the regulation of liver lipid metabolism. Sex and gender factors affecting metabolic homeostasis. Diabetes Obes 2017:227–56. 10.1007/978-3-319-70178-3_12 [DOI] [PMC free article] [PubMed]
- 51.Fu W, Gao X-P, Zhang S, Dai Y-P, Zou W-J, Yue L-M. 17β-estradiol inhibits PCSK9-mediated LDLR degradation through GPER/PLC activation in HepG2 cells. Front Endocrinol (Lausanne). 2020;10:930. 10.3389/fendo.2019.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arner P, Viguerie N, Massier L, Rydén M, Astrup A, Blaak E, Langin D, Andersson DP. Sex differences in adipose insulin resistance are linked to obesity, lipolysis and insulin receptor substrate 1. Int J Obes (Lond). 2024;48:934–40. 10.1038/S41366-024-01501-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Divoux A, Sandor K, Bojcsuk D, Yi F, Hopf ME, Smith JS, et al. Fat distribution in women is associated with depot-specific transcriptomic signatures and chromatin structure. J Endocr Soc. 2020;4:bvaa042. 10.1210/jendso/bvaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6:60–75. 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Björnson E, Adiels M, Taskinen M-R, Borén J. Kinetics of plasma triglycerides in abdominal obesity. Curr Opin Lipidol. 2017;28:11–8. 10.1097/MOL.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 56.Stadler JT, Marsche G. Obesity-related changes in high‐density lipoprotein metabolism and function. Int J Mol Sci. 2020;21:1–28. 10.3390/IJMS21238985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu L, Luu T, Emfinger CH, Parks BA, Shi J, Trefts E, et al. CETP inhibition improves HDL function but leads to fatty liver and insulin resistance in CETP-expressing transgenic mice on a high-fat diet. Diabetes. 2018;67:2494–506. 10.2337/DB18-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Varbo A, Nordestgaard BG. Remnant cholesterol and risk of ischemic stroke in 112,512 individuals from the general population. Ann Neurol. 2019;85:550–9. 10.1002/ANA.25432. [DOI] [PubMed] [Google Scholar]
- 59.Duran EK, Aday AW, Cook NR, Buring JE, Ridker PM, Pradhan AD. Triglyceride-rich lipoprotein cholesterol, small dense LDL cholesterol, and incident cardiovascular disease. J Am Coll Cardiol. 2020;75:2122–35. 10.1016/J.JACC.2020.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Roeters JE, Tokgözoǧlu LS, Badimon L, Dumanski SM, Gulati M, Hess CN, Holven KB, Kavousi M, Kaylkçloǧlu M, Lutgens E, Michos ED, Prescott E, Stock JK, Tybjaerg-Hansen A, Wermer MJH, Benn M. Women, lipids, and atherosclerotic cardiovascular disease: a call to action from the European atherosclerosis society. Eur Heart J. 2023;44:4157–73. 10.1093/EURHEARTJ/EHAD472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang A, Tian X, Zuo Y, Chen S, Meng X, Chen P, Li H, Wu S, Wang Y. Age dependent association between remnant cholesterol and cardiovascular disease. Atherosclerosis Plus. 2021;45:18–24. 10.1016/J.ATHPLU.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Inaraja V, Thuissard I, Andreu-Vazquez C, Jodar E. Lipid profile changes during the menopausal transition. Menopause. 2020;27:780–7. 10.1097/GME.0000000000001532. [DOI] [PubMed] [Google Scholar]
- 63.Quispe R, Martin SS, Michos ED, Lamba I, Blumenthal RS, Saeed A, et al. Remnant cholesterol predicts cardiovascular disease beyond LDL and apob: a primary prevention study. Eur Heart J. 2021;42:4324–32. 10.1093/eurheartj/ehab432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kraaijenhof JM, Kerkvliet MJ, Nurmohamed NS, Grefhorst A, Kroon J, Wareham NJ, Hovingh GK, Stroes ESG, Boekholdt SM, Reeskamp LF. The role of systemic inflammation in remnant cholesterol associated cardiovascular risk: insights from the EPIC-Norfolk study. Eur J Prev Cardiol. 2025. 10.1093/EURJPC/ZWAF037. [DOI] [PubMed] [Google Scholar]
- 65.Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. Low triglycerides-high high-density lipoprotein cholesterol and risk of ischemic heart disease. Arch Intern Med. 2001;161:361–6. 10.1001/ARCHINTE.161.3.361. [DOI] [PubMed] [Google Scholar]
- 66.Chiriacò M, Nesti L, Natali A, Santoro N, Caprio S, Tricò D. Proatherogenic changes in lipoprotein particles associated with a high triglyceride to high-density lipoprotein cholesterol ratio in youths. Obes (Silver Spring). 2023;31:1894–902. 10.1002/OBY.23767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Z, Zhong S, Wu M, Shao X, Gu T, Xu M, et al. The relationship between remnant cholesterol and visceral adipose tissue: a National Cross-Sectional study. Horm Metab Res. 2025;57:47–54. 10.1055/A-2357-2579. [DOI] [PubMed] [Google Scholar]
- 68.Wilson PWF, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–72. 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 69.Marcos PJT, López PJT, López-González ÁA, Rifá EMA, Oliveira HP, Sánchez CM, et al. Estimation of cardiovascular risk using SCORE2, REGICOR and vascular age scales in Spanish healthcare workers: a retrospective longitudinal study. Healthcare. 2025. 10.3390/HEALTHCARE13040375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.