Abstract
Background
In recent years, the prevalence of AIDS has shown a high increase, which has become a major public health problem of international concern. According to research, the quality of life(QoL) of AIDS patients is often lower than that of patients with other chronic diseases, so how to improve the QoL of AIDS patients has become a problem that needs to be urgently focused on at present. The purpose of this study is to develop and validate the AIDS scale among the System of Quality of Life Instruments for Chronic Diseases(QLICD), name as QLICD-HIV V2.0.
Methods
QLICD-HIV V2.0 was developed using a programmed modular approach with multiple nominal and focus group discussions, in-depth interviews and quantitative statistical procedures. Pre-surveys and formal surveys were conducted on the preliminary version of the scale, thus completing the scale development and validation process. The reliability of the scale was analyzed using the correlation coefficient method, the variability method, and the Cronbach’s α coefficient method of classical test theory(CTT), the validity of the scale was analyzed using factor analysis and correlation analysis with the SF-36 as a criterion, and the responsiveness of the scale and of each domain/facet was assessed through paired t-tests of before and after admission scores, and calculation of the standardized response mean (SRM).
Results
QLICD-HIV V2.0 ultimately retained 43 items, resulting in a structure of 4 domains, subdivided into 12 facets. The scale was shown to have high reliability overall by several methods of CTT. The Cronbach’s α coefficient and test-retest reliability for the total scale were 0.90 and 0.88, respectively, with domain Cronbach’s α coefficient ranging from 0.74 to 0.85 and test-retest reliabilities ranging from 0.69 to 0.89. Factor analysis results showed KMO = 0.852, with three common factors extracted from 15 items of the specific module, accounting for 55.2% of the cumulative variance, and correlation and factor analyses confirmed good structural and criterion-related validity.
Conclusions
QLICD-HIV V2.0 was developed in a systematic and scientific way, and showed good reliability and validity after preliminary application, which can be further promoted and used as a new QoL scale for AIDS patients with Chinese characteristics.
Keywords: Human immunodeficiency virus, Quality of life, Classical test theory, Scale
Background
Human Immunodeficiency Virus (HIV) remains a major public Health threat, globally 39 million people Living with HIV in 2022 [1]. By the end of 2020, a total of 1.053 million people were infected with HIV in China, and 351,000 deaths were reported [2]. The acquired immune deficiency syndrome (AIDS) is no longer an incurable fatal disease, but a chronic disease that requires lifelong treatment. The concept of Quality of Life (QOL) originates from the new concept of health promoted by the World Health Organization (WHO). According to the WHO definition, health is not merely the absence of disease or infirmity, but a state of overall well-being that encompasses the physical, mental, and social dimensions of life [3]. AIDS patients have lower QoL than the general population, even where the majority of those living with HIV have virological control and are immunologically stable [4]. AIDS patients commonly showed the self-stigma and social stigma, and self-stigma was associated with QoL and health-related QoL [5]. Evaluation and improve QoL were central to the care and support of AIDS patients. Developing a valid, reliable and responsive tools are required to evaluate the QoL [6].
There are several tools for the assessment of the QOL in HIV patients, mostly designed in North America or Europe. These include the general scales EQ-5D [7], SF-36 [8], and WHOQOL-BREF [9], in addition to specific scales for HIV-infected individuals, such as MOS-HIV [10], MQo L-HIV [11], PROQOL-HIV [12], and WHOQOL HIV-BREF [13]. Cultural perceptions and values play an important role in understanding the concept and the content of QOL by an individual [14]. Studies have shown that the QoL of people with AIDS is severely affected by the disease. In addition to physical pain, patients are also affected by influences from the outside world, and these pressures can cause negative emotions such as fear, depression and anxiety, which have a significant impact on treatment and adherence, and often, foreign QOL scales for AIDS seldom provide information on the impact of disease-specific psychology on QoL. Therefore, when carrying out the development of QoL scales for AIDS patients, it is first necessary to clarify the basic assessment elements, such as including physical health status, psychological stress, social support etc. At the same time, designers need to consider the possible inapplicability of conventional QoL scales in AIDS patients. For this reason, the actual situation and needs of AIDS patients need to be taken into account when developing the scale items. The selection and determination of the items of the QoL scale for AIDS patients need to be based on relevant psychosocial theories and combined with the real situation of the patients. Usually, this work requires the joint efforts of a team of experts. In addition, in order to ensure that the scale can accurately and comprehensively evaluate the QoL of patients, it is also necessary to screen, evaluate and revise the test questions.
Compared with western scales that focus on individual physical functions, the Chinese scales emphasize more on spiritual pursuits (e.g., collective value orientations such as the perception of the meaning of life, the family support network, and the responsibility of social roles). For example, the QLICP-GM scale adds a domain on “psychological functioning” and “social relations”, which translates abstract social functioning into behavioral observations such as “participation in family gatherings”, “Fulfilling the obligations of traditional festivals”, etc [15]; The Chinese version of the MOS-HIV scale adds “occupational anxiety due to health problems” based on the cognitive characteristics of local patients, and adjusts the level of social functioning in the mental health dimension [16]. Considering this distinctive culturally adapted feature of the Chinese QoL scale, disease-specific scales should be developed that conform to the Chinese way of expression and reflect the QoL that is most valued by Chinese people. People living with HIV and AIDS patients are at different stages of HIV infection, and their diagnoses are different but in a continuous progression. In view of the fact that patients who have progressed to the diagnosis of AIDS have significant discomfort and impaired QoL, and that there are no international reports on QoL scales for Chinese AIDS patients,, the purpose of this study is to develop and validate a disease-specific QoL scale for Chinese AIDS patients, name as QLICD-HIV (V2.0), based on QLICD (Quality of Life Instruments for Chronic Diseases) system developed by Wan’s team [17].
Methods
Patients
The pre-study phase (including literature, reference scales, scale conceptualisation, interview phase, etc.) extensively collected case characteristics and information on people living with HIV and AIDS patients, and the validation phase after development chose an infectious disease hospital as the sampling site to administer questionnaires to inpatients with AIDS who fulfilled the inclusion criteria, using the convenience sampling method. The informed consent had been obtained before the data collection. The ethics committee of Guangdong Medical University approved this study.
