Abstract
Tree xylem formation is highly dependent on non-structural carbohydrates content and microenvironments. However, it is still less well understood how the key variables regulate cambial activity and xylem formation under different environmental conditions, or the specific contribution of each variable to the number of cells in different stages of xylogenesis. Here, we monitored the xylogenesis and xylem non-structural carbohydrates dynamics of Picea crassifolia during the growing seasons of 2021 and 2022 along an altitude gradient in the Qilian Mountains. We found that the date of maximum cell production rate was about a week later in 2021 than in 2022, and that was later at 2950 and 3200 m than at 2700 m. High altitude sites developed significantly more cambial cells, driving substantially higher cell production rates. Notably, non-structural carbohydrates remained stable early in the growing season before accumulating to peak levels in 2021, whereas 2022 showed a pronounced decrease followed by recovery. The altitude-independent contrast reveals seasonal non-structural carbohydrates dynamics, as the 2022 decline connects stored carbohydrates to sustained xylem formation under drought stress. Linear mixed-effects models showed that, in 2021, cambium cells were predominantly influenced by soil water content and tree individuality, enlargement cells primarily by air temperature (AT), wall-thickening cells by both air and soil temperatures, and the rates of cell production were most significantly affected by AT, soil temperature, and tree individuality. However, in 2022, the cambium cells enlargement cells, and cell production rates were constrained by starch and soluble sugars, while the wall-thickening cells were limited primarily by soluble sugars. Our findings demonstrate that drought triggers a physiological transition from environmental to non-structural carbohydrates mediated control of xylogenesis, highlighting the critical role of carbon reserves for tree resilience in arid regions.
Keywords: carbon allocation, environmental factors, trade-off, water stress, xylogenesis
Introduction
Non-structural carbohydrates (NSC) are the products of photosynthesis, mainly including starch and soluble sugars, which serve as the primary energy substances for tree growth (Deslauriers et al. 2016, A. Wang et al. 2018). As an irreversible process, tree xylem formation is an important indicator of long-term tree growth status (Lu et al. 2022, Wu et al. 2022). During stem growth, non-structural carbohydrates in the xylem are transported to the cambium region, where they support cell division/differentiation and serve as osmotic agents for cell expansion (Klein et al. 2014, Deslauriers et al. 2016). Environmental changes, particularly temperature (T) and moisture variations, directly influence non-structural carbohydrates conversion and allocation for xylem formation (Hartmann and Trumbore 2016, Adams et al. 2017). For example, early growing season warming accelerates starch-to-sugar conversion, while increased precipitation during the growing season enhances photosynthetic product transport—both processes alter non-structural carbohydrates availability for xylem formation (Simard et al. 2013, Adams et al. 2017). However, previous studies have primarily focused on the effect of environmental factors on xylem formation, with limited integration of regulatory mechanisms of non-structural carbohydrates (Muller et al. 2011, Buttò et al. 2020).
The distribution and conversion of non-structural carbohydrates among tree organs directly regulate tree growth processes (Simard et al. 2013). Previous studies have found that higher xylem non-structural carbohydrates levels correlate with faster stem growth (Deslauriers et al. 2016). Similarly, during sprouting or leaf expansion, starch in adjacent branches will be converted to soluble sugars to prioritize energy supply to growing points (Fajardo et al. 2012). However, as global climate change accelerates, extreme drought events are leading to profound consequences for the accumulation of organic matter and tree growth in climate sensitive regions. For instance, in early drought stages, non-structural carbohydrates stored in xylem are rapidly utilized to sustain respiration and restore hydraulic conductivity, whereas prolonged drought ultimately causes xylem embolism and limits tree growth (Martínez-Vilalta et al. 2016). Therefore, it is important to study the regulation of xylem formation by non-structural carbohydrates to deepen the understanding of the stem growth process of trees and their response mechanisms to climate change.
The Qilian Mountains, located on the northeastern edge of the Qinghai-Tibetan Plateau, are a typical representative of mountain ecosystems in the arid regions of northwestern China, and the mountain forest plays an important role in water conservation and maintaining the regional ecological balance. Over the past 50 years in this region, the average annual T has increased at a rate of 0.26 °C every 10 years, the interannual variability of the number of extremely high-T days is 0.79 days per year, and the average annual precipitation is on an increasing trend (Du et al. 2014, Li et al. 2024). As the dominant tree species, Picea crassifolia (Qinghai spruce) accounts for more than 80% of the total tree area, and is mostly distributed as pure forests on shady and semi-shady slopes. The sensitivity of Qinghai spruce to climate change has led to its widespread use in dendroclimatological growth–climate relationship studies and past climate reconstruction (Gao et al. 2018, Wang et al. 2021). However, there is still a lack of research on how xylem non-structural carbohydrates affect the xylem formation process of Qinghai spruce, which limits the understanding of the physiological mechanisms by which carbon supply affects stem growth.
In this study, xylogenesis and xylem non-structural carbohydrates dynamics of Qinghai spruce and environmental factors were monitored at three altitudes in the wetter 2021 and drier 2022 within the Qilian Mountains. The objectives were to (i) examine the differences in xylem formation and non-structural carbohydrates dynamics along the altitude gradient. (ii) evaluate the effects of drought on seasonal variation of xylem formation and non-structural carbohydrates, and (iii) quantify the effects of non-structural carbohydrates and environmental factors on the number of cells in the cambium, enlargement, wall-thickening and rates of cell production. Considering the distinction in dry/wet conditions during study years, we hypothesized that (H1) drought reshapes seasonal stem growth dynamics and depletes non-structural carbohydrates reserves, and (H2) drought shifts the primary drivers of xylogenesis by suppressing T dependency and intensifying non-structural carbohydrates limitation of cell differentiation.
Materials and methods
Study site and experimental design
The study area is located in the Pailugou watershed in Xishui Nature Reserve in the middle part of Qilian Mountains in China (100°17′6″–100°18′26″E, 38°31′42″–38°33′29″N). The watershed covers an area of 2.91 km2, with an altitude of 2640–3800 m, and the soil type is dominated by mountain chestnut calcium soil and gray-brown soil, with an average thickness of ~80 cm. According to the records of the Qilian Mountains Forest Ecosystem Observatory located at 2700 m since 2001, the average annual T is 0.5 °C, the average annual precipitation is 376 mm, of which May–October precipitation accounts for more than 80% of the annual precipitation, the annual evaporation is up to 1400 mm, the annual sunshine duration is 1893 h and the region belongs to the temperate continental climate. The vegetation in this area is distributed along the elevation gradient, with Qinghai spruce as the dominant tree species, accounting for more than 70% of the total tree area, and mostly pure forests distributed in patches or strips on shady slopes and semi-shady slopes at an altitude of 2640–3200 m. In this study, to minimize growth variability within each altitude group, five Qinghai spruce trees were selected at altitudes 2700, 2950 and 3200 m based on their similarity in height, diameter at breast height, and age (Table S1 available as Supplementary Data at Tree Physiology Online). The process of xylem formation and the xylem non-structural carbohydrate content were monitored from 25 April to 15 September, 2021, and from 15 April to 10 September, 2022, respectively.
Xylem activity
To monitor xylem formation during the growing season, we collected tree microcore samples every 5–10 days using a trephor tool from each sample tree at breast height. Two microcores were collected from each sample tree to ensure that they were not broken. The collected microcores were quickly put into FAA solution. In the laboratory, each microcore sample was individually placed into numbered embedding boxes. The samples underwent dehydration through sequential immersion in 75%, 90%, 95% and 100% ethanol, followed by D-Limonene, each for 90 min. After dehydration, the samples were embedded in paraffin. Using the rotary microtome (Leica Microsystems, Wetzlar, Germany), the embedded microcores were sectioned into 9–12 μm thickness and mounted onto glass slides. For dewaxing, the sections were stained with D-Limonene and ethanol for 15 min, respectively. Subsequently, composite staining was performed by immersing the sections in 3% safranine (Merck, Darmstadt, Germany) and 0.5% Astra Blue (Sigma-Aldrich, Steinheim, Germany) solutions sequentially for 20 min each. Finally, permanent tissue sections were prepared using a permanent mounting medium (Rossi et al. 2006).
