Abstract
Clostridium difficile, the primary cause of nosocomial diarrhea in the United States and many other industrialized countries, is recognized as a major health concern because of its ability to cause severe intestinal disease leading to complications such as relapses and infections due to vancomycin-resistant enterococci. The disease results from two toxins, toxins A and B, produced by this pathogen. In this study, we evaluated the TOX A/B TEST, a new 1-h enzyme immunoassay (EIA) that detects toxins A and B. We compared the test with the tissue culture assay, which is recognized as the “gold standard” for C. difficile testing. Evaluations were performed in-house at TechLab, Inc. (Blacksburg, Va.) and off-site at four clinical laboratories. Of 1,152 specimens tested, 165 were positive by the TOX A/B TEST and tissue culture and 973 were negative by both tests. The sensitivity and specificity were 92.2 and 100%, respectively. The positive and negative predictive values were 100 and 98.6%, respectively, and the correlation of the TOX A/B TEST with tissue culture was 98.8%. When discrepant samples were resolved by culture, the sensitivity and specificity were 93.2 and 98.9%, respectively. The positive and negative predictive values were 100 and 98.8%, respectively, with a correlation of 99.0%. There were no specimens that were positive by the TOX A/B TEST and negative by tissue culture. Fourteen specimens were negative by the TOX A/B TEST but positive by tissue culture. Of these, two were negative by toxigenic culture, five were positive by toxigenic culture, and seven were not available for further testing. There were no indeterminate results, since the test does not have an indeterminant zone. In a separate study, 102 specimens that were positive by tissue culture and the TOX A/B TEST were examined in toxin A-specific EIAs. Two specimens that presumptively contained toxin A-negative, toxin B-positive (toxA−/toxB+) isolates were identified. One specimen was from a patient with a clinical history consistent with C. difficile infection. Isolates obtained from these specimens by selective culture on solid media and in broth tested toxA−/toxB+ when grown in brain heart infusion dialysis flasks, which stimulate in vitro production of both toxins. Our findings show that the TOX A/B TEST is suitable as a diagnostic aid for C. difficile disease because it correlates well with tissue culture and detects isolates that may be missed with toxin A-specific EIAs.
Clostridium difficile, which causes virtually all cases of pseudomembranous colitis, is now recognized as the primary cause of nosocomial diarrhea in the United States and many other industrialized countries (3, 21). Health care costs for this disease, which run into the hundreds of millions of dollars each year in the United States, are continuing to rise. Relapses, which can be extremely difficult to treat, occur in 10 to 20% of patients with C. difficile disease. Fortunately, most relapse patients can be effectively treated with metronidazole and vancomycin, but these treatments have triggered new problems, such as the emergence of vancomycin-resistant enterococci. New therapeutic approaches are being investigated for C. difficile disease, but these therapies are still under development. The strategy used by the health care profession in the management of C. difficile disease is not limited simply to the treatment of hospitalized patients with diarrhea. Many hospitalized patients acquire C. difficile asymptomatically as carriers, raising the question of whether these persons should be treated to minimize their chances of disease and spread of the organism (6, 24). In some instances, asymptomatic elderly persons who are positive for C. difficile are denied entrance into facilities such as nursing homes because of the potential risk of outbreaks (4).
Although C. difficile disease continues to be a major challenge for the health care profession, our basic understanding of the toxins of this organism and significant advances in new and improved diagnostic testing are leading to better diagnosis of and therapy against this opportunistic pathogen. The two toxins that it produces, toxins A and B, have been well characterized (9, 20, 21, 28–30). Toxins A and B are the largest bacterial toxins known, with molecular weights of 308,000 and 279,000, respectively. Both toxins have contiguous repeating units at the COOH terminus. The repeating units on toxin A represent the portion that binds to galactose receptors (15, 18). These repeating units also represent the portion of the toxin recognized by the monoclonal antibody used in most of the diagnostic enzyme immunoassays (EIAs) on the market (10). Overall, the two toxins exhibit 45% homology at the amino acid level, strongly suggesting that the toxA and toxB genes resulted from gene duplication (2, 9, 29, 30). This possibility is substantiated further by the recent finding that both toxins are glucosyltransferases which glucosylate factor rho, a small GTP-binding protein involved in the regulation of the cytoskeletal system in mammalian cells (1, 13). Toxin A is a potent enterotoxin that is lethal and cytotoxic, and it probably causes most of the clinical symptoms. Toxin B also is lethal and is much more cytotoxic than toxin A. In the present study, we developed a new EIA, the TOX A/B TEST, designed to detect both toxin A and toxin B and examined its potential use as an in vitro diagnostic aid in C. difficile disease. The test incorporates highly specific affinity-purified polyclonal and monoclonal antibodies against toxin A and highly specific polyclonal antibodies against toxin B. The test was evaluated through a multicenter study comparing its performance to the tissue culture test, considered the “gold standard” in C. difficile testing. During this study, we identified two isolates that tested negative for toxin A and positive for toxin B (toxA−/toxB+) and showed that these isolates are not detected by toxin A-specific EIAs.
