Abstract
Background
Chlamydia psittaci pneumonia (CPP) typically presents with exudative and consolidative changes on chest computed tomography (CT), including lung consolidation, bronchial air trapping, pleural effusion, and ground-glass opacities. Here, we report an atypical case of CPP manifesting as pulmonary nodules and cavitary lesions, which was subsequently diagnosed through targeted next-generation sequencing (tNGS) of bronchoalveolar lavage fluid (BALF).
Case presentation
A previously healthy 16-year-old male adolescent developed a severe cough with scant mucoid sputum, nasal congestion, and rhinorrhea after cleaning his dormitory, with symptoms lasting nearly one month. Initial laboratory tests revealed normal infection-related markers, including white blood cell (WBC) count, interleukin-6 (IL-6), and procalcitonin (PCT), while chest CT showed pulmonary nodules and a small cavitary lesion. Based on the clinical presentation of persistent cough and the absence of significant inflammatory markers, empirical therapy with intravenous doxycycline was initiated, resulting in significant symptom improvement. After discharge, failure to follow the recommended dosing schedule and non-standardized use of oral doxycycline (including missed doses and co-administration with milk) compromised treatment efficacy. A follow-up CT demonstrated partial improvement in the right lung lesion but progression in the left, prompting a switch to cephalosporin, which proved ineffective. Referred to our hospital, bronchoscopy and tNGS of BALF identified Chlamydia psittaci (11 nucleic acid sequence reads), confirming psittacosis. A 14-day course of oral doxycycline led to significant clinical improvement and resolution of pulmonary lesions on follow-up imaging.
Conclusion
In clinical practice, CPP should be included in the differential diagnosis for patients presenting with pulmonary nodules and cavitary lesions on imaging, even in the absence of confirmed direct avian exposure. This consideration is particularly crucial when patients exhibit persistent respiratory symptoms coupled with poor clinical response to broad-spectrum antibiotic therapy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-025-11676-x.
Keywords: Chlamydia psittaci, Next-generation sequencing, Computed tomography of the chest, Pulmonary nodules, Pulmonary cavities
Background
CPP, caused by the gram-negative bacterium Chlamydia psittaci (C. psittaci), is a zoonotic infectious disease that is typically acquired through exposure to infected birds and their feces. CPP has been identified in numerous countries and regions worldwide. It is estimated that CPP accounts for approximately 1% of all cases of community-acquired pneumonia (CAP) [1]. Although most patients present with mild symptoms, some individuals experience a rapid decline in health, potentially leading to death due to conditions like severe pneumonia, acute respiratory distress syndrome (ARDS), or multi-organ dysfunction syndrome (MODS). This can be attributed to the lack of both timely identification of the disease and targeted antipathogen treatment [2, 3]. The imaging findings of CPP on thoracic CT are often nonspecific, with common features including consolidations, ground-glass opacities and pleural effusion [4]. While these features are frequently observed in CPP, the presence of pulmonary nodules and cavitary lesions in our case expands the spectrum of CT imaging findings associated with this infection, as such features have not been previously documented in the literature.
Case presentation
A previously healthy 16-year-old boy developed cough with scant mucoid sputum, nasal congestion, and rhinorrhea after cleaning his dormitory in early June 2024. After nearly one month of persistent cough, evaluation at a local hospital showed normal infection markers including WBC count (7.41 × 10⁹/L), PCT (0.060 ng/ml), and IL-6 levels (4.39 pg/ml), as well as normal results for other basic parameters (Table 1). Chest CT revealed pulmonary nodules in both lower lobes and a small cavitary lesion in the right lower lobe (Fig. 1a-b), prompting hospitalization for further evaluation and management. No pathogens were identified in sputum samples obtained during hospitalization (Table 1). Mycoplasma pneumoniae (M. pneumoniae) is a major cause of CAP in China, especially in younger populations [5]. In this case, the patient’s persistent dry cough and lab findings (no significant leukocytosis, normal infection markers) aligned with Mycoplasma/Chlamydia infection, prompting the treating physician initiated empirical therapy with doxycycline (100 mg q12h IVgtt). After 4 days of treatment, his cough improved significantly, allowing for discharge. Following discharge, the patient was prescribed oral doxycycline (100 mg q12h) but did not adhere to the recommended dosing schedule. Additionally, he often took the medication with milk, which significantly reduced the bioavailability of doxycycline due to impaired absorption. Approximately 2 weeks later, a follow-up CT revealed a reduction in the size of the right lung lesion but an increase in the size of the left lung lesion (Fig. 1c-d). Due to the absence of significant radiographic improvement with doxycycline, the treatment was switched to cephalosporin for 2 weeks. However, this did not relieve his cough, and a subsequent CT revealed a new cavity in the left lower lobe (Fig. 1e). As a consequence of the radiographic progression, the boy was referred to our hospital for comprehensive evaluation and management. Upon admission, the patient’s vital signs were recorded as follows: height, 172 cm; weight, 64 kg; blood pressure, 139/106 mmHg; pulse rate, 117 bpm; and temperature, 36.8 °C. No abnormalities were detected on cardiopulmonary examination. Laboratory tests revealed a normal WBC count (8.14 × 10⁹/L), CRP (<0.28 mg/L), and PCT (0.029 ng/ml). The (1–3)-β-D-glucan index, T-SPOT test as well as liver, renal, and coagulation function tests were also within normal ranges. Serological tests for hepatitis B surface antigen, hepatitis C virus antibodies, and human immunodeficiency virus antibodies were negative. No pathogens, including bacteria, fungi, and Mycobacterium tuberculosis (M. tuberculosis), were detected in sputum samples. The patient’s imaging presentation coupled with non-response to broad-spectrum antibiotics raised clinical suspicion for ANCA-associated vasculitis. However, negative results for both cytoplasmic (c-ANCA) and perinuclear (p-ANCA) anti-neutrophil cytoplasmic autoantibodies, as well as normal levels of anti-myeloperoxidase (MPO) and anti-proteinase 3 (PR3) antibodies, effectively excluding vasculitis as a potential etiology. On the third day after admission, fiberoptic bronchoscopy was performed, with the BALF subjected to tNGS for respiratory pathogen detection. The test identified 11 nucleic acid sequence reads corresponding to C. psittaci, 62 nucleic acid sequence reads of Klebsiella pneumoniae (K. pneumoniae), and 5 nucleic acid sequence reads of Pseudomonas aeruginosa (P. aeruginosa) (Table 2). Given that C. psittaci is an obligate intracellular bacterium and the low probability of sample contamination with C. psittaci, we established the diagnosis of CPP. The relatively low number of C. psittaci nucleic acid sequences detected by tNGS may be due to its obligate intracellular nature, which limits bacterial shedding from host cells into body fluids such as BALF, thereby reducing detection sensitivity. The patient completed a 14-day course of oral doxycycline (100 mg q12h), resulting in significant cough improvement, and follow-up chest CT showing reduced pulmonary inflammation, lesion absorption, and cavity resolution (Fig. 1f-g). A follow-up image 1 month after discharge revealed that the pulmonary lesions had been almost completely absorbed (Fig. 1h-i).
Table 1.
Laboratory test results
| Laboratory test results | Result | Reference Interval |
|---|---|---|
| White blood cell (×109/L) | 7.41 | 4.0–10.0 |
| Hemoglobin (g/L) | 152.0 | 120–170 |
| Platelet (×109/L) | 293.00 | 100–300 |
| Procalcitonin (ng/ml) | 0.060 | 0.000-0.076 |
| Interleukin-6 (pg/ml) | 4.39 | 0.00-5.50 |
| Erythrocyte sedimentation rate (mm/1 h) | 7.00 | 0.00–15.0 |
| Total Protein (g/L) | 64.1 | 62–85 |
| Albumin (g/L) | 44.3 | 35–53 |
| Globulin (g/L) | 20 | 20–33 |
| Alanine Aminotransferase (U/L) | 10 | 0–38 |
| Aspartate Aminotransferase (U/L) | 13 | 0–38 |
| (1–3)-β-D-glucan index (Pg/ml) | <37.50 | 0–70 |
| Routine sputum culture for bacteria and fungi | Negative | Negative |
| Routine bacterial smear examination of sputum | Negative | Negative |
| Smear examination of sputum for Mycobacterium tuberculosis | Negative | Negative |
Fig. 1.