Inclusion criteria: (1) Patients with a definitive diagnosis of AIDS according to the latest national health industry standard: Diagnosis of AIDS (WS-293-2019) issued by the National Health Commission; (2) Literacy of primary school and above, with certain reading ability and comprehension, able to fill in the questionnaire by themselves.
Exclusion criteria: (1) Patients who are critically ill, with other serious diseases, mental diseases, etc.;(2) Cognitive dysfunction; (3) Illiteracy; (4) Those who refused to participate in the study or had a low level of cooperation.
The case data were collected in strict accordance with the inclusion and exclusion criteria, the data collected were collated and verified and double-entered using Epidata, and SPSS 21.0 software was used for preliminary data processing and statistical description. If there was a default value on the response of an item (unanswered), the missing item was replaced with the median score of the item.
Instrument: QLICD-HIV(V2.0)
Development of QLICD-HIV (V2.0)
The QLICD-HIV is a self-administered Likert-type rating questionnaire which utilized as the instrument for this study. The QLICD-HIV scale includes sociodemographic questionnaire, general module, and AIDS specific module. The sociodemographic questionnaire compiled information on the following Variables: gender, age, nation, occupation, education, income, marital status and payment format. The general module (QLICD-GM) is scale section containing core health-related quality of life dimensions applicable to all populations (regardless of health status, disease type, age, gender, etc.). Specific modules are tailored to the symptoms of the patient or infected person, the possible side effects of the treatment (intervention), and possible special psychosocial roles and impacts, and are used to measure health problems, symptoms or concerns that are unique to a particular population and are not adequately covered by the common modules.
The general module (QLICD-GM) includes three domains, mental function domain consisted of 11 items, physical function domain consisted of 9 items, and social function domain consisted of 8 items [18]. Items are rated on a 5-point Likert Scale, with 1 meaning no such symptoms, 2 mild symptoms, 3 medium levels of symptoms, 4 relatively severe symptoms, and 5 severe symptoms. The higher standard score represents a better QoL, and the standard score (SS) can be defined as SS= (RS-Min)×100/R, where RS refers to the raw score of the domain/facet/total, Min is the minimum value of the domain/facet/total, and R represents the range of scale scores (R = Max-Min) [19]. There have been some international articles reporting our team’s development of multiple disease-specific scales, formed by combining QLICD-GM (V2.0) with specific modules, which have been validated through a large number of developments and shown to have good reliability and validity [19–22].
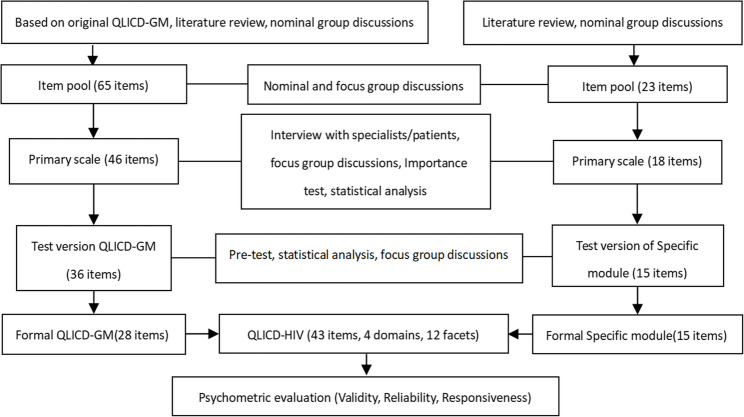
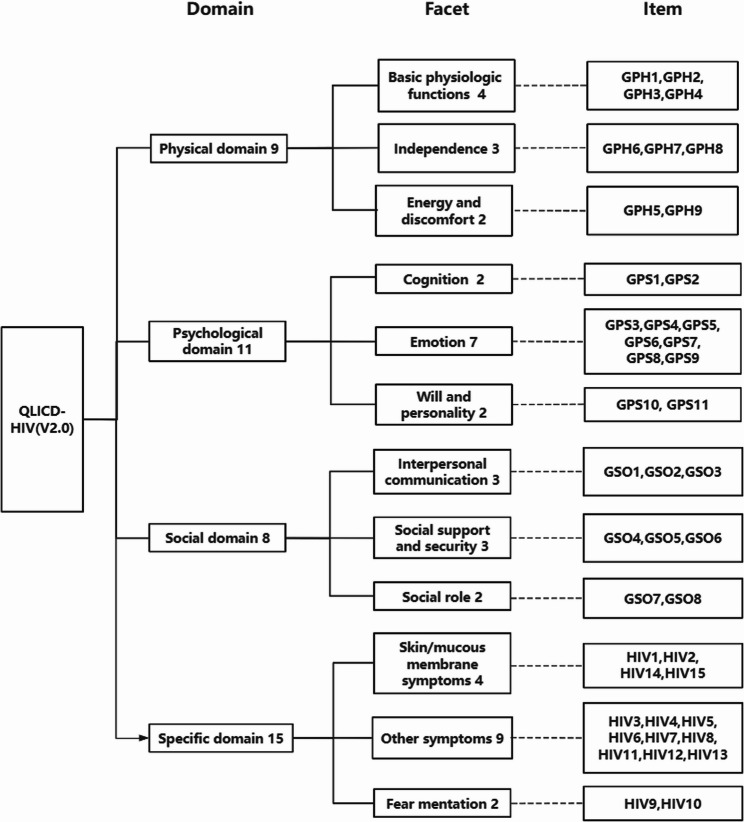
This study focused on the specific module development for Patients with AIDS. The QLICD-HIV(V2.0) was conducted in a standardized manner, a review of the existing literature, conceptualization, item development, pilot testing, and psychometric validation(Fig. 1). First, a nominal Group of hospital physicians, AIDS and quality-of-life oriented specialists conducted an extensive collection of suggestions and qualitative decision making regarding the content of the scale components and items. Next, 45 participants (mainly postgraduate students of this research group, doctors and nurses of the hospital where the survey was implemented) were convened to participate in five focus Groups, and a total of 116 responses were recorded after interviews. The responses were then content-analyzed, based on an arbitrary frequency criterion in which responses Listed at least four times were Grouped. A total 18 items were written and included in the specific module. Ten physicians and researchers with knowledge and experience in the area were invited in the experts’ panel meeting to review these 18 items. Finally, 15 items were retained for the specific module, and the scale structure was refined in the form of domains → facets → items (the scale structure is shown in Fig. 2). Each Item was rated on a 5-point Likert Scale, with 1 meaning no such symptoms, 2 mild symptoms, 3 medium level of symptoms, 4 relatively severe symptoms, and 5 severe symptoms.