MATERIALS AND METHODS
Study sites and stool specimens.
Six separate studies were performed in the TOX A/B TEST evaluation: (i) study 1, performed in-house at TechLab with stool specimens from the in-house fecal log; (ii) study 2, performed in-house at TechLab with specimens supplied by an outside clinical laboratory; (iii) study 3, performed at Indiana University Hospital, Indianapolis; (iv) study 4, performed at the Hershey Medical Center, Hershey, Pa.; (v) study 5, performed at PinnacleHealth, Harrisburg, Pa.; and (vi) study 6, performed at the School of Medicine at the University of Maryland, Baltimore. All specimens used in the study were stored either at 2°C to 8°C for ≤72 hours before assay or frozen at −20°C or −70°C. Specimens included in the study were submitted to the laboratory for routine testing and included specimens from patients who had recently received antibiotics and from patients not on antibiotics.
Bacterial strains and toxin reagents.
C. difficile and Clostridium sordellii VPI strains were obtained from the anaerobe collection at Virginia Polytechnic Institute and State University (Blacksburg, Va.). Strain CCUG 8864 was kindly supplied by Peter Borriello (PHLS Central Public Health Laboratory, London, England). Serogroup type F strains IS37 and IS73 were kindly provided by Jon Brazier (Public Health Laboratory Service, Health Park, Cardiff, Wales). Normal intestinal bacteria and enteric pathogens were obtained from the American Type Culture Collection (Rockville, Md.). Toxins A and B were purified from C. difficile VPI 10463 as previously described (19).
EIAs.
The TOX A/B TEST (TechLab, Inc.) is a microwell EIA that utilizes affinity-purified mouse monoclonal antibody against toxin A and affinity-purified goat polyclonal antibody against toxins A and B. Microwells provided with the test are coated with affinity-purified goat polyclonal antibodies against toxins A and B. The detecting antibody consists of monoclonal antibody against toxin A and affinity-purified goat polyclonal antibody against toxin B, each conjugated to horseradish peroxidase. The test was performed according to the manufacturer’s instructions. Briefly, stool specimens were diluted 1:5 in kit diluent. One drop of conjugate per microwell was added, followed by the addition of 2 drops of diluted stool. Microwells were incubated at 37°C for 50 min and washed five times with 1× wash solution. One drop of substrate A was added to each well followed by 1 drop of substrate B. After 10 min at room temperature, reactions were stopped by the addition of 1 drop of stop solution. The positive control consisted of the addition of positive control reagent to a well containing conjugate. The negative control consisted of the addition of diluent to a well also containing conjugate. Test results were interpreted as follows: (i) for visual readings, a colorless result was considered negative and any yellow color was considered positive; (ii) for a spectrophotometric single wavelength at 450 nm, negative was <0.120 and positive was ≥0.120; and (iii) for a spectrophotometric dual wavelength at 450/620 nm or 450/550 nm, negative was <0.080 and positive was ≥0.080. EIA titers were determined for C. difficile strains for a more accurate assessment of the sensitivity of the TOX A/B TEST compared with tissue culture results. For determination of EIA titers, serial 10-fold dilutions of test samples were prepared and assayed as described in the package insert. EIA titers were expressed as the reciprocal of the highest dilution giving a positive reaction.