Under a high magnification microscope, three rows of cells were selected from tissue sections where the process of xylem differentiation was evident, and the cells characteristics (numbers, onset, end and duration) of cambium, cell enlargement, cell wall thickening and mature cell were observed in each sample tree. Based on the histological observations, the growing seasons in 2021 ranged from day of year (DOY) 132 ± 3 to DOY 239 ± 7, while in 2022 they ranged from DOY 128 ± 10 to DOY 236 ± 5 at three altitudes. The Gompertz equation was used to fit the cell number of each sample tree:
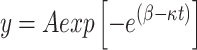 |
(1) |
where y is the total number of xylem cells, t is the day of year, A is the upper asymptote, and β and κ are the x-axis intercept and curve change rate parameter, respectively. The cell division rates were calculated from the first-order difference of the Gompertz equation.
Non-structural carbohydrates
To monitor the changes in xylem non-structural carbohydrates content during the growing season, we collected tree-ring cores from approximately the outer 3 cm of xylem at the breast height of each sample tree every 10–15 days using a 12 mm increment borer. Once collected, samples were immediately placed in ice before transport to the laboratory (Gričar et al. 2019, Signori et al. 2021). In the laboratory, samples were put into a microwave oven to inactivate enzymes at high T for 90 s, and dried in an oven at 70 °C until constant weight, then crushed and passed through a 0.25 mm sieve. The anthrone-sulfate method was used to measure the soluble sugar (SS, %) and starch (ST, %), and the total non-structural carbohydrate (%). Then 0.05 g of the powder sample was dissolved in 80% ethanol, and the soluble sugar test solution was extracted by filtration, decolorization, and volume determination. The filtered precipitate was pasted in distilled water, and then the starch test solution was extracted by dissolving in perchloric acid and fixing the volume. Finally, the anthrone-sulfate reagent was added to the test solution after dilution with distilled water, and water bath at 100 °C for 10 min. The absorbance values were read at 625 nm using an enzyme-labeling measuring instrument (Multiskan FC; Thermo, Waltham, MA, USA), and the contents of non-structural carbohydrates were calculated based on the standard curve for glucose (Mei et al. 2023).
Environmental data
During the 2021–2022 growing season, automatic weather stations were installed in open areas within 50 m of the sample plots at each altitude to record precipitation (P, mm) (TRWS205, MPS systém Ltd, Bratislava, Slovakia), air temperature (AT, °C) and relative air humidity (RH, %) (HMP155, Vaisala Oyj, Vantaa Finland) automatically. Meteorological data were recorded by a data logger CR1000 (Campbell Scientific, Inc., Logan, UT, USA). Daily mean AT was extracted from the values of AT. The vapor pressure deficit (VPD, kPa) was calculated from the AT and relative air humidity values. According to the main rooting zone of sampled tree species, soil temperature (Ts, °C) and soil water content (SWC, m3 m−3) at a depth of 20 cm soil layer were continuously monitored using 5TM sensors (Decagon Devices, Inc., Pullman, WA, USA) in each sample plot. All the above environmental factors were recorded every 30 min in synchrony, and subsequent analyses were completed using the mean or cumulative values (P) of the sampling intervals.
Comparative analysis of environmental conditions during the study period (DOY 110–263) reveals 2022 was markedly drier than 2021, characterized by a 0.5–0.8 °C mean T increase, 6.1–12.7% P reduction and 0.01–0.04 m3 m−3 soil water content decline. Crucially, rainfall patterns were frequent and evenly distributed throughout May to June 2021. In contrast, the same period in 2022 saw fewer but heavier downpours. During that time, rainy days decreased by 31–61% compared with 2021, and over 80% of the seasonal rainfall occurred in just several events, punctuated by prolonged dry spells. Given the combined effects of elevated temperatures, reduced precipitation frequency, and accelerated soil moisture depletion, this study designates 2022 as a relative drought year.
Statistical analyses
ANOVA and Tukey’s test were used to evaluate differences in environmental factors and cell numbers for three altitudes. Generalized additive models for location scale and shape (GAMLSS) were fitted using the ‘gamlss’ function of the R package Gamlss, Version 5.4–20 to describe seasonal changes in soluble sugar, starch and total non-structural carbohydrate of xylem for three altitudes. The model enabled us to formulate the mean (μ) and the standard deviation (σ) of the fitted distribution as functions of the explanatory variables: DOY and the altitudes. In all cases, we first modeled μ as a function of altitudes and DOY as a smoother term. The models were chosen depending on the Akaike information criterion (AIC), goodness of fit was accepted when residuals mean and coefficient of skewness were close to 0, residuals variance was nearly 1 and coefficient of kurtosis was nearly 3.
Linear Mixed-Effects Models (LMM) were fitted using the ‘lmer’ function of the R package lme4, Version 1.1–34 to quantify the effect of environmental factors and non-structural carbohydrates on cambium cells, enlargement cells, wall-thickening cells and rates of cell production during 2021 and 2022, respectively. Previous to the LMMs all the numeric variables were standardized (z-score). In the full LMMs, all the environmental factors and non-structural carbohydrates were treated as fixed factors, while tree ID were treated as a random factor. Then, a variance inflation factor (VIF) analysis was performed to detect multicollinearity and the variables were removed until the highest VIF value was < 4. (R package ‘car’, Version 3.1–2). Finally, the best model was chosen depending on the AIC, marginal and conditional R2 for LMMs were computed with the ‘r.squaredGLMM’ function (R package ‘MuMIn’, Version 1.47.5), and the contribution of independent and random variables to the xylogenesis was calculated with the ‘partR2’ function (R package ‘partR2’, Version 0.9.1).