Fig. 1.
Steps towards development and validation procedure of QLICD-HIV (V2.0)
Fig. 2.
Structure of QLICD-HIV (V2.0)
Validation of QLICD-HIV (V2.0)
A pilot test was given the QLICD-HIV V2.0 paper and pencil questionnaire to 30 AIDS patients, all of whom gave informed consent. A pretest of the questionnaire was conducted before the actual study to check for errors and readability. After pretesting, patients were able to understand the content of the items well and complete the responses on their own. Upon verifying that the questionnaire was free from errors and comprehension problems, the researcher proceeded to conduct the actual study in the designated study locations.
According to the paired design sample size calculation formula 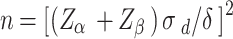 , in this study α = 0.05, β = 0.1, with a tolerance errorδ = 2(2% change), and the standard deviation of the difference between the pre- and post-treatment scores of quality of life for the QLICD/QLICP series,
, in this study α = 0.05, β = 0.1, with a tolerance errorδ = 2(2% change), and the standard deviation of the difference between the pre- and post-treatment scores of quality of life for the QLICD/QLICP series,  ,which is usually 6.07–9.58 [23, 24], a sample size range of 91–226 could be calculated. The final sample size consisted of 124 patients with AIDS, who had been diagnosed as AIDS in Chinese Hospital.
,which is usually 6.07–9.58 [23, 24], a sample size range of 91–226 could be calculated. The final sample size consisted of 124 patients with AIDS, who had been diagnosed as AIDS in Chinese Hospital.
The informed consent had been obtained before the data collection. The researcher obtained information about the patients from the hospital system and screened the hospitalised patients who met the inclusion exclusion criteria. When the formal survey was implemented, the investigator appeared as a doctor, and when the patient was just hospitalized, the investigator sent the scale to the patient to fill in, which included the patient’s general information, the QLICD-HIV (V2.0) scale, and was accompanied by the internationally recognized universality scale SF-36 to facilitate the evaluation of criterion-related validity with the scale developed in this study. The investigator provided brief explanations and instructions to the patients to ensure that they understood the purpose of the questionnaire and the method of completing it, and then retrieved the scale and checked its completeness after completion. If there were any missing items, the patients were asked to complete them in time. Next, the researcher continued to follow the patients, and the questionnaire for the patients was a longitudinal measure, with three measurements required for all patients. The first measurement was taken on the day of admission (before treatment). The second measurement was taken on the second day of admission by repeating the questionnaire used for the first time, so as to facilitate the calculation of the test-retest reliability. The third measurement was conducted before the patients were discharged from the hospital (after treatment), completing the same questionnaire as the first and second. Finally, the investigator fills in the diagnosis and treatment of the disease of the patient in question, including the clinical stage of AIDS, the clinical diagnosis, and the treatment, and the investigator signs the questionnaire when it is completed.
Classical Test Theory (CTT), also known as True Score Theory, is one of the leading test theories to be developed and widely used [25]. Currently, the methods of CTT are commonly used for analyzing the reliability of scales. The reliability of the QLICD-HIV was assessed using the CTT approach, reliability was analyzed as internal consistency through the degree of variation (standard deviation), test-retest reliability coefficients (Pearson r and Intra-class correlations ICC), and Cronbach’s α coefficient.
The content validity was held by six experts who work in AIDS-related health organization with knowledge and experience in this area. And only the expert recommends items remain for statistics. Exploratory factor analysis was used to validate the construct validity of the scale. The result from the KMO and Bartlett’s test of sphericity was used to test the assumption of ‘sufficient significant correlations in the data matrix’. The Chinese version of SF-36 was used for the comparative analysis of the criterion-related validity. The SF-36 scale is a widely used universal self-assessment tool for assessing health-related quality of Life, covering a total of 36 items in eight dimensions [26]. The correlation coefficients between the same/similar domains of the two scales were analyzed by letting the same sample answer the QLICD-HIV(V2.0) and the SF-36 at the same time.
Responsiveness is the ability of a scale to measure changes in QoL over a longitudinal period of time, which allows visualization of the changes in QoL after a patient has undergone treatment [27]. In the present study, mean scores were calculated for each domain/facet before and after treatment (first and third assessment). In this paper we used a paired t-test and calculated the standardized reactivity mean (SRM) to assess reactivity [28, 29].
Results
Sociodemographic characteristics of the participants
The sample was composed of 124 AIDS patients in this study. There were 69 male participants (55.6%) and 55 female participants (44.4%). The mean age of the participants was 36.3 (SD = 6.7) years. The educational level, 17.7% had completed primary education, 54% had completed junior high school, 24.3% had completed high school or vocational training, and 4% had completed university education. For marital status, 36.3% were single, 46.8% were married, 12.9% were divorced, 5% were widowed. Regarding to nations, 80.6% were the Han nationality, 7.3% were the Yi nationality, 0.8% were the Bai nationality, 7.3% were the Hui nationality, 4% were other nationality. Nearly 82.4% participants’ income were lower than 5000 RMB/month. The occupation of participants, 14.5% were worker, 15.3% were farmer, 1.6% were cadre, 6.5% were self-employed, 62.1% were other occupations. For the medical expenses, 12.9% were self-paying, 55.6% were social medical insurance, 25.8% were rural Cooperative medical care system, 7% were other payment.
Reliability analysis
Comprehensively analyzing the reliabilities of the total scale and the domains and facets, the detailed results are shown in Table 1. From the results, it can be seen that the scale has high internal consistency reliabilities in general, with the Cronbach’s α coefficient of the total scale of 0.9, and Cronbach’s α coefficients of the domains ranging from 0.74 to 0.85. The test-retest reliability for each domain ranged from 0.69 to 0.89, and the test-retest reliability for the total scale was 0.88, which fulfills the requirement of test-retest reliability > 0.7.
Table 1.