The Tox-A Test (TechLab, Inc.) and the Premier C. difficile Toxin A (Meridian Diagnostics, Inc., Cincinnati, Ohio), and Cytoclone A+B (Cambridge Biotech Corp., Worcester, Mass.) tests were used according to each manufacturer’s instructions. The Tox-A Test and the Premier test detect toxin A; the Cytoclone A+B test detects both toxins A and B. Specimens were prepared as directed by each manufacturer, and positive and negative results were determined as specified by each manufacturer.
Tissue culture assay.
Tissue culture assays were performed according to individual in-house testing procedures: (i) Indiana University Hospital used the C. difficile Tox-B Test (TechLab, Inc.) with human foreskin monolayers, (ii) Hershey Medical Center used the C. difficile Toxin/Antitoxin Kit (TechLab, Inc.) with MRC-5 cells, (iii) PinnacleHealth used the Toxi-Titer (Bartels Immunodiagnostics) test with human foreskin monolayers, (iv) the University of Maryland School of Medicine used the C. difficile Tox-B Test with human foreskin monolayers, and (v) TechLab used the C. difficile Tox-B Test with Chinese hamster ovary K-1 cells. At each site, a standardized dilution of toxin B was used as the positive control as recommended with each kit. Specimens were diluted and prepared according to each manufacturer’s instructions, and results were determined at 24 and 48 h. For the Tox-B Test, a 1/50 dilution (final concentration in the tissue culture well) of stool specimen was tested, and for the Toxi-Titer a 1/40 dilution was tested. With the C. difficile Toxin/Antitoxin Kit, the final dilution was 1/100. Cytotoxicity titers were determined for individual C. difficile strains for a more accurate assessment of the sensitivity of the TOX A/B TEST compared to tissue culture. For determination of cytotoxicity titers, serial 10-fold dilutions of test samples were prepared and assayed as previously described (19). Cytotoxic titers were expressed as the reciprocal of the highest dilution giving a positive reaction.
Culture.
Prereduced anaerobically sterilized brain heart infusion broth (Carr-Scarborough, Stone Mountain, Ga.) was supplemented with cycloserine and cefoxitin (CC-BHI) (Oxoid, Ogdensburg, N.Y.). The final concentrations of cycloserine and cefoxitin were 500 μg/ml and 16 μg/ml, respectively. For inoculation of broth media, 1 drop of stool specimen was added to a tube containing 5.0 ml of supplemented broth. Inoculated media were incubated at 37°C for 3 to 4 days. Selective cycloserine-cefoxitin-fructose agar (11) (CCFA) (Oxoid) was prepared as recommended by the manufacturer. Presumptive colonies were characterized by a yellowish color, flat morphology, yellow-green fluorescence, and a horsey smell. CCFA plates were incubated anaerobically at 37°C for a minimum of 3 days. Selected strains were grown in brain heart infusion dialysis flasks as previously described (19).
PAGE and crossed immunoelectrophoresis.
Isolates were grown at 37°C for 96 h in brain heart infusion dialysis flasks as previously described (19). After incubation, cultures were collected and centrifuged to remove cells and debris, and the supernatant fluids were passed through 0.2-μm-pore-size membranes. Filtrates were stored at 2°C to 8°C. Polyacrylamide gel electrophoresis (PAGE) was performed in nondenaturing 3 to 27% polyacrylamide gradient gels (Jule Biotechnologies, Inc., New Haven, Conn.). Samples (20 μl) of filtrate were mixed with bromphenol blue-glycerol (5 μl) and loaded into sample wells. Electrophoresis was performed in Tris-borate-EDTA buffer, pH 8.3, at a constant voltage and 40 mA. After electrophoresis, gels were stained with Coomassie blue R-250 and destained. Crossed immunoelectrophoresis was performed in 1.2% agarose gels as previously described (19).
Gas chromatography.
Isolates were characterized according to their cellular fatty acid profiles with the Microbial Identification System (Microbial ID, Inc.), as previously described (25).
RESULTS
Reactions of toxins A and B and C. difficile strains in the TOX A/B TEST.
The reactions of highly purified toxins A and B in the TOX A/B TEST were compared with those in the Cytoclone A+B test, the Tox-A Test, and the Premier C. difficile Toxin A test. All four tests detected toxin A at approximately 1 ng/ml. The minimum level of toxin B detected by the TOX A/B TEST was 2 to 5 ng/ml, whereas the minimum level detected by Cytoclone A+B was 5 to 10 ng/ml. Toxin B did not react in either the Tox-A Test or the Premier C. difficile Toxin A test.