Results
Environmental conditions
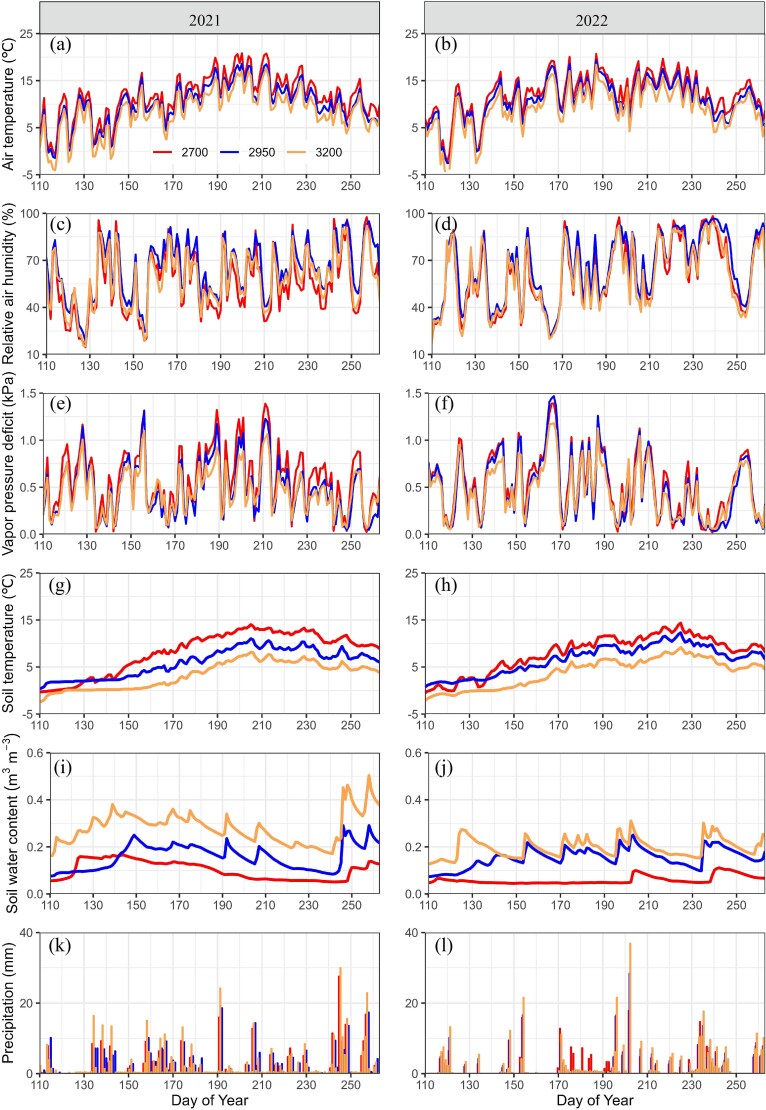
Significant differences in environmental factors were observed at the three altitudes from May to September of the study years (Figure 1, Table 1). From high to low altitudes, the mean AT ranged from 8.41 to 11.61 °C and from 9.24 to 12.12 °C in 2021 and 2022, respectively, with the highest AT occurring in July. The trend of the soil temperatures was dependent on the AT. The mean relative air humidity was 55.68, 64.22 and 57.99% in 2021 and 60.09, 65.60 and 59.73% in 2022 at the three altitudes, respectively. The mean VPD was with the highest values occurring in July. There were significant differences in soil water content at the three altitudes with mean values of 0.10, 0.16 and 0.28 m3 m−3 in 2021 and 0.06, 0.15 and 0.20 m3 m−3 respectively, with the highest at 3200 m. The cumulative P at the three altitudes was 281.5, 297.6 and 413.6 mm in 2021 and 264.4, 277.8 and 361.2 in 2022, respectively.
Figure 1.
Seasonal variation of AT (a, b), relative air humidity (c, d), VPD (e, f), Ts (g, h), soil water content (i, j) and P (k, l) at the three altitudes during 2021–2022.
Table 1.
Environmental characteristics observed at the three altitudes during the growing season of 2021–2022. Different letters mean significantly different at P < 0.05. P: total precipitation during growing season.
| Year | Altitude | T | RH | VPD | Ts | SWC | P |
|---|---|---|---|---|---|---|---|
| 2021 | 2700 m | 11.61 ± 4.67c | 55.68 ± 19.92a | 0.59 ± 0.32b | 8.31 ± 4.44c | 0.10 ± 0.04a | 281.5 |
| 2950 m | 9.96 ± 4.44b | 64.22 ± 17.96b | 047 ± 0.34a | 5.99 ± 2.97b | 0.16 ± 0.05b | 297.6 | |
| 3200 m | 8.41 ± 4.60a | 57.99 ± 17.67ab | 0.46 ± 0.24a | 3.38 ± 2.85a | 0.28 ± 0.07c | 413.6 | |
| 2022 | 2700 m | 12.12 ± 4.38c | 60.09 ± 21.11ab | 0.54 ± 0.33b | 8.95 ± 3.95c | 0.06 ± 0.02a | 264.4 |
| 2950 m | 10.76 ± 4.34b | 65.60 ± 21.07b | 0.48 ± 0.34a | 5.65 ± 3.07b | 0.15 ± 0.04b | 277.8 | |
| 3200 m | 9.24 ± 4.44a | 59.73 ± 20.96a | 0.46 ± 0.29a | 3.91 ± 3.06a | 0.20 ± 0.04c | 361.2 |
At 2700 m, AT, Ts and VPD were significantly higher, and soil water content and P were lower than at other altitudes. Compared with the 2 years, AT and Ts were lower, and soil water content and precipitation were higher in 2021 than in 2022.
Seasonal variation of xylem formation
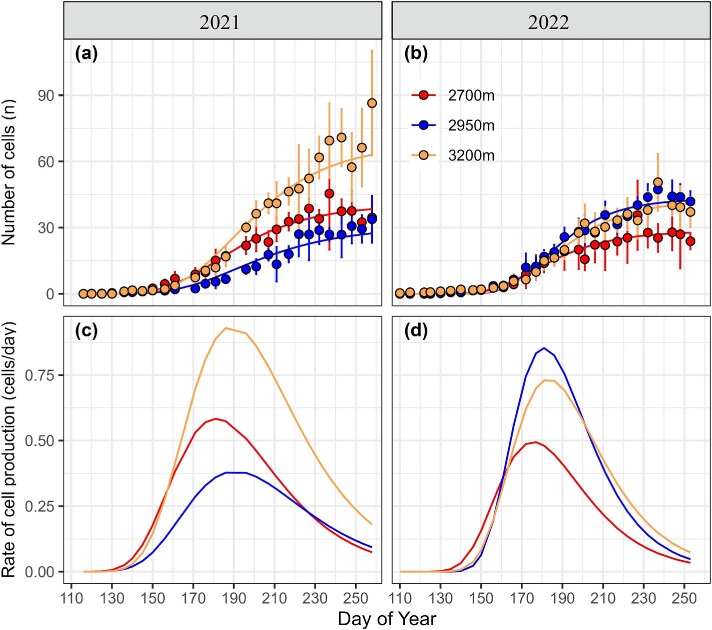
From low to high altitudes, the accumulated xylem cell numbers were 34.56 ± 6.34, 33.77 ± 10.63 and 86.44 ± 23.76 in 2021 and 23.83 ± 3.66, 41.85 ± 4.72 and 37.10 ± 6.82 in 2022, respectively (Figure 2a and b). Xylem growth patterns were fitted by the Gompertz function with a high goodness of fit, explaining 95–98% of the variations. From low to high altitudes, the rate of cell production simulated by Gompertz functions showed a mean cell production rate of 0.36, 0.23 and 0.57 cells per day in 2021 and 0.30, 0.52 and 0.45 cells per day in 2022, respectively (Figure 2c and d). The date of maximum production rate appeared at DOY 187 ± 4 in 2021, while the maximum rate occurred about a week earlier, specifically at DOY 179 ± 3 in 2022.
Figure 2.
Observed (dots) and Gompertz functions fitted (lines) to cumulative xylem growth (a, b) and the estimated the growth rates (c, d) at the three attitudes during 2021–2022. Error bars present mean ± SD (n = 5).
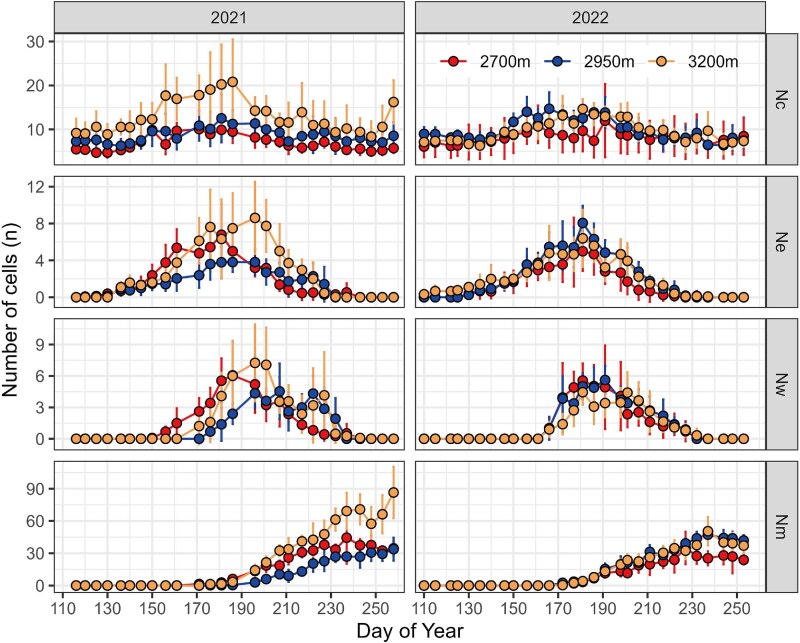
In 2021 and 2022, the cell number in cambium, enlargement and wall-thickening showed an inverted ‘U’ curve over time, and the mature showed an ‘S’ curve (Figure 3). Comparing the two years, the number of cells in the cambium and mature varied significantly with altitude in 2021 but showed no altitudinal difference between 2950 m and 3200 m in 2022 (Table 2). Additionally, xylem differentiation cells decreased at 2700 m and 3200 m but increased at 2950 m in 2022.