Reliability of the quality of life instrument QLICD-HIV(V2.0)
| Domains/facets | Internal consistency coefficient α | Test-retest reliability correlation r | ICC (95%CI) |
|---|---|---|---|
| Physical domain (PHD) | 0.80 | 0.88 | 0.87 (0.79–0.92) |
| Basic physiologic functions (BPF) | 0.57 | 0.75 | 0.75 (0.62–0.84) |
| Independence (IND) | 0.88 | 0.86 | 0.85 (0.76–0.91) |
| Energy and discomfort (EAD) | 0.53 | 0.56 | 0.55 (0.35–0.70) |
| Psychological domain (PSD) | 0.74 | 0.69 | 0.69 (0.53–0.80) |
| Cognition (COG) | 0.42 | 0.78 | 0.78 (0.66–0.86) |
| Emotion (EMO) | 0.84 | 0.59 | 0.59 (0.41–0.73) |
| Will and personality (WIP) | 0.53 | 0.46 | 0.46 (0.24–0.63) |
| Social domain (SOD) | 0.79 | 0.84 | 0.84 (0.74–0.90) |
| Interpersonal communication (INC) | 0.63 | 0.80 | 0.79 (0.68–0.87) |
| Social support and security (SSS) | 0.582 | 0.81 | 0.81 (0.71–0.88) |
| Social role (SOR) | 0.36 | 0.74 | 0.74 (0.60–0.83) |
| Sub-total (QLICD-GM) | 0.85 | 0.89 | 0.88 (0.81–0.93) |
| Specific domain (SPD) | 0.84 | 0.79 | 0.79 (0.68–0.87) |
| Skin/mucous membrane symptoms(SMS) | 0.73 | 0.79 | 0.79 (0.67–0.86) |
| Other symptoms(OTS) | 0.86 | 0.81 | 0.80 (0.69–0.87) |
| Fear mentation(FEM) | 0.41 | 0.63 | 0.63 (0.45–0.76) |
| Total (TOT) | 0.90 | 0.88 | 0.88 (0.81–0.93) |
ICC Intra-class correlation, CI Confidence interval
For the specific module analysis, standard deviation, correlation coefficient, and Cronbach’s alphas were calculated to evaluate items for the specific module. As can be seen from Table 2, the standard deviation for all 15 items are greater than 0.9, indicating that the sensitivity of the items is high [30]. Except for HIV11, the correlation coefficients between the items and the domains were all greater than 0.5, and the Cronbach’s α coefficients of the items were all greater than 0.8, indicating that the items had good reliability.
Table 2.
Item analysis of the AIDS-specific module based on methods under CTT
| Items | Coefficient of variation | Correlation coefficient | Cronbach’s α coefficient |
|---|---|---|---|
| HIV1 | 0.906* | 0.568* | 0.825* |
| HIV2 | 1.415* | 0.648 | 0.826* |
| HIV3 | 1.539* | 0.643* | 0.817* |
| HIV4 | 1.539* | 0.268 | 0.826* |
| HIV5 | 1.441* | 0.633* | 0.830* |
| HIV6 | 1.438* | 0.554* | 0.817* |
| HIV7 | 1.509* | 0.671* | 0.820* |
| HIV8 | 1.239* | 0.541* | 0.830* |
| HIV9 | 1.226* | 0.477* | 0.842* |
| HIV10 | 1.356* | 0.533* | 0.854 |
| HIV11 | 1.146* | 0.433 | 0.820* |
| HIV12 | 1.169* | 0.706* | 0.818* |
| HIV13 | 1.436* | 0.603* | 0.823* |
| HIV14 | 1.187* | 0.527* | 0.829* |
| HIV15 | 1.030* | 0.522* | 0.830* |
The * means P < 0.05
Validity analysis
Construct validity
Construct validity was evaluated using item-domain Pearson correlation coefficients and factor analysis. Correlation analysis of the data measured at admission showed strong correlations between the item and the domains (mostly above 0.40). However, the relationship between the item and other domains was weak (see Table 3 for details). For example, the correlation coefficients of the Social domain with GSO1-GSO8 ranged from 0.519 to 0.753 (bolded portions), which is higher than the correlation coefficients with other items.
Table 3.
Correlation coefficients r among items and domains of QLICD-HIV(V2.0) (n = 124)
| Items | Items brief description | Physical | Psychological | Social | Specific |
|---|---|---|---|---|---|
| GPH1 | Appetite | 0.628 | 0.158 | 0.168 | 0.358 |
| GPH2 | Sleep | 0.438 | 0.166 | 0.166 | 0.292 |
| GPH3 | Sexual function | 0.592 | 0.434 | 0.432 | 0.405 |
| GPH4 | Excrement | 0.528 | 0.253 | 0.185 | 0.311 |
| GPH5 | Pain | 0.681 | 0.373 | 0.249 | 0.567 |
| GPH6 | Daily activities | 0.744 | 0.382 | 0.394 | 0.401 |
| GPH7 | Work | 0.781 | 0.400 | 0.526 | 0.352 |
| GPH8 | Walk | 0.739 | 0.312 | 0.422 | 0.322 |
| GPH9 | Fatigue | 0.443 | 0.421 | 0.234 | 0.331 |
| GPS1 | Attention | 0.713 | 0.451 | 0.481 | 0.371 |
| GPS2 | Memory deterioration | 0.307 | 0.637 | 0.187 | 0.211 |
| GPS3 | Joy of life | 0.102 | 0.084 | 0.085 | 0.216 |
| GPS4 | Restless | 0.210 | 0.072 | 0.369 | 0.063 |
| GPS5 | Family burden | 0.393 | 0.683 | 0.381 | 0.450 |
| GPS6 | State of health | 0.364 | 0.723 | 0.422 | 0.284 |
| GPS7 | Depression | 0.289 | 0.624 | 0.278 | 0.282 |
| GPS8 | Disappointment | 0.441 | 0.734 | 0.279 | 0.399 |
| GPS9 | Fear | 0.424 | 0.741 | 0.362 | 0.385 |
| GPS10 | Positive attitude | 0.246 | 0.300 | 0.365 | 0.151 |
| GPS11 | Termagancy | 0.436 | 0.245 | 0.514 | 0.230 |
| GSO1 | Social contact | 0.507 | 0.358 | 0.647 | 0.298 |
| GSO2 | Family relationship | 0.246 | 0.169 | 0.601 | 0.150 |
| GSO3 | Friend relationship | 0.466 | 0.229 | 0.638 | 0.220 |
| GSO4 | Family support | 0.168 | 0.187 | 0.698 | 0.122 |