The reactivity of eight C. difficile strains which varied in their in vitro production of toxins A and B was examined. Included were seven toxigenic strains ranging from 101 to 106 in cytotoxic titer when grown anaerobically in prereduced brain heart infusion broth. Six of the toxigenic strains were toxA+/toxB+. The other toxigenic strain, CCUG 8864, was toxA−/toxB+ and carried a truncated toxA gene that lacked the repeating units (5, 17, 27). All seven toxigenic strains, including CCUG 8864, reacted strongly (A450 > 2.500) in the TOX A/B TEST and the Cytoclone A+B test. The six toxA+/toxB+ strains reacted in the Tox-A Test and the Premier test, whereas strain CCUG 8864 was negative in these tests. Also included in the analysis was VPI 11186, a nontoxigenic strain that lacks the toxA and toxB genes but which cross-reacts extensively with nontoxic antigens from VPI 10463. VPI 11186 was negative in all of the EIAs and noncytotoxic in the tissue culture test.
Further analyses were done to examine the reactions of VPI 10463 and VPI 11186 in stool specimens of different consistencies. For the analyses, cultures of each strain were prepared and centrifuged and the supernatant fluids were used to spike negative stool specimens of differing consistencies. Filtrates from VPI 10463 (toxA+/toxB+) were positive at dilutions of 5 × 103 or lower in liquid, semisolid, and solid stool specimens. VPI 11186 filtrates (toxA−/toxB−) were consistently negative when tested in specimens of different consistencies.
Forty species of organisms representing members of the normal intestinal flora and various enteric pathogens were tested in the TOX A/B TEST. The only organisms that reacted in the test were toxigenic C. difficile and C. sordellii. The C. sordellii strain that was positive, VPI 9048, produces toxin HT (hemorrhagic toxin) and toxin LT (lethal toxin), which have extensive identity with toxin A and toxin B, respectively, at the gene and protein levels. Toxins HT and LT have been shown previously to cross-react extensively with antibodies against toxins A and B (22, 23, 26). A nontoxigenic strain of C. sordellii did not react in the TOX A/B TEST.
Performance characteristics.
A total of 1,152 stool specimens submitted to the clinical laboratory were assayed in the TOX A/B TEST and tissue culture assay. Included were 177 specimens positive by tissue culture. Of these, 165 were positive in the TOX A/B TEST. A total of 973 specimens were negative in both tests. Included in the analysis were 10 specimens that were initially identified as tissue culture positive but were subsequently confirmed as tissue culture negative and ruled false positive. Three of these were neutralized by neutral goat serum, and seven caused atypical stretching of the cells uncharacteristic of C. difficile toxin. The seven specimens that caused atypical reactions were all from one test location, and the reactions were observed with human foreskin cells. For the purposes of this study, these samples were considered negative. When compared to tissue culture, the TOX A/B TEST exhibited sensitivity and specificity of 92.2 and 100%, respectively, with positive and negative predictive values of 100 and 98.6%, respectively. The correlation between the TOX A/B TEST and tissue culture was 98.6%. Of the 14 specimens that were tissue culture positive but negative in the TOX A/B TEST, 2 were negative by toxigenic culture, 5 were positive by toxigenic culture, and 7 were not available for further testing. The 2 specimens that were negative by toxigenic culture were ruled false positive. No specimens tested positive in the TOX A/B TEST but negative by tissue culture. When discrepancies were resolved by toxigenic culture, the TOX A/B TEST exhibited sensitivity and specificity of 93.2% and 100%, respectively. The positive and negative predictive values were 100 and 98.8%, respectively, with a correlation of 99.0%. Table 1 shows the results from each individual study.
TABLE 1.