Figure 3.
Seasonal variation of cells number in Nc (cambium), Ne (enlargement), Nw (wall-thickening) and Nm (mature) at the three altitudes during 2021–2022. Error bars present mean ± SD (n = 5).
Table 2.
Seasonally mean number of cells in cambium, enlargement, wall-thickening and mature at the three altitudes during the growing season of 2021–2022. Different letters mean significantly different at P < 0.05.
| Year | Altitude | Cambium | Enlargement | Wall-thickening | Mature |
|---|---|---|---|---|---|
| 2021 | 2700 m | 6.88 ± 1.74a | 1.80 ± 2.09a | 1.43 ± 1.90a | 14.4 ± 15.94b |
| 2950 m | 8.61 ± 1.63b | 1.48 ± 1.34a | 1.22 ± 1.62a | 8.70 ± 11.33a | |
| 3200 m | 12.74 ± 3.68c | 2.66 ± 2.89a | 1.73 ± 2.33a | 21.69 ± 26.03c | |
| 2022 | 2700 m | 8.05 ± 1.10a | 1.36 ± 1.64a | 1.29 ± 1.84a | 11.13 ± 11.85a |
| 2950 m | 9.60 ± 2.23b | 2.04 ± 2.33a | 1.34 ± 1.77a | 16.65 ± 17.95b | |
| 3200 m | 9.42 ± 2.38b | 2.00 ± 1.89a | 1.14 ± 1.53a | 15.7 ± 16.93b |
Seasonal variation of non-structural carbohydrates
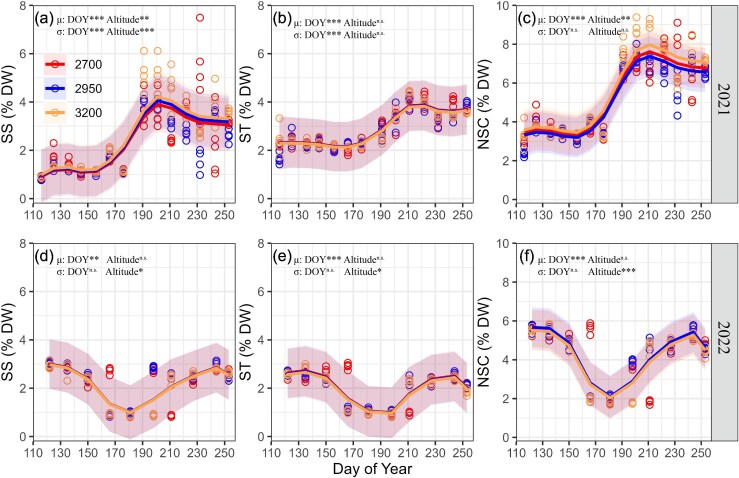
The generalized additive model showed that xylem non-structural carbohydrates (soluble sugar, starch and total non-structural carbohydrate) showed significant seasonal variations (χ2, P < 0.01 for DOY) (Figure 4). In 2021, non-structural carbohydrates maintained relatively stable levels until DOY 170, followed by accumulation peaking around DOY 200, and finally declining toward the season end. Conversely, in 2022, sustained depletion persisted until DOY 180, then recovery initiated culminating in peak accumulation at DOY 240, prior to late-season decline. The average content of soluble sugar, starch and total non-structural carbohydrate were 2.38 ± 1.32, 3.03 ± 0.83 and 5.41 ± 1.91 in 2021, and 2.31 ± 0.77, 2.06 ± 0.67 and 4.37 ± 1.37 in 2022, respectively. The average content of soluble sugar and total non-structural carbohydrate differed significantly among the three altitudes in 2021 (χ2, P < 0.01 for Altitude), with the lowest at 2700 m and the highest at 3200 m. Starch in 2021 and non-structural carbohydrates in 2022 did not differ significantly among the altitudes (χ2, P > 0.05 for Altitude). Comparing the two years of non-structural carbohydrates, the average contents were higher in 2021 than in 2022.
Figure 4.
Seasonal variation of soluble sugar (a, d), starch (b, e) and total non-structural carbohydrate (c, f) of xylem in Qinghai spruce according to the fitted GAM models at the three altitudes during 2021–2022. Dots indicate observed data. Lines and shaded areas indicate mean and SD. Significance of DOY and altitude is shown for μ and σ (*P < 0.05, **P < 0.01, ***P < 0.001, and n.s. represent not significant).
Response of stem growth to environmental factors and non-structural carbohydrates
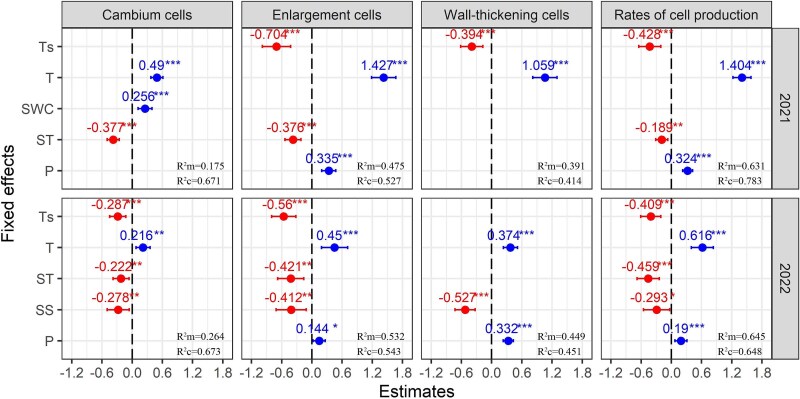
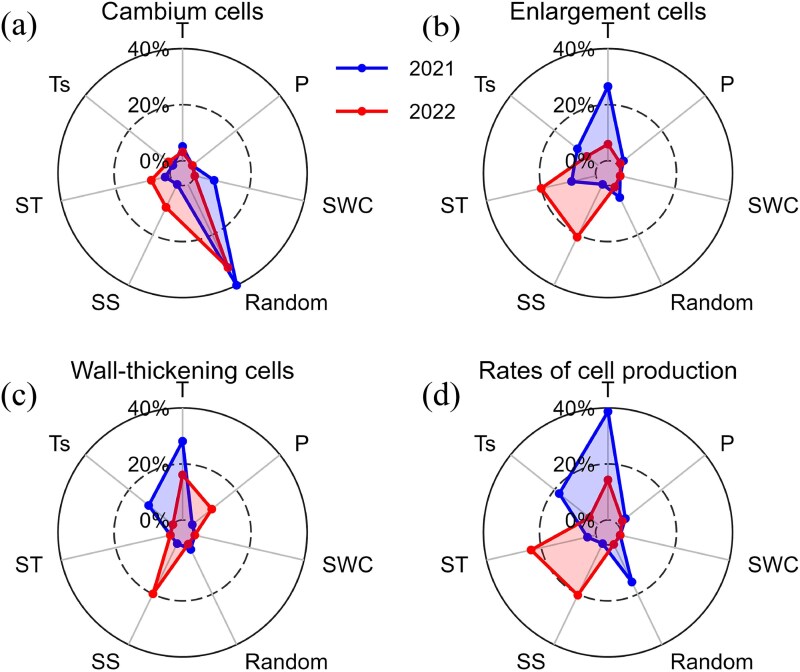
Linear mixed-effects modeling results showed that the fixed part explained (marginal R2) 17.5%, 47.5%, 39.1% and 63.1% of the cambium cells, enlargement cells, wall-thickening cells and rates of cell production in 2021, and 26.4%, 53.2%, 44.9% and 64.5% in 2022, respectively (Figure 5, Table S2 available as Supplementary Data at Tree Physiology Online). The random part explained 49.6%, 5.2%, 2.3% and 15.2% in 2021, and 40.9%, 1.1%, 0.2% and 0.3% in 2022, respectively (difference between conditional R2 and marginal R2). In 2021, cambium cells showed positive associations with AT and soil moisture, but negative correlation with starch. Enlargement cells and the rates of cell production increased with AT and precipitation, while decreasing with Ts and starch. Wall-thickening cells affected by T, which positively correlated with AT and negatively correlated with Ts. The contributions analysis highlighted soil moisture and tree individuality as primary drivers of cambium activity, while AT dominated cells enlargement (Figure 6). A dual thermal influence (air and soil temperatures) governed wall-thickening processes, and rates of production were most complexly regulated by T and tree individuality.