| GSO5 | Other people’s care | 0.211 | 0.167 | 0.687 | 0.082 |
| GSO6 | Economic hardship | 0.389 | 0.422 | 0.519 | 0.351 |
| GSO7 | Labor status | 0.417 | 0.454 | 0.589 | 0.361 |
| GSO8 | Family role | 0.274 | 0.111 | 0.753 | 0.148 |
| HIV1 | Oral leukoplakia | 0.260 | 0.182 | 0.099 | 0.588 |
| HIV2 | Skin ulceration and abscess | 0.268 | 0.233 | 0.214 | 0.572 |
| HIV3 | Nausea and vomiting | 0.468 | 0.360 | 0.211 | 0.704 |
| HIV4 | Diarrhea | 0.313 | 0.184 | 0.041 | 0.564 |
| HIV5 | Cough and expectoration | 0.336 | 0.232 | 0.110 | 0.551 |
| HIV6 | Chest pain and tightness | 0.487 | 0.287 | 0.262 | 0.698 |
| HIV7 | Dyspnea | 0.455 | 0.278 | 0.233 | 0.670 |
| HIV8 | Catches cold frequently | 0.337 | 0.217 | 0.098 | 0.507 |
| HIV9 | Feeling discriminated against | 0.260 | 0.372 | 0.367 | 0.373 |
| HIV10 | Afraid to be recognized | 0.168 | 0.330 | 0.216 | 0.246 |
| HIV11 | Fever | 0.390 | 0.127 | 0.162 | 0.660 |
| HIV12 | Abnormal sweating | 0.460 | 0.314 | 0.279 | 0.689 |
| HIV13 | Significant weight loss | 0.484 | 0.246 | 0.339 | 0.618 |
| HIV14 | Skin blisters with local pain | 0.186 | 0.119 | 0.025 | 0.531 |
| HIV15 | Intractable skin itching | 0.151 | 0.160 | 0.111 | 0.498 |
#Correlations between each item and its designated scale are in bold type
Factor analysis was conducted for specific modules. The KMO and Bartlett’s test of sphericity tested the null hypothesis that the 15 items are uncorrelated. The result obtained from the KMO = 0.852 and Bartlett’s test of sphericity is highly significant, p < 0.001. supports the applicability of factor analysis. After using the extraction criterion of eigenvalues > 1, three common factors, accounting for 55.2% of the cumulative variance, were extracted from the 15 items of the specific module using Varimax rotation and reconstructed for three different facets of the specific module (Table 4). The contributions of the first, second and third common factors were 34.3%, 11.5% and 9.4%, respectively.
Table 4.
Common factors and factor loadings of the specific module of QLICD-HIV (n = 124)*
| Items | Common factors and variance contribution (55.2%) | ||
|---|---|---|---|
| P1(34.3%) | P2(11.5%) | P3(9.4%) | |
| HIV1 | 0.70 | ||
| HIV2 | 0.59 | ||
| HIV3 | 0.53 | ||
| HIV4 | 0.79 | ||
| HIV5 | 0.86 | ||
| HIV6 | 0.85 | ||
| HIV7 | |||
| HIV8 | 0.68 | ||
| HIV9 | 0.76 | ||
| HIV10 | 0.61 | ||
| HIV11 | |||
| HIV12 | 0.56 | ||
| HIV13 | 0.74 | ||
| HIV14 | 0.75 | ||
| HIV15 | 0.70 | ||
*Less than 0.5 is not displayed
Criterion‑related validity
Table 5 shows the Pearson correlation coefficients between the domain scores of QLICD-HIV (V2.0) and SF-36. The results indicate a positive correlation. The correlation coefficients between the same domains or similar domains are significantly higher than the coefficient values between unrelated domains in the same column, and most of the coefficient values are greater than 0.4, indicating that the QLICD-HIV (V2.0) has good convergent validity and discriminant validity, wherein the correlation coefficients with all the domains of the SF-36 can be seen due to the content of the specific module that deals with the physiology, pain, and the special psychology of the disease in question.
Table 5.
Correlation coefficients among domains scores of QLICD-HIV(V2.0) and SF-36 (n = 124)
| Domain | SF-36 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Physical function | Role-physical | Body pain | General health | Vitality | Social function | Role-emotional | Mental health | |||
| Physical | 0.29 | 0.31 | 0.38 | 0.30 | 0.37 | 0.44 | 0.26 | 0.43 | ||
| Psychological | 0.36 | 0.45 | 0.47 | 0.42 | 0.34 | 0.56 | 0.39 | 0.41 | ||
| Social | 0.42 | 0.41 | 0.59 | 0.39 | 0.51 | 0.44 | 0.37 | 0.40 | ||
| Specific | 0.63 | 0.50 | 0.71 | 0.54 | 0.56 | 0.48 | 0.44 | 0.42 | ||
Correlations in bold were that for similar domains
*There was a significant correlation at the level of 0.05 for all correlation coefficients
Responsiveness analysis
The responsiveness of the scale was evaluated by detecting the change in the mean scores of the domains/facets before and after the treatment using paired t-test and response index SRM and the detailed results are shown in Table 6. It can be seen that the pre-treatment and post-treatment scores of the specific domains were statistically significantly different (p < 0.05) and there was no statistically significant difference between the pre- and post-scores of the other domains/facets. The domain level SRM ranged from 0.04 to 0.20. The unsatisfactory results in responsiveness may be due to the short observation period and the long treatment period, which is not curable and is best followed up for a long period of time.
Table 6.