Results from individual studies of performance characteristics of the TOX A/B TESTa
| Study (location) | Test results
|
Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Correlation (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| ntotal | npositive | nnegative | ndiscrepant | ||||||
| 1 (TechLab, Blacksburg, Va.)b | 436 | 45 | 389 | 2 | 95.7 | 100 | 100 | 99.5 | 99.5 |
| 2 (TechLab)b | 166 | 8 | 157 | 1 | 88.9 | 100 | 100 | 99.4 | 99.4 |
| 3 (Indiana University Hospital, Indianapolis)b | 300 | 25 | 273 | 2 | 92.6 | 100 | 100 | 99.3 | 99.3 |
| 4 (Hershey Medical Center, Hershey, Pa.) | 50 | 24 | 25 | 1 | 96.0 | 100 | 100 | 96.2 | 98.0 |
| 5 (PinnacleHealth, Harrisburg, Pa.) | 98 | 48 | 45 | 5 | 90.6 | 100 | 100 | 90.0 | 94.9 |
| 6 (University of Maryland School of Medicine, Baltimore)b | 102 | 15 | 84 | 3 | 83.3 | 100 | 100 | 96.6 | 97.1 |
Abbreviations: ntotal, total number of specimens in the study; npositive, number of specimens positive by tissue culture and the TOX A/B TEST; nnegative, number of specimens negative by both tests; ndiscrepant, number of specimens positive by tissue culture and negative by the TOX A/B TEST; PPV, positive predictive value; NPV, negative predictive value. Confidence intervals (P = 0.05): sensitivity, 87.4 to 95.0%; NPV, 93.8 to 99.8%; correlation, 96.6 to 99.4%.
Incidence rates for studies 1, 2, 3, and 6 were 10.3, 4.8, 8.3, and 14.7%, respectively. Incidence rates for studies 4 and 5 are not included because these groups included selected specimens, and results were not necessarily indicative of the incidence in stools submitted for routine testing.
Comparative studies of centrifuged versus noncentrifuged specimens and visual versus spectrophotometric readings (A450 and A450/620) were done. For the analysis, 337 specimens that included 30 TOX A/B TEST-positive specimens and 307 TOX A/B TEST-negative specimens were tested. Initially, specimens were prepared and a portion of each diluted specimen was tested according to the directions in the package insert. The remaining portion of each diluted specimen was centrifuged (5,000 × g) for 5 min, and the supernatant fluid was assayed in the TOX A/B TEST according to the directions. The results showed a 100% correlation between centrifuged and noncentrifuged specimens and between visual and spectrophotometric readings.
Identification of toxA−/toxB+ isolates.
A total of 102 specimens that were positive by tissue culture and TOX A/B TEST were examined further in the Tox-A Test for toxA−/toxB+ isolates. Included in the evaluation were specimens from each of the studies listed in Table 1. Ten of the 102 specimens tested negative in the Tox-A Test. Eight of these gave low readings (A450 values in the range of 0.120 to 0.260 in the TOX A/B TEST), were only weakly cytotoxic, and tested negative in both the Premier and Cytoclone A+B tests, suggesting to us that these specimens contained only trace levels of toxins A and B. These specimens were subcultured into selective CC-BHI broth and incubated at 37°C for 96 h, and the cultures were tested. Six of the eight cultures reacted positively in both the Tox-A Test and the TOX A/B TEST and were cytotoxic, demonstrating that they were true positives for toxigenic C. difficile. The other two cultures were negative.
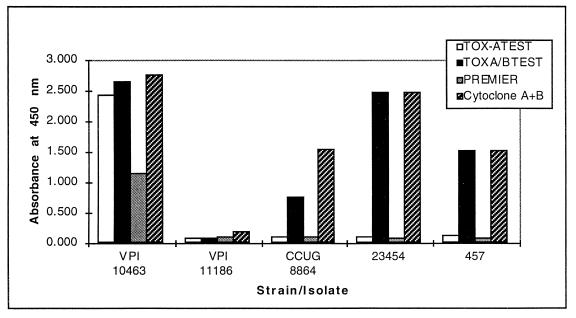
The remaining two stool specimens that were positive in the TOX A/B TEST and negative in the Tox-A Test gave A450 values of >0.500. One of the specimens, no. 23454, from Indiana University Hospital, was from a patient on antibiotic therapy. The other specimen, no. 457, was from our in-house fecal log and was originally obtained from the Hershey Medical Center. When tested in the Cytoclone A+B test, both specimens were positive. In the Premier C. difficile Toxin A test, both specimens were negative. These results suggested to us the possibility that the specimens contained toxA−/toxB+ isolates. Isolates from each specimen were obtained by selective culture in CC-BHI broth followed by further selection on CCFA. For CCFA selection, approximately 5 to 10 colonies exhibiting typical C. difficile morphology were picked for analysis and identification by gas chromatography. Isolates were identified by their cellular fatty acid profiles. Each was subsequently grown in brain heart infusion dialysis flasks, which enhances toxin production approximately 10- to 100-fold compared to toxin levels in tube broth cultures. An analysis of the dialysis sac filtrates from each isolate in each of the EIAs is shown in Fig. 1. Filtrates from the two isolates were strongly positive in the TOX A/B TEST and the Cytoclone A+B test (A450 > 0.500) and negative in the Tox-A Test and the Premier C. difficile Toxin A test. Further quantitative analysis of the toxin A and toxin B titers was performed with the Tox-A Test and the TOX A/B TEST and by tissue culture assay (Table 2). Again, the results showed the absence of toxin A.