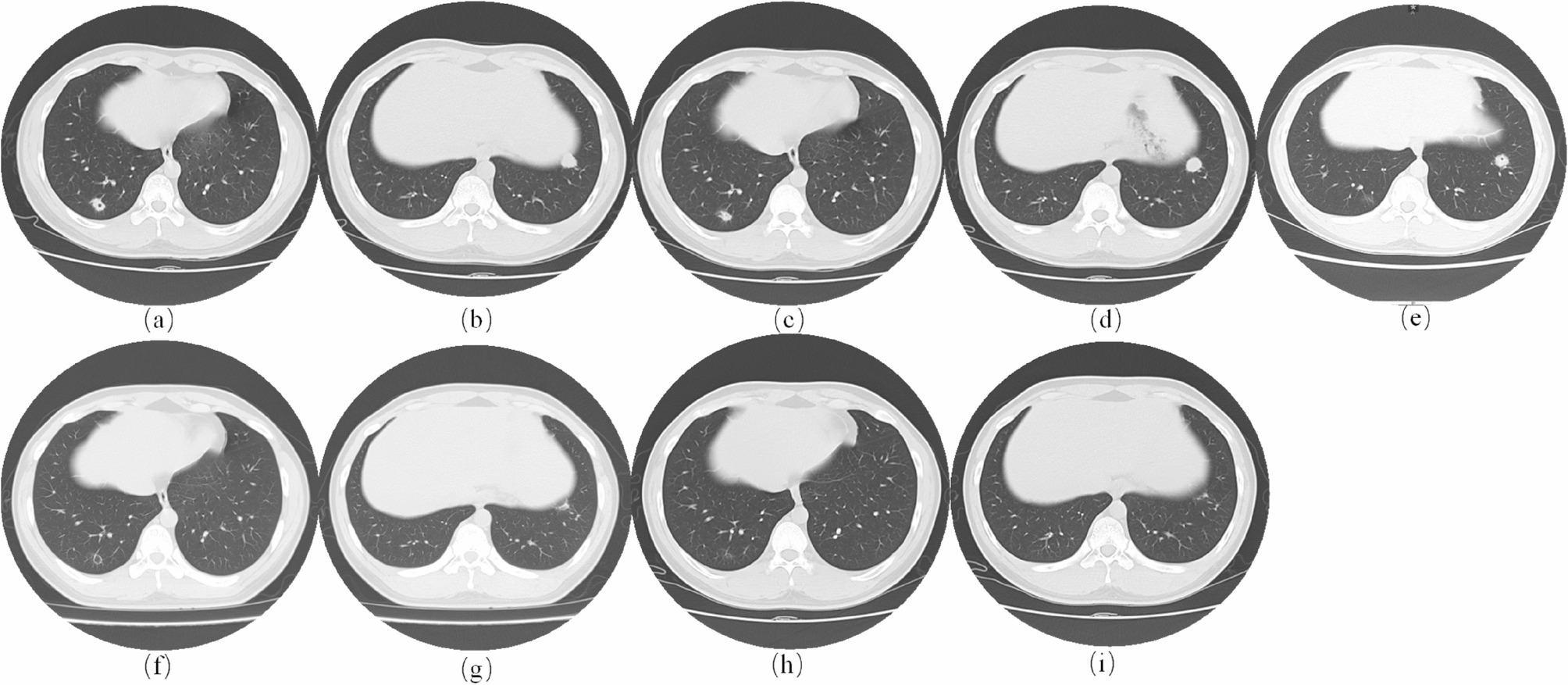
Serial Chest CT Imaging Demonstrates change of Pulmonary Lesions. (a, b) show the baseline chest CT obtained at the local hospital during the patient’s initial evaluation. (c, d) show pulmonary CT after a period of doxycycline treatment. (e) shows chest CT following 2 weeks of cephalosporin therapy. (f, g) present the image after completing 14-day of regular doxycycline treatment. (h, i) show the CT scans 1 month after post-discharge. (a, b) show pulmonary nodules in both lower lobes and a small cavity in the right lower lobe. (c, d) show a reduction in the size of the right lung lesion but an increase in the size of the left lung lesion. (e) shows a new cavity in the left lower lobe. (f, g) show reduced pulmonary inflammation, lesion absorption, and cavity resolution. (h, i) show near-complete resolution of pulmonary lesions. Abbreviation: CT, computed tomography
Table 2.