Responsiveness of the quality of life instrument QLICD-HIV(V2.0) (n = 124)
| Domains/facets | Before treatment Mean SD |
After treatment Mean SD |
Differences Mean SD |
t | p | SRM | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Physical domain | 62.61 | 17.11 | 63.73 | 16.11 | −1.12 | 11.33 | −1.10 | 0.273 | 0.10 | |
| Basic physiologic functions | 50.66 | 17.20 | 52.82 | 18.66 | −2.17 | 15.02 | −1.61 | 0.111 | 0.14 | |
| Independence | 78.43 | 26.40 | 79.03 | 23.02 | −0.60 | 15.22 | −0.44 | 0.659 | 0.04 | |
| Energy and discomfort | 62.80 | 23.12 | 62.60 | 21.59 | 0.20 | 21.32 | 0.11 | 0.916 | 0.01 | |
| Psychological domain | 52.44 | 13.05 | 53.39 | 12.19 | −0.95 | 8.58 | −1.24 | 0.219 | 0.11 | |
| Cognition | 57.56 | 23.74 | 58.47 | 23.73 | −0.91 | 19.07 | −0.53 | 0.597 | 0.05 | |
| Emotion | 53.43 | 16.41 | 54.18 | 15.95 | −0.75 | 11.68 | −0.71 | 0.477 | 0.06 | |
| Will and personality | 43.85 | 16.68 | 45.56 | 17.83 | −1.71 | 16.89 | −1.13 | 0.261 | 0.10 | |
| Social domain | 60.18 | 19.94 | 59.70 | 20.55 | 0.48 | 13.07 | 0.41 | 0.684 | 0.04 | |
| Interpersonal communication | 68.75 | 19.64 | 66.73 | 19.69 | 2.02 | 15.19 | 1.48 | 0.142 | 0.13 | |
| Social support and security | 53.63 | 24.28 | 54.30 | 25.29 | −0.67 | 17.35 | −0.43 | 0.667 | 0.04 | |
| Social role | 57.16 | 28.21 | 57.26 | 27.84 | −0.10 | 22.68 | −0.05 | 0.961 | 0.00 | |
| Sub-total (QLICD-GM) | 57.92 | 13.25 | 58.52 | 12.90 | −0.60 | 7.54 | −0.88 | 0.379 | 0.08 | |
| Specific domain | 71.21 | 16.44 | 73.35 | 15.53 | −2.14 | 10.59 | −2.25 | 0.026 | 0.20 | |
| Skin/mucous membrane symptoms(SMS) | 77.02 | 21.53 | 79.33 | 20.85 | −2.32 | 15.09 | −1.71 | 0.090 | 0.15 | |
| Other symptoms(OTS) | 73.68 | 19.26 | 75.40 | 17.47 | −1.72 | 14.09 | −1.36 | 0.175 | 0.12 | |
| fear mentation(FEM) | 49.29 | 27.62 | 51.41 | 27.48 | −2.12 | 26.56 | −0.89 | 0.377 | 0.08 | |
| Total (TOT) | 62.56 | 12.78 | 63.69 | 12.30 | −1.13 | 6.94 | −1.82 | 0.071 | 0.16 | |
Discussions
The development of the QoL Scale for AIDS patients is a very important and complex process. The background to this work lies in the specificity of AIDS. The HIV virus, which requires lifelong treatment once it has infected the body, attacks the immune system, thereby increasing the patient’s susceptibility to the disease and causing a number of serious complications. As the infection progresses to the stage of AIDS, patients suffer from the disease and have a severely reduced quality of life, which has a profound impact on their physical and mental health. Traditional quality-of-life assessment tools often fail to fully cover the problems and needs specific to AIDS patients. Therefore, the development of a QoL scale specifically for AIDS patients will have a positive impact on clinical practice and scientific research. This study was driven by the motivation to improve the QoL of AIDS patients to develop and validate a QoL measurement scale suitable for Chinese AIDS patients. Based on Wan’s well-established methodology of developing the QLICD series of instruments, we have developed the QLICD-HIV (V2.0), a QoL measurement scale for Chinese AIDS patients, through the procedural modeling method, which has more outstanding advantages than the QoL measurement scales currently used at home and abroad: (1) The design of the scale takes into account the inapplicability of the conventional QoL scale to AIDS patients, and the content of the items is developed with the consideration of the possible unsuitability of the conventional QoL scale to AIDS patients. (2) The specific module developed based on the QLICD system can satisfy the sensitivity and specificity required for the measurement, and at the same time, it can also compare the variability of the domains or facets in the general module of different diseases within the QLICD system, which is in line with the general trend of the research on QoL scales [31, 32].
In terms of item setting, in view of the special psychosocial aspects of AIDS patients, the initial item bank of QLICD-HIV (V2. 0) contains these issues, such as social stigmatization and discrimination against AIDS patients, low self-esteem and pressure of AIDS patients on themselves, and self-reproach and shame of AIDS patients on their families, and so on [33]. During the development process, after layers of selection and discussion, the psychological domain, social domain and specific modules in QLICD-HIV (V2.0) can reflect these issues in a more comprehensive way. For example, the general module GPS5 “Do you worry about being seen as a burden to your family?”, GSO7 “Does the illness and treatment affect your status or role in work or labor?” etc.; HIV9 “Do you feel discriminated against?” in specific modules. etc. Such a QoL scale with Chinese cultural characteristics will be more applicable to Chinese AIDS patients and will be conducive to the popularization and application of the scale.
The reliability assessment of the macro of the scale focused on internal consistency reliability and test-retest reliability, and the results proved that the total scale, including all domains and facets, had a high level of reliability, indicating that the QLICD-HIV (V2.0) is reliable and stable as a specific scale for the QoL of AIDS patients. Next, the 15 items of the specific module were individually analyzed for reliability, which showed that there was good differentiation between the items and that there was correlation and consistency between the items and their domains and dimensions. In summary, the items are statistically sound and plausible.
The validity of the scale includes content validity, structural validity and criterion related validity. The development process of QLICD-HIV(V2.0) was discussed and supervised by experts related to the field, and the content of the scale was repeatedly modified after many researches and pre-tests, so it has a good content validity. At the same time, after factor analysis, we can see that the relationship between the items in the scale is in line with the theoretical expectations set by the main factor expression content, with satisfactory construct validity. The SF-36, the most widely used generic QoL scale, was used as the criterion scale, and the correlation between each domain of the scale and the corresponding domain of the criterion scale was calculated. The results of the criterionrelated were favorable, indicating that the results measured by the QLICD-HIV(V2.0) were consistent with the reality.