Figure 5.
Fixed effects of the final LMM models on cambium cells, enlargement cells, wall-thickening cells and rates of cell production during 2021–2022. R2c and R2m represent conditional R2 and marginal R2, respectively. Error bars present mean ± SE. Significance is shown for a given term (*P < 0.05, **P < 0.01, ***P < 0.001). P: precipitation.
Figure 6.
Partition of the variables on cambium cells (a), enlargement cells (b), wall-thickening cells (c) and rates of cell production (d) during 2021–2022. P: precipitation; Random: random variable of altitude.
The 2022 growing season exhibited marked shifts in regulatory mechanisms. Cambium cells became decoupled from soil moisture but showed negative correlations with Ts and soluble sugars. Enlargement cells and rates of cell production negatively correlated with soluble sugar. Wall-thickening cells did not correlated with Ts, but positively correlated with precipitation and negatively correlated with soluble sugar. Notably, tree individuality gained prominence in cambium regulation, while carbohydrate metabolism emerged as a systemic constraint across all developmental stages in 2022.
Discussion
Seasonal xylem formation and non-structural carbohydrates dynamics
This study on Qinghai spruce showed that seasonal variation of cambial activity and xylem formation, as well as non-structural carbohydrates contents, changed between years and altitudes, supporting the hypothesis H1 that drought reshapes seasonal stem growth dynamics and depletes non-structural carbohydrates reserves. In the drier 2022 year, the mean cell production rate declined at all altitudes except 2950 m. This was because the mean soil water content at 2950 changed little over the two years, while it decreased more at the other altitudes. Thus, relatively weak changes in moisture conditions had a low impact on growth and could ensure growth requirements. A comparison of the date of maximum cell production rates revealed that it was about a week earlier in the drier year (2022) than in the wetter year (2021) and earlier in the same year at the drier lower altitude (2700 m) than at the wetter higher altitudes (2950 m and 3200 m). Early studies generally recognized that the date of maximum cell production rates was related to the photoperiod and synchronized with the summer solstice (Duchesne et al. 2012, Kalliokoski et al. 2013). However, more and more studies have found that photoperiod may only roughly regulate the date of maximum cell production rates. Concurrently, the microenvironment, light demand for photosynthesis and carbon supply also have an effect on the peak growth rates (Rathgeber et al. 2011, Jyske et al. 2014, Tombesi et al. 2015, Pasqualotto et al. 2021). In this study, in 2022, the combination of higher temperatures, poorer moisture conditions and lower non-structural carbohydrates contents at 2700 m may have led to an earlier timing of maximum growth rates, which was consistent with the previous study (Tian et al. 2024).
Comparing the two years, cambial and mature cell counts became similar at 2950 and 3200 m in 2022. This convergence likely resulted from differing precipitation declines. The sharpest rainfall reduction occurred at 3200 m, disproportionately suppressing its growth. Meanwhile, smaller precipitation losses at 2950 m better maintained cell production. Additionally, the decrease in xylem differentiation cells at 2700 and 3200 m during the drier 2022 contrasted with increased production at 2950 m. This divergence likely stemmed from site-specific stress thresholds. At 2700 m, extreme drought stress pushed soil water content to critically low levels, directly constraining cell differentiation. Meanwhile, 3200 m faced drought combined with relatively low soil temperatures, slowing metabolic activity despite moderate moisture. In contrast, 2950 m experienced milder water reduction and maintained optimal thermal conditions, allowing sustained cell differentiation. Previous studies have also found that warming during periods of high moisture availability favors tree growth in the subalpine forest, while increased T exacerbates the stressful effects of moisture when moisture is the limiting factor (Jyske et al. 2014, Andrus et al. 2018). Moreover, water deficit reduces turgor in the cells in the dry season, affecting cell division and expansion, ultimately leading to lower growth (Peters et al. 2021).
Non-structural carbohydrates, as the products of photosynthesis, are the energy substances that supply the growth of trees, and their seasonal changes will regulate the growth (Deslauriers et al. 2016, A. Wang et al. 2018). Drought is a key driver causing the seasonal variations of non-structural carbohydrates, and carbon storage plays an important role in maintaining tree function during droughts (Wiley and Helliker 2012, Liu et al. 2018). In this study, seasonal dynamics of xylem non-structural carbohydrates in Qinghai spruce diverged markedly between years, with 2022 exhibiting a substantial depletion until DOY 180 whereas 2021 maintained stable levels during the same period. This divergence arose from drought-induced carbon imbalance in 2022, activating Qinghai spruce’s inherent capacity to sacrifice stored carbohydrates for growth maintenance under hydraulic stress. In the wetter year of 2021, photosynthesis was strong, the source activity was greater than the sink activity, assimilation carbon was sufficient to meet growth requirements and respiration, and soluble sugars accumulated as starch (Y. Wang et al. 2018, Guo et al. 2022). However, in May–June 2022, reduced precipitation frequency combined with prolonged dry spells created persistent drought stress. This continuous water deficit suppressed photosynthetic carbon gain, leading to insufficient carbon supply relative to growth demands. Consequently, starch reserves were progressively converted to soluble sugars to sustain metabolic activity, driving a substantial decline in non-structural carbohydrate storage (Sala 2009, McDowell et al. 2008, Palacio et al. 2014). Previous studies have shown that lower photosynthetic rates in the early growth season are unable to meet the demands of growth and respiration, and non-structural carbohydrates stored in plant organs are remobilized for growth, causing a reduction in non-structural carbohydrates (Martínez-Vilalta et al. 2016, Furze et al. 2019). Moreover, as a gymnosperm, Qinghai spruce has a wide hydraulic safety boundary and can sacrifice stored non-structural carbohydrates to maintain growth during drought (Johnson et al. 2012, Adams et al. 2017, Piper and Paula 2020). This also suggested that non-structural carbohydrates near the cambium can be homeostatically maintained within relatively tight limits to ensure metabolic stability for tree growth (Carteni et al. 2018). Non-structural carbohydrates started to accumulate in 2022 from July (about DOY 180). This may be due to the fact that cell numbers in enlargement and wall-thickening decreased and the rate of cell production reduced, where assimilated carbon from photosynthesis is sufficient for growth and excess carbon supply is stored as non-structural carbohydrates (Furze et al. 2019). In addition, the accumulation is also an adaptive mechanism for plants to improve cold tolerance and buffer against periods of carbon deficit, which plays an important role in winter dormancy and spring resumption of growth (Lintunen et al. 2016).