FIG. 1.
Reactions of C. difficile strains in the TechLab TOX A/B TEST, TechLab Tox-A Test, Meridian Premier C. difficile Toxin A test, and Cambridge Cytoclone A+B test. For the analysis, strains were grown at 37°C for 72 h in brain heart infusion dialysis flasks and culture filtrates were assayed in each EIA. Strains VPI 10463, VPI 11186, and CCUG 8864 have been described previously (19, 20). Isolates 23454 and 457 were obtained in this study.
TABLE 2.
Quantitative EIA and cytotoxic titers of C. difficile strains
| C. difficile strain | EIA titera
|
Cytotoxic titerb | |
|---|---|---|---|
| Tox-A Test | TOX A/B TEST | ||
| VPI 10463 | 104 | 105 | 106 |
| VPI 11186 | −c | − | − |
| CCUG 8864 | − | 105 | 106 |
| 457 | − | 102 | 104 |
| 23454 | − | 102 | 105 |
EIA titers are expressed as the reciprocal of the highest dilution giving a positive reaction, as defined in the package insert.
Cytotoxic titers are expressed as the reciprocal of the highest dilution giving a positive reaction. Results were confirmed by neutralization of the cytotoxic activity by specific C. difficile antiserum.
−, negative.
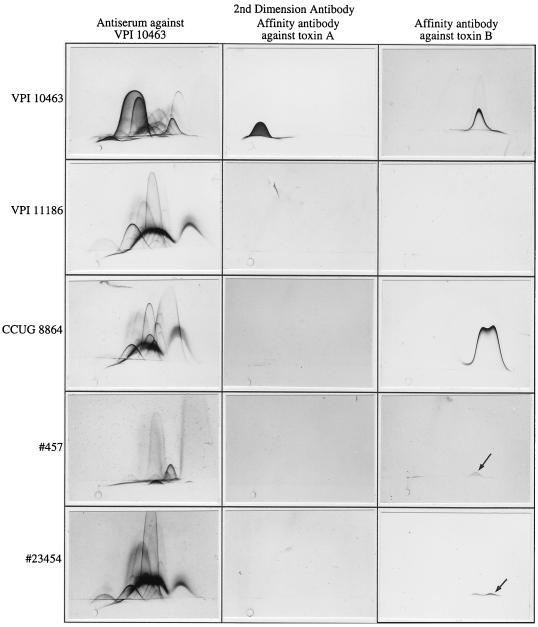
Further analyses were done by crossed immunoelectrophoresis to examine the antigenic profiles of these strains (Fig. 2). Isolates 23454 and 457 cross-reacted extensively with antiserum against VPI 10463. Filtrates from both isolates tested weakly positive for toxin B, as shown by the presence of a small precipitin arc in the toxin B location. No precipitin arcs were detected for either isolate with affinity-purified toxin A antibody.
FIG. 2.
Antigenic profiles of C. difficile isolates. Filtrates were subjected to crossed immunoelectrophoresis with antiserum against VPI 10463 or affinity-purified antibody against toxin A or toxin B. Strains VPI 10463, VPI 11186, and CCUG 8864 have been described previously (19, 20). Isolates 23454 and 457 were obtained in this study. Toxin A and toxin B arcs were observed with VPI 10463. Neither toxin was detected with VPI 11186, which does not carry either the toxA or the toxB gene. This strain produces an antigen that migrates similarly to toxin A, but the antigen is distinct from toxin A. Toxin B arcs were observed with CCUG 8864 and isolates 457 and 23454. The 457 and 23454 toxin B arcs (designated by arrows) were considerably smaller than the toxin B arcs from VPI 10463 and CCUG 8864. The double precipitin arc observed with toxin B from CCUG 8864 and isolate 23454 are occasionally observed with toxin B from VPI 10463. Toxin A arcs were not detected with CCUG 8864, isolate 457, or isolate 23454.