Due to the long duration of the disease and the complexity and severity of the complications of AIDS, which is a difficult problem for medical treatment, most patients are admitted to hospitals for early detection by physical examination or for treatment of serious complications, which results in poor treatment outcomes during hospitalization and the need for long-term maintenance treatment to slow down the progression of the disease [34, 35], so the results of the responsiveness of the scales we measured were not satisfactory. The difference between the scores measured at admission and before discharge was rarely statistically significant, and only the total score of the specific module showed variability, which may be due to the fact that the specific module focuses on the typical symptoms of AIDS, such as somatic pain, mucous membrane leukoplakia, fever, night sweats, and diarrhea, rather than the generic module, which focuses on describing physical, psychological, and social functioning, and the content of what is described by these items may be difficult to detect in a short period of time in patients with AIDS. This point suggests that long-term observational follow-up is more recommended for QoL measurements in patients with AIDS, and therefore subsequent studies could apply the QLICD-HIV (V2.0) for long-term longitudinal responsiveness studies.
In this study, we developed a QoL scale applicable to Chinese AIDS patients with good reliability and validity, reflecting its scientific, appropriateness, and practical value. QLICD-HIV V2.0 provides a multidimensional assessment framework (physical, psychological, social, and specific modules) that can be used not only to assess all aspects of a patient’s health at the cross-sectional level, but also to monitor treatment outcomes longitudinally, enabling clinicians to tailor interventions to unmet needs (e.g., for psychological distress), which also has great potential for advancing AIDS care.
In addition, the development of this scale will provide a richer and more accurate source of data for related research, such as using this scale to develop Minimal Clinically Important Difference (MCID) values to provide clinicians with efficacy references, to analyze the QoL at different stages of disease progression, and to analyze the factors affecting the QoL of patients. As the survey sites of this study were concentrated in hospitals, and the participants of the scale validation were inpatients with confirmed AIDS diagnosis, the sample was representative but the insufficient sample size and the limitation of the survey sites were also the limitations of this study. We hope that in the future the scale can be applied not only to patients at the stage of diagnosis of AIDS, but also to include people living with HIV at all stages of the disease, so in the future we will further expand the sample size, expand the sample population, and continue to validate and adjust.
Conclusions
QLICD-HIV (V2.0) is a QoL scale for AIDS patients, which has been rigorously developed and validated, has good validity and reliability, and improves the important contents in the QLICD system, can be widely used to measure the QoL of Chinese AIDS patients.
Acknowledgements
We have received substantial assistance from the staff of the Affiliated Hospital of Guangdong Medical University. We sincerely acknowledge all the support.
Abbreviations
- AIDS
Acquired Immune Deficiency Syndrome
- CTT
Classical test theory
- HIV
Human Immunodeficiency Virus
- QoL
Quality of life
- QLICD
Quality of Life Instruments for Chronic Diseases
- ICC
Intra-class correlations
- SRM
Standardized Response Mean
- MCID
Minimal Clinically Important Difference
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by CW, LQ and XS. The first draft of the manuscript was written by LQ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 71373058) and Science andTechnology Plan of Guangdong Province (Grant No. 2013B021800074) and Guangdong Basic and Applied Basic Research Foundation (Grant No.2020A1515110608).
Data availability
The datasets generated and/or analyzed in this study are not publicly available due to confidentiality but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethical approval for this study was obtained from the Ethics Committee of the Affiliated Hospital of Guangdong Medical University (REC: PJ2015050KT). Participants gave informed consent to participate in the study before taking part. The statistical methods used in this study are in accordance with the relevant guidelines and regulations and several published articles are available for reference.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaoyuan Sun and Liyuan Qiao are as the first co-author with the same contributions.
Contributor Information
Ying Chen, Email: 1284958937@qq.com.
Chonghua Wan, Email: wanchh@hotmail.com.
References
- 1.Organization WH. People (all ages) living with HIV estimates by WHO region: World Health Organization; 2022. Available from: http://apps.who.int/gho/data/view.main.22100WHO?lang=en
- 2.He N. New progress of AIDS epidemiology in China. Chin J Disease Control Prev. 2021;25(12):5. [Google Scholar]
- 3.Group W. Study protocol for the world health organization project to develop a quality of life assessment instrument (WHOQOL). quality of life research: an international journal of quality of life aspects of treatment. Care Rehabilitation. 1993;2(2):153–9. [PubMed] [Google Scholar]
- 4.Miners A, Phillips A, Kreif N, Rodger A, Speakman A, Fisher M, et al. Health-related quality-of-life of people with HIV in the era of combination antiretroviral treatment: a cross-sectional comparison with the general population. Lancet HIV. 2014;1(1):e32–40. [DOI] [PubMed] [Google Scholar]
- 5.Nobre N, Pereira M, Roine RP, Sutinen J, Sintonen H. HIV-Related Self-Stigma and Health-Related quality of life of people living with HIV in Finland. J Association Nurses AIDS Care: JANAC. 2018;29(2):254–65. [DOI] [PubMed] [Google Scholar]
- 6.Cooper V, Clatworthy J, Harding R, Whetham J. Measuring quality of life among people living with HIV: a systematic review of reviews. Health Qual Life Outcomes. 2017;15(1):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72. [DOI] [PubMed] [Google Scholar]
- 8.McHorney CA, Ware JE Jr., Lu JF, Sherbourne CD. The MOS 36-item Short-Form health survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. [DOI] [PubMed] [Google Scholar]
- 9.Group W. Development of the world health organization WHOQOL-BREF quality of life assessment. Psychol Med. 1998;28(3):551–8. [DOI] [PubMed] [Google Scholar]
- 10.Wu AW, Revicki DA, Jacobson D, Malitz FE. Evidence for reliability, validity and usefulness of the medical outcomes study HIV health survey (MOS-HIV). Qual Life Research: Int J Qual Life Aspects Treat Care Rehabilitation. 1997;6(6):481–93. [DOI] [PubMed] [Google Scholar]