Effects of environmental factors and non-structural carbohydrates on xylem formation
This study on Qinghai spruce showed that environmental factors affect the entire process of xylem formation during the two years, while soluble sugar and starch contents mainly affect xylem formation in the drier 2022, which supports hypothesis H2 that drought shifts the primary drivers of xylogenesis by suppressing T dependency and intensifying non-structural carbohydrates limitation of cell differentiation. The AT affects the entire process of xylem formation, and previous studies have suggested that air AT is positively correlated with radial growth in cold environments because it can accelerate cell production (Balducci et al. 2016, Dolezal et al. 2019). Other study has shown that xylem cell production rates are greatest when temperatures are in the range of 10–25 °C (Körner 2003). During the growing season in this study, the mean daily temperatures were largely within this range, providing sufficient heat for tree growth. Soil moisture is directly absorbed by the roots and Ts mainly influences soil evapotranspiration, and both can affect plant’s water availability (Ni et al. 2019). Precipitation impacts canopy transpiration and roots’ water availability by changing air humidity and soil moisture, respectively (Wu et al. 2021). Soluble sugars and starch provide the solutes for maintaining cell turgor and carbon for xylem formation during tree growth (Deslauriers et al. 2016, A. Wang et al. 2018).
In 2021, the number of cambium cells exhibited a positive association with AT and soil water content, while displaying a negative correlation with starch. However, in 2022, this pattern changed, revealing a negative correlation with Ts and soluble sugars, rather than soil moisture content. Increasing temperatures have contributed to the early initiation of cambium activity (Zhang et al. 2023). By observing different altitudes, we noticed a corresponding increase in T with decreasing altitude, which led to an earlier start to cambium activity at low altitudes. In addition, we found that the mean number of cambium cells increased irregularly with altitude. This phenomenon suggested that low temperatures delay growth onset and shorten cambium activity duration, causing disproportionate cell production changes. Such shifts may accelerate cell division rates temporarily for environmental adaptation (Rossi et al. 2014). At the same time, higher Ts increases soil evapotranspiration and thus reduces soil water content, which will affect water availability by plants and control cell division in the cambium. Other studies have shown that AT and moisture conditions together determine the cambium activity, with activity beginning when both reached thresholds (Ren et al. 2018, Cabon et al. 2020). However, the number of cambium cells was not correlated with soil water content, but was negatively correlated with Ts and soluble sugars in the drier 2022. This implies that soil water promotes cambium activity only under sufficient carbon supply.
The enlargement stage of xylem formation is particularly sensitive to moisture conditions, and the rate of cell expansion significantly influences the annual wood production (Garcia-Forner et al. 2019). Thus, we observed consistent factors affecting the enlargement cells and rates of cell production. Perturbation of soil moisture conditions by precipitation and Ts will significantly affect cell expansion. Previous studies have also shown that T and moisture conditions affect wood yield by controlling the rate of cell expansion (Ren et al. 2019, ). In addition, water and soluble sugars absorbed by the plant act as solvents and solutes respectively to maintain cell turgor, and adequate water and high soluble sugar content will contribute to cell expansion (Deslauriers et al. 2016, A. Wang et al. 2018, Garcia-Forner et al. 2019). In our study, cell expansion was negatively correlated with starch in 2021 and with both starch and soluble sugars in 2022. This pattern reflects a carbon allocation trade-off where storage is prioritized over radial growth in wet years, while during drought starch converts to sugars to support xylem formation.
Cell wall-thickening during xylem formation is the stage of greatest carbon demand, and most of the carbon fixation in the xylem occurs during this process (Cuny et al. 2015). The cell wall-thickening process utilizes soluble sugars, however it was not correlated with xylem non-structural carbohydrates in 2021 and was only negatively correlated with starch in 2022. This discrepancy may stem from the adequacy of carbon fixed through photosynthesis, which was sufficient for the thickening of cell walls in wetter years. However, in drier years, the carbon fixed by photosynthesis as well as the carbon stored in the xylem were insufficient to support the thickening of cell walls, thereby restricting growth (Garcia-Forner et al. 2019, Guo et al. 2022). This study has also revealed that Ts exhibited a negative correlation with cell wall thickening in 2021, whereas precipitation demonstrated a positive correlation with the process in 2022. Both Ts and precipitation have a significant impact on root water uptake, which subsequently influences cell turgor and hence cell wall thickening (Cavender-Bares 2016, Carteni et al. 2018, Vieira et al. 2020).
Overall, cambium activity and xylem formation were primarily influenced by T and moisture conditions in the wetter 2021, whereas they were predominantly shaped by non-structural carbohydrates stored in the xylem during the drier 2022. This change resulted from a shift in rainfall patterns from steady light rain to long dry spells with only several heavy downpours. These processes deplete non-structural carbohydrates reserves, which in turn restricts xylem formation through carbon limitation. Over the past decades, the Qilian Mountains have experienced a significant increase in T and extreme precipitation frequency, with projections indicating intensified seasonal precipitation disparities (Du et al. 2014, Li et al. 2024). These precipitation seasonality shifts will amplify ecological risks to tree growth stability through carbon allocation disruption.
Conclusions
Our study showed that in drought environments, the date of maximum cell production rate advanced to adapt to the change of microenvironment, the number of cells in the cambium and xylem differentiation reduced due to lower soil water content. Cambium activity and xylem formation were mainly influenced by T and moisture conditions in the wetter 2021, whereas they were mainly influenced by non-structural carbohydrates stored in the xylem in the drier 2022. The trend in xylem formation and non-structural carbohydrates suggest that there is a trade-off between carbon accumulation and xylem formation in wetter years, while stored carbon in xylem serves as a carbon source for xylem formation in drier years, reflecting the adaptive strategy of Qinhai spruce to ensure a steady carbon supply for growth. Considering the increasing trend in precipitation and AT in the Qilian Mountains over the past decades, it is likely that climate changes in this region may promote the growth of Qinghai spruce.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 42277481, 42007410, 42261134537, 42171167, 42207537), the Russian Science Foundation (RSF-23-44-00067) and the Ministry of Science and Higher Education of the Russian Federation (FSRZ-2023-0007).
Contributor Information
Quanyan Tian, State Key Laboratory of Ecological Safety and Sustainable Development in Arid Lands, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Linze Inland River Basin Research Station, Chinese Ecosystem Research Network, Lanzhou 730000, China.
Zhibin He, State Key Laboratory of Ecological Safety and Sustainable Development in Arid Lands, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Linze Inland River Basin Research Station, Chinese Ecosystem Research Network, Lanzhou 730000, China.
Shengchun Xiao, State Key Laboratory of Ecological Safety and Sustainable Development in Arid Lands, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Linze Inland River Basin Research Station, Chinese Ecosystem Research Network, Lanzhou 730000, China.
Bao Yang, School of Geography and Ocean Science, Nanjing University, Nanjing 210023, China.
Xiaomei Peng, State Key Laboratory of Ecological Safety and Sustainable Development in Arid Lands, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Linze Inland River Basin Research Station, Chinese Ecosystem Research Network, Lanzhou 730000, China.
Xiangyan Feng, Key Laboratory of Restoration and Reconstruction of Degraded Ecosystems in Northwestern China of Ministry of Education, Ningxia University, Yinchuan 750021, China.
Pengfei Lin, State Key Laboratory of Ecological Safety and Sustainable Development in Arid Lands, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Linze Inland River Basin Research Station, Chinese Ecosystem Research Network, Lanzhou 730000, China.
Xi Zhu, State Key Laboratory of Ecological Safety and Sustainable Development in Arid Lands, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Linze Inland River Basin Research Station, Chinese Ecosystem Research Network, Lanzhou 730000, China.