Targeted next-generation sequencing of BALF
| Microorganisms | Specific Read Counts | |
|---|---|---|
| List of Detected Pathogens | Klebsiella pneumoniae | 62 |
| Chlamydia psittaci | 11 | |
| Pseudomonas aeruginosa | 5 | |
| List of Detected Suspected/Background Microorganisms | Streptococcus intermedius | 232 |
| Streptococcus anginosus | 168 | |
| Corynebacterium striatum | 109 |
Discussion and conclusion
CPP is relatively rare among patients with CAP [1], and clinical diagnosis is challenging due to the limitations of conventional laboratory culture and serological methods [6]. In recent years, increased pet bird ownership has led to a rising incidence of pneumonia caused by C. psittaci, and the development of next-generation sequencing (NGS) technology has facilitated its detection. Notably, in this case, the patient denied any direct contact with birds or poultry, including pets, visits to markets or farms. However, he reported cleaning his dormitory extensively before symptom onset, including dusting window sills, cleaning ventilation systems, and handling accumulated debris. Although no definitive exposure source was confirmed, we speculate that infection may have occurred through inhalation of aerosolized particles from contaminated dust or residual bird droppings in the dormitory environment.
Contrasting with typical CPP presentations, this patient’s CT scan displayed unusual features of pulmonary nodules and cavitary lesions. The specific mechanisms underlying these pulmonary abnormalities remain unclear and require further investigation. Although tNGS of BALF detected only 11 nucleic acid sequence reads of C. psittaci, this result holds significant diagnostic value, especially considering this pathogen’s unique biological characteristics as an obligate intracellular bacterium. C. psittaci relies on viable host cells for replication both in vivo and in vitro, resulting in minimal release of free nucleic acids into body fluids and consequently posing technical challenges for nucleic acid extraction [7]. Several clinical factors further contributed to the limited detection of nucleic acids of C. psittaci. First, the patient’s pulmonary lesions were relatively small and peripherally located, potentially compromising the effectiveness of bronchoalveolar lavage. Second, empirical doxycycline treatment had been initiated prior to specimen collection, which may have reduced the bacterial load in the sample. Given these considerations, tNGS results demonstrating even minimal nucleic acid sequence reads of C. psittaci should be interpreted as clinically significant, as this pathogen can cause severe infections requiring prompt therapeutic intervention.
In this case, based on the radiographic features of pulmonary cavitary lesions, pneumonia caused by fungi, M. tuberculosis, K. pneumoniae and P. aeruginosa were considered in the differential diagnosis. Pulmonary tuberculosis was deemed unlikely due to negative sputum tuberculosis culture, a negative T-SPOT test, and the absence of M. tuberculosis sequences on tNGS. Fungal infection was also ruled out based on a negative sputum fungal smear and culture and a normal (1–3)-β-D-glucan index. As for K. pneumoniae and P. aeruginosa, although tNGS of BALF detected 62 and 5 nucleic acid sequences of K. pneumoniae and P. aeruginosa respectively, these pathogens were unlikely responsible for the pulmonary cavitary lesions based on multiple lines of evidence. First, the patient’s clinical presentation, characterized by persistent dry cough, and laboratory findings, which showed no significant elevation in infection markers, were inconsistent with typical infections caused by these bacterial pathogens. Second, the progression of lesions despite cephalosporin treatment and the significant clinical and radiological improvement achieved with doxycycline monotherapy provided compelling evidence against K. pneumoniae and P. aeruginosa as causative agents. Therefore, we concluded that these pathogens did not contribute to the formation of pulmonary nodules and cavitary lesions in this case.
The radiological manifestations of CPP are diverse and often nonspecific, making diagnosis challenging. Common CT findings include consolidation and ground-glass opacities (GGOs), which are present in the majority of cases [8]. The consolidation patterns often appear as lobar or spherical pneumonia, frequently accompanied by air bronchogram signs indicating preserved airway architecture [9]. GGOs predominantly distributed along perivascular regions and subpleural zones, reflecting interstitial involvement [10]. Pleural effusions are usually unilateral, though severe cases can develop bilateral involvement [11]. Other characteristic radiographic manifestations encompass bronchiectasis, “fine mesh sign”, “halo sign”, “reversed halo sign”. Although these typical imaging patterns are most common, clinicians should be aware that CPP may occasionally present with exceptionally rare patterns, such as pulmonary nodules and cavitary lesions observed in our case, which have limited documentation in the literature. When such atypical radiological findings are accompanied by characteristic clinical features, CPP should be considered in the differential diagnosis. Importantly, this consideration should persist even in the absence of documented direct poultry exposure history, as the lack of classic epidemiological risks does not definitively exclude the diagnosis, and atypical manifestations may delay appropriate treatment.