- 11.Smith KW, Avis NE, Mayer KH, Swislow L. Use of the MQoL-HIV with asymptomatic HIV-positive patients. Qual Life Research: Int J Qual Life Aspects Treat Care Rehabilitation. 1997;6(6):555–60. [DOI] [PubMed] [Google Scholar]
- 12.Duracinsky M, Herrmann S, Berzins B, Armstrong AR, Kohli R, Le Coeur S, et al. The development of PROQOL-HIV: an international instrument to assess the health-related quality of life of persons living with HIV/AIDS. J Acquir Immune Defic Syndr. 2012;59(5):498–505. [DOI] [PubMed] [Google Scholar]
- 13.O’Connell KA, Skevington SM. An international quality of life instrument to assess wellbeing in adults who are HIV-positive: a short form of the WHOQOL-HIV (31 items). AIDS Behav. 2012;16(2):452–60. [DOI] [PubMed] [Google Scholar]
- 14.Alavi NM, Ghofranipour F, Ahmadi F, Emami A. Developing a culturally valid and reliable quality of life questionnaire for diabetes mellitus. East Mediterranean Health J = La Revue De Sante De La Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2007;13(1):177–85. [PubMed] [Google Scholar]
- 15.Wan C, Yang Z, Meng Q, Feng C, Wang H, Tang X, et al. Development and validation of the general module of the system of quality of life instruments for cancer patients. Int J Cancer. 2008;122(1):190–6. [DOI] [PubMed] [Google Scholar]
- 16.Lau JT, Tsui HY, Patrick LC, Rita CW, Molassiotis A. Validation of a Chinese version of the medical outcomes study HIV health survey (MOS-HIV) among Chinese people living with HIV/AIDS in Hong Kong. Qual Life Research: Int J Qual Life Aspects Treat Care Rehabilitation. 2006;15(6):1079–89. [DOI] [PubMed] [Google Scholar]
- 17.Wan C, Tu X, Messing S, Li X, Yang Z, Zhao X, et al. Development and validation of the general module of the system of quality of life instruments for chronic diseases and its comparison with SF-36. J Pain Symptom Manag. 2011;42(1):93–104. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Chang Y, Wan D, Li W, Xu C, Wan C. Development and validation of a disease-specific quality of life measure QLICD-HY (V2.0) for patients with hypertension. Sci Rep. 2023;13(1):12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quan P, Yu L, Yang Z, Lei P, Wan C, Chen Y. Development and validation of quality of life instruments for chronic diseases-Chronic gastritis version 2 (QLICD-CG V2.0). PLoS ONE. 2018;13(11):e0206280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Q, Liu X, Zhang P, Xie X, Wan C, Wang X, et al. Development and assessment of the quality of life instruments for chronic Diseases-Gout (QLICD-GO) (V2.0). Clin Rheumatol. 2023;42(2):501–9. [DOI] [PubMed] [Google Scholar]
- 21.Wan C, Chen Y, Gao L, Zhang Q, Li W, Quan P. Development and validation of the chronic gastritis scale under the system of quality of life instruments for chronic diseases QLICD-CG based on classical test theory and generalizability theory. J Clin Gastroenterol. 2022;56(2):e137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q, Feng L, Wan C, Tan J, Yu J, Wang L. Development and validation of the psoriasis scale among the system of quality of life instruments for chronic diseases QLICD-PS (V2.0). Health Qual Life Outcomes. 2022;20(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li F, Zhou J, Wan C, Yang Z, Liang Q, Li W, et al. Development and validation of the breast cancer scale QLICP-BR V2.0 based on classical test theory and generalizability theory. Front Oncol. 2022;12:915103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Y, Chi W, Ding M, Ding H, Xu C, Wan C. Development and validation of the epilepsy scale among the system of quality of life instruments for chronic diseases QLICD-EP (V2.0):A multicenter longitudinal study. Epilepsy Behavior: E&B. 2025;169:110444. [DOI] [PubMed] [Google Scholar]
- 25.Vaccarino AL, Kalali AH, Blier P, Gilbert Evans S, Engelhardt N, Foster JA, et al. THE DEPRESSION INVENTORY DEVELOPMENT SCALE: assessment of psychometric properties using classical and modern measurement theory in a CAN-BIND trial. Innovations Clin Neurosci. 2020;17(7–9):30–40. [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z, Li W, Tu X, Tang W, Messing S, Duan L, et al. Validation and psychometric properties of Chinese version of SF-36 in patients with hypertension, coronary heart diseases, chronic gastritis and peptic ulcer. Int J Clin Pract. 2012;66(10):991–8. [DOI] [PubMed] [Google Scholar]
- 27.Stevens MW, Hainsworth KR, Weisman SJ, Layde PM. Health-related quality of life in pediatric minor injury: reliability, validity, and responsiveness of the pediatric quality of life inventory in the emergency department. Arch Pediatr Adolesc Med. 2012;166(1):74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terwee CB, Dekker FW, Wiersinga WM, Prummel MF, Bossuyt PM. On assessing responsiveness of health-related quality of life instruments: guidelines for instrument evaluation. Qual Life Research: Int J Qual Life Aspects Treat Care Rehabilitation. 2003;12(4):349–62. [DOI] [PubMed] [Google Scholar]
- 29.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102–9. [DOI] [PubMed] [Google Scholar]
- 30.Wan C, Yu Y, Tan J, Meng Q, Huang X. Introductions on quality of life Rescarch-Measurements·Assessments·Improvements (in Chinese). Beijing: Science; 2016. [Google Scholar]
- 31.Wan C, Chen Y, Gao L, Zhang Q, Quan P, Sun X. Development and validation of the peptic ulcer scale under the system of quality of life instruments for chronic diseases based on classical test theory and generalizability theory. BMC Gastroenterol. 2020;20(1):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marmura H, Tremblay PF, Getgood AMJ, Bryant DM. The knee injury and osteoarthritis outcome score does not have adequate structural validity for use with young, active patients with ACL tears. Clin Orthop Relat Res. 2022;480(7):1342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu X, Chen J, Huang H, Liu Z, Li X, Wang H. Perceived stigma, medical social support and quality of life among people living with HIV/AIDS in hunan, China. Appl Nurs Research: ANR. 2015;28(2):169–74. [DOI] [PubMed] [Google Scholar]
- 34.Wang P, Gao JY, Zhou HJ, Wu J, Wang Y. Health-related quality of life of Chinese AIDS patients: a multi-region study. Qual Life Research: Int J Qual Life Aspects Treat Care Rehabilitation. 2023;32(4):1005–14. [DOI] [PubMed] [Google Scholar]
- 35.Miyada S, Garbin AJI, Wakayama B, Saliba TA, Garbin CAS. Quality of life of people with HIV/AIDS - the influence of social determinants and disease-related factors. Rev Soc Bras Med Trop. 2019;52:e20180157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed in this study are not publicly available due to confidentiality but are available from the corresponding author on reasonable request.