Jingjing Liu, State Key Laboratory of Ecological Safety and Sustainable Development in Arid Lands, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Linze Inland River Basin Research Station, Chinese Ecosystem Research Network, Lanzhou 730000, China.
Eugene A Vaganov, Institute of Fundamental Biology and Biotechnology, Siberian Federal University, Krasnoyarsk 660041, Russia.
Vladimir V Shishov, Institute of Fundamental Biology and Biotechnology, Siberian Federal University, Krasnoyarsk 660041, Russia.
Liliana V Belokopytova, Institute of Fundamental Biology and Biotechnology, Siberian Federal University, Krasnoyarsk 660041, Russia.
Author contributions
Q.T. wrote the manuscript. Z.H. and S.X. designed the experiment. X.P. and X.F. analyzed the sample. P.L., X.Z., and J. L. conducted field experiments and B.Y., E.A. V., V.V.S. and L.V.B. reviewed the manuscript.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
None declared.
Data availability
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Adams H, Zeppel M, Anderegg W et al. (2017) A multi-species synthesis of physiological mechanisms in drought induced tree mortality. Nat Ecol Evol 1:1285–1291. 10.1038/s41559-017-0248-x. [DOI] [PubMed] [Google Scholar]
- Andrus R, Harvey B, Rodman K, Hart SJ, Veblen TT (2018) Moisture availability limits subalpine tree establishment. Ecology 99:567–575. 10.1002/ecy.2134. [DOI] [PubMed] [Google Scholar]
- Balducci L, Cuny H, Rathgeber C, Deslauriers A, Giovannelli A, Rossi S (2016) Compensatory mechanisms mitigate the effect of warming and drought on wood formation. Plant Cell Environ 39:1338–1352. 10.1111/pce.12689. [DOI] [PubMed] [Google Scholar]
- Buttò V, Deslauriers A, Rossi S, Rozenberg P, Shishov V, Morin H (2020) The role of plant hormones in tree-ring formation. Trees 34:315–335. 10.1007/s00468-019-01940-4. [DOI] [Google Scholar]
- Cabon A, Peters R, Fonti P, Martínez-Vilalta J, de Cáceres M (2020) Temperature and water potential co-limit stem cambial activity along a steep elevational gradient. New Phytol 226:1325–1340. 10.1111/nph.16456. [DOI] [PubMed] [Google Scholar]
- Carteni F, Deslauriers A, Rossi S et al. (2018) The physiological mechanisms behind the earlywood-to-latewood transition: a process-based modeling approach. Front Plant Sci 9:1053. 10.3389/fpls.2018.01053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavender-Bares J (2016) Diversity, distribution, and ecosystem services of the north American oaks. Int Oaks 27:37–48. [Google Scholar]
- Cuny H, Rathgeber C, Frank D et al. (2015) Woody biomass production lags stem-girth increase by over one month in coniferous forests. Nat Plants 1:15160. 10.1038/nplants.2015.160. [DOI] [PubMed] [Google Scholar]
- Deslauriers A, Huang J, Balducci L, Beaulieu M, Rossi S (2016) The contribution of carbon and water in modulating wood formation in black spruce saplings. Plant Physiol 170:2072–2084. 10.1104/pp.15.01525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezal J, Kopecky M, Dvorsky M et al. (2019) Sink limitation of plant growth determines tree line in the arid Himalayas. Funct Ecol 33:553–565. 10.1111/1365-2435.13284. [DOI] [Google Scholar]
- Du J, He Z, Yang J et al. (2014) Detecting the effects of climate change on canopy phenology in coniferous forests in semi-arid mountain regions of China. Int J Remote Sens 35:6490–6507. 10.1080/01431161.2014.955146. [DOI] [Google Scholar]
- Duchesne L, Houle D, D’Orangeville L (2012) Influence of climate on seasonal patterns of stem increment of balsam fir in a boreal forest of Quebec, Canada. Agric For Meteorol 162-163:108–114. 10.1016/j.agrformet.2012.04.016. [DOI] [Google Scholar]
- Fajardo A, Piper F, Pfund L, Körner C, Hoch G (2012) Variation of mobile carbon reserves in trees at the alpine treeline ecotone is under environmental control. New Phytol 195:794–802. 10.1111/j.1469-8137.2012.04214.x. [DOI] [PubMed] [Google Scholar]
- Furze M, Huggett B, Aubrecht D, Stolz CD, Carbone MS, Richardson AD (2019) Whole-tree nonstructural carbohydrate storage and seasonal dynamics in five temperate species. New Phytol 221:1466–1477. 10.1111/nph.15462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Gou X, Deng Y, Wang Z, Gu F, Wang F (2018) Increased growth of Qinghai spruce in northwestern China during the recent warming hiatus. Agric For Meteorol 260-261:9–16. 10.1016/j.agrformet.2018.05.025. [DOI] [Google Scholar]
- Garcia-Forner N, Vieira J, Nabais C, Carvalho A, Martínez-Vilalta J, Campelo F (2019) Climatic and physiological regulation of the bimodal xylem formation pattern in Pinus pinaster saplings. Tree Physiol 39:2008–2018. 10.1093/treephys/tpz099. [DOI] [PubMed] [Google Scholar]
- Gričar J, Zavadlav S, Jyske T, Lavrič M, Laakso T, Hafner P, Eler K, Vodnik D (2019) Effect of soil water availability on intra-annual xylem and phloem formation and non-structural carbohydrate pools in stem of Quercus pubescens. Tree Physiol 39:222–233. 10.1093/treephys/tpy101. [DOI] [PubMed] [Google Scholar]
- Guo X, Liu S, Wang H et al. (2022) Divergent allocations of nonstructural carbohydrates shape growth response to rainfall reduction in two subtropical plantations. For Ecosyst 9:100021. 10.1016/j.fecs.2022.100021. [DOI] [Google Scholar]
- Hartmann H, Trumbore S (2016) Understanding the roles of nonstructural carbohydrates in forest trees-from what we can measure to what we want to know. New Phytol 211:386–403. 10.1111/nph.13955. [DOI] [PubMed] [Google Scholar]
- Johnson D, McCulloh K, Woodruff D, Meinzer FC (2012) Hydraulic safety margins and embolism reversal in stems and leaves: why are conifers and angiosperms so different? Plant Sci 195:48–53. 10.1016/j.plantsci.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Jyske T, Makinen H, Kalliokoski T et al. (2014) Intra-annual tracheid production of Norway spruce and scots pine across a latitudinal gradient in Finland. Agric For Meteorol 194:241–254. 10.1016/j.agrformet.2014.04.015. [DOI] [Google Scholar]
- Kalliokoski T, Makinen H, Jyske T, Nojd P, Linder S (2013) Effects of nutrient optimization on intra-annual wood formation in Norway spruce. Tree Physiol 33:1145–1155. 10.1093/treephys/tpt078. [DOI] [PubMed] [Google Scholar]
- Klein T, Hoch G, Yakir D, Korner C (2014) Drought stress, growth and nonstructural carbohydrate dynamics of pine trees in a semi-arid forest. Tree Physiol 34:981–992. 10.1093/treephys/tpu071. [DOI] [PubMed] [Google Scholar]
- Körner C (2003) Carbon limitation in trees. J Ecol 91:4–17. 10.1046/j.1365-2745.2003.00742.x. [DOI] [Google Scholar]
- Li Y, Zhang Z, Zhou X, Gao M, Duan J, Xue Y, Shang H, Liu S (2024) Transformation and mechanisms of climate wet/dry change on the northern Tibetan plateau under global warming: a perspective from paleoclimatology. Sci China Earth Sci 67:1932–1951. 10.1007/s11430-023-1260-6. [DOI] [Google Scholar]