The diagnostic challenge posed by the nonspecific and variable radiological patterns observed in pulmonary infections has positioned NGS as a transformative diagnostic solution. By performing sequencing of microbial nucleic acids in respiratory samples [12], NGS overcomes the limitations of traditional diagnostic approaches, delivering precise pathogen identification particularly in diagnostically challenging cases with atypical imaging features. This technology has significantly enhanced our diagnostic capabilities for complex pulmonary infections, allowing for the identification of less common pathogens and supporting more targeted treatment approaches.
In conclusion, while CPP typically manifests radiologically as consolidation, ground-glass opacities, and pleural effusion, clinicians should maintain awareness of atypical radiological presentations, including pulmonary nodules and cavitary lesions. This consideration is particularly crucial when evaluating patients with persistent cough showing poor response to conventional antibiotics. The integration of NGS technology into clinical practice is essential for accurate and timely diagnosis, particularly in cases with unusual radiological features.
Supplementary Information
Acknowledgements
None.
Authors’ contributions
HSZ, DHH and YYL wrote the main manuscript and shared the first authorship as co-authors; PY and HGS helped manage the patient; SY, SXC and HMD were involed in the diagnostic process and his treatment. All the authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of.
China (No. 82270024), the Guangdong Basic and Applied Basic Research Foundation, China (Grant No. 2023A1515110216) and the China Postdoctoral Science Foundation (Certification Number: 2023M731546).
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Written consent was obtained from the patient to publish this case report in an online open-access publication. All identifying information has been removed from the manuscript and figures.
Consent for publication
Written informed consent for publication was obtained from all participants.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haishan Zhong, Danhui Huang and Yuying Lin contributed equally to this work.
Contributor Information
Shuang Yang, Email: 329336215@qq.com.
Shaoxi Cai, Email: hxkc@smu.edu.cn.
Hangming Dong, Email: dhm@smu.edu.cn.
References
- 1.Hogerwerf L, DE Gier B, Baan B, VAN DER Hoek W. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect. 2017;145(15):3096–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beeckman DS, Vanrompay DC. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clin Microbiol Infect. 2009;15(1):11–7. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Shi Z, Chen W, Du X, Zhan L. Extracorporeal membrane oxygenation in severe acute respiratory distress syndrome caused by chlamydia psittaci: A case report and review of the literature. Front Med (Lausanne). 2021;8:731047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coutts II, Mackenzie S, White RJ. Clinical and radiographic features of psittacosis infection. Thorax. 1985;40(7):530–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Xiao Y, Zhang G, Li H, Zhao J, Chen M, et al. Identification of priority pathogens for aetiological diagnosis in adults with community-acquired pneumonia in china: a multicentre prospective study. BMC Infect Dis. 2023;23(1):231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rybarczyk J, Versteele C, Lernout T, Vanrompay D. Human psittacosis: a review with emphasis on surveillance in Belgium. Acta Clin Belg. 2020;75(1):42–8. [DOI] [PubMed] [Google Scholar]
- 7.Tang J, Tan W, Luo L, Xu H, Li N. Application of metagenomic Next-Generation sequencing in the diagnosis of pneumonia caused by chlamydia psittaci. Microbiol Spectr. 2022;10(4):e02384–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J, Pan J, Han C, Liu C, Huang J, Yan J, Zhang K, Chen YC. Clinical and CT diagnosis of 50 cases of chlamydia psittaci pneumonia. Quant Imaging Med Surg. 2023;13(4):2053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ni Y, Zhong H, Gu Y, Liu L. Clinical features, treatment, and outcome of psittacosis pneumonia: A multicenter study. Open Forum Infect Dis. 2023;10(2):ofac518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan L, Chen Q, Zhu XY, Lai LM. Evaluation of clinical characteristics and risk factors associated with chlamydia psittaci infection based on metagenomic next-generation sequencing. BMC Microbiol. 2024;24(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang N, Ou Z, Sun Q, Pan J, Wu J, Xue C. Chlamydia psittaci pneumonia - evolutionary aspects on chest CT. BMC Infect Dis. 2025;25(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simner PJ, Miller S, Carroll KC. Understanding the promises and hurdles of metagenomic Next-Generation sequencing as a diagnostic tool for infectious diseases. Clin Infect Dis. 2018;66(5):778–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.