- Lintunen A, Paljakka T, Jyske T et al. (2016) Osmolality and nonstructural carbohydrate composition in the secondary phloem of trees across a latitudinal gradient in Europe. Front Plant Sci 7:726. 10.3389/fpls.2016.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Su J, Li S, Lang X, Huang X (2018) Non-structural carbohydrates regulated by season and species in the subtropical monsoon broad-leaved evergreen forest of Yunnan province. China Sci Rep 8:1083. 10.1038/s41598-018-19271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Liang E, Babst F, Camarero JJ, Büntgen U (2022) Warming-induced tipping points of Arctic and alpine shrub recruitment. Proc Natl Acad Sci U S A 119:e2118120119. 10.1073/pnas.2118120119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Vilalta J, Sala A, Asensio D, Galiano L, Hoch G, Palacio S, Piper FI, Lloret F (2016) Dynamics of non-structural carbohydrates in terrestrial plants: a global synthesis. Eco Monogr 86:495–516. 10.1002/ecm.1231. [DOI] [Google Scholar]
- McDowell N, Pockman W, Allen C et al. (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739. 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- Mei Z, Li Z, Lu X et al. (2023) Supplementation of natural light duration promotes accumulation of sugar and anthocyanins in apple (Malus domestica Borkh.) fruit. Environ Exp Bot 205:105133. 10.1016/j.envexpbot.2022.105133. [DOI] [Google Scholar]
- Muller B, Pantin F, Genard M et al. (2011) Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. J Exp Bot 62:1715–1729. 10.1093/jxb/erq438. [DOI] [PubMed] [Google Scholar]
- Ni J, Cheng Y, Wang Q, Ng CWW, Garg A (2019) Effects of vegetation on soil temperature and water content: field monitoring and numerical modelling. J Hydrol 571:494–502. 10.1016/j.jhydrol.2019.02.009. [DOI] [Google Scholar]
- Palacio S, Hoch G, Sala A, Körner C, Millard P (2014) Does carbon storage limit tree growth? New Phytol 201:1096–1100. 10.1111/nph.12602. [DOI] [PubMed] [Google Scholar]
- Pasqualotto G, Ascari L, Bicego G, Carraro V, Huerta ES, de Gregorio T, Siniscalco C, Anfodillo T (2021) Radial stem growth dynamics and phenology of a multi-stemmed species (Corylus avellana L.) across orchards in the northern and southern hemispheres. Tree Physiol 41:2022–2033. 10.1093/treephys/tpab069. [DOI] [PubMed] [Google Scholar]
- Peters R, Steppe K, Cuny H, de Pauw DJW, Frank DC, Schaub M, Rathgeber CBK, Cabon A, Fonti P (2021) Turgor-a limiting factor for radial growth in mature conifers along an elevational gradient. New Phytol 229:213–229. 10.1111/nph.16872. [DOI] [PubMed] [Google Scholar]
- Piper F, Paula S (2020) The role of nonstructural carbohydrates storage in forest resilience under climate change. Curr For Rep 6:1–13. 10.1007/s40725-019-00109-z. [DOI] [Google Scholar]
- Rathgeber C, Rossi S, Bontemps J (2011) Cambial activity related to tree size in a mature silver-fir plantation. Ann Bot 108:429–438. 10.1093/aob/mcr168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren P, Rossi S, Camarero J, Ellison AM, Liang E, Peñuelas J (2018) Critical temperature and precipitation thresholds for the onset of xylogenesis of Juniperus przewalskii in a semi-arid area of the north-eastern Tibetan plateau. Ann Bot 121:617–624. 10.1093/aob/mcx188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren P, Ziaco E, Rossi S, Biondi F, Prislan P, Liang E (2019) Growth rate rather than growing season length determines wood biomass in dry environments. Agric For Meteorol 271:46–53. 10.1016/j.agrformet.2019.02.031. [DOI] [Google Scholar]
- Rossi S, Anfodillo T, Menardi R (2006) Trephor: a new tool for sampling microcores from tree stems. IAWA J 27:89–97. 10.1163/22941932-90000139. [DOI] [Google Scholar]
- Rossi S, Girard M, Morin H (2014) Lengthening of the duration of xylogenesis engenders disproportionate increases in xylem production. Glob Chang Biol 20:2261–2271. 10.1111/gcb.12470. [DOI] [PubMed] [Google Scholar]
- Sala A (2009) Lack of direct evidence for the carbon-starvation hypothesis to explain drought-induced mortality in trees. Proc Natl Acad Sci U S A 106:E68 10.1073/pnas.0904580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signori M, Oliveira R, Barros F et al. (2021) Non-structural carbohydrates mediate seasonal water stress across Amazon forests. Nat Commun 12:2310. 10.1038/s41467-021-22378-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard S, Giovannelli A, Treydte K, Traversi ML, King GM, Frank D, Fonti P (2013) Intra-annual dynamics of non-structural carbohydrates in the cambium of mature conifer trees reflects radial growth demands. Tree Physiol 33:913–923. 10.1093/treephys/tpt075. [DOI] [PubMed] [Google Scholar]
- Tian Q, He Z, Xiao S, Peng X, Lin P, Zhu X, Feng X (2024) Intra-annual stem radial growth of Qinghai spruce and its environmental drivers in the Qilian Mountains, northwestern China. Sci Total Environ 915:170093. 10.1016/j.scitotenv.2024.170093. [DOI] [PubMed] [Google Scholar]
- Tombesi S, Palliotti A, Poni S, Farinelli D (2015) Influence of light and shoot development stage on leaf photosynthesis and carbohydrate status during the adventitious root formation in cuttings of Corylus avellana L. Front Plant Sci 6:973. 10.3389/fpls.2015.00973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J, Carvalho A, Campelo F (2020) Tree growth under climate change: evidence from xylogenesis timings and kinetics. Front Plant Sci 11:90. 10.3389/fpls.2020.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Han S, Zhang J, Wang M, Yin XH, Fang LD, Yang D, Hao GY (2018) The interaction between nonstructural carbohydrate reserves and xylem hydraulics in Korean pine trees across an altitudinal gradient. Tree Physiol 38:1792–1804. 10.1093/treephys/tpy119. [DOI] [PubMed] [Google Scholar]
- Wang B, Chen T, Xu G, Li C, Wu G, Liu G (2021) Stand age related dissimilarity in radial growth of a moisture-sensitive forest tree species is greater under a lower drought limitation. For Ecol Manage 482:118895. 10.1016/j.foreco.2020.118895. [DOI] [Google Scholar]
- Wang Y, Mao Z, Bakker M et al. (2018) Linking conifer root growth and production to soil temperature and carbon supply in temperate forests. Plant Soil 426:33–50. 10.1007/s11104-018-3596-7. [DOI] [Google Scholar]
- Wiley E, Helliker B (2012) A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytol 195:285–289. 10.1111/j.1469-8137.2012.04180.x. [DOI] [PubMed] [Google Scholar]
- Wu W, Li H, Feng H, Si B, Chen G, Meng T, Li Y, Siddique KHM (2021) Precipitation dominates the transpiration of both the economic forest (Malus pumila) and ecological forest (Robinia pseudoacacia) on the loess plateau after about 15 years of water depletion in deep soil. Agric For Meteorol 297:108244. 10.1016/j.agrformet.2020.108244. [DOI] [Google Scholar]
- Wu X, Liu H, Hartmann H et al. (2022) Timing and order of extreme drought and wetness determine bioclimatic sensitivity of tree growth. Earths Future 10:e2021EF002530. 10.1029/2021EF002530. [DOI] [Google Scholar]
- Zhang J, Gou X, Rademacher T, Wang L, Li Y, Sun Q, Wang F, Cao Z (2023) Interaction of age and elevation on xylogenesis in Juniperus przewalskii in a cold and arid region. Agric For Meteorol 337:109480. 10.1016/j.agrformet.2023.109480. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.