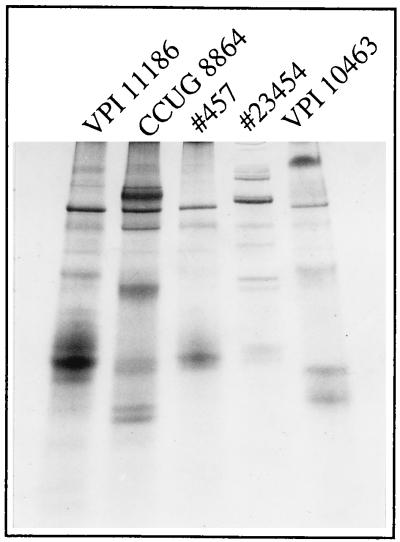
Filtrates from isolates 23454 and 457 were prepared from brain heart infusion dialysis flask cultures and analyzed by gradient PAGE to determine their protein profiles (Fig. 3). We have used this method in other studies to type C. difficile isolates and examine their prevalence in outbreaks of antibiotic-associated diarrhea (21a). The two isolates gave protein profiles distinct from each other and from CCUG 8864, VPI 10463, and VPI 11186 (Fig. 3).
FIG. 3.
Protein profiles of C. difficile isolates. Filtrates were analyzed by PAGE in a 3 to 27% gradient gel under nondenaturing conditions. Strains VPI 10463, VPI 11186, and CCUG 8864 have been described previously (19, 20). Isolates 457 and 23454 were identified in this study.
Two C. difficile strains from serogroup type F, strains IS37 and IS73, were grown in brain heart infusion dialysis flasks, and culture filtrates from each strain were analyzed in the TOX A/B TEST, the Tox-A Test, and the Tox-B Test. The filtrates from these strains were negative in the Tox-A Test. When tested in the TOX A/B TEST, the filtrates had EIA titers of 102 and undiluted test samples from each strain gave A450 values of >2.0. In the Tox-B Test, the cytotoxic titers of filtrates from each strain were consistently 104 or higher, and the cytotoxic activity was neutralized by C. difficile antitoxin.
DISCUSSION
Our study was undertaken to evaluate the performance of the TOX A/B TEST, a new EIA that detects C. difficile toxins A and B. Our goal in developing the TOX A/B TEST was to produce an EIA that closely matched the performance of the tissue culture test, which represents the most sensitive test on the market. The test was designed to be simple, by using the microwell EIA format of the Tox-A Test, and rapid, by maintaining the 1-h turnaround time. The interpretation of test results was simplified by removal of the indeterminant zone, which is present in most of the C. difficile EIAs now on the market. Our results show that the TOX A/B TEST detects toxin A at levels similar to other EIAs on the market and toxin B at levels lower than the Cytoclone A+B test. The TOX A/B TEST was highly specific for toxigenic C. difficile strains and detected toxins A and B produced in vitro in broth cultures by weakly cytotoxigenic strains. The only non-C. difficile organism found to cross-react was a toxigenic strain of C. sordellii. This finding was not surprising, because certain strains of C. sordellii produce toxins that are highly related immunologically to toxins A and B. These C. sordellii strains react positively in other C. difficile EIAs and by tissue culture. In our clinical evaluations involving 1,152 stool specimens in six different studies, the TOX A/B TEST exhibited a correlation of 98.8% with tissue culture.
During our evaluation, we identified two specimens, no. 23454 and no. 457, that exhibited properties consistent with a toxA−/toxB+ phenotype. These specimens gave positive reactions in the TOX A/B TEST, the Cytoclone A+B test, and tissue culture but were negative in toxin A-specific EIAs. At least one of the specimens was from a patient who had a clinical history consistent with C. difficile disease. We subsequently isolated toxigenic C. difficile from both specimens and showed that the isolates continued to test toxA−/toxB+ when grown under conditions optimal for toxin production. The reaction of toxin B from these isolates with polyclonal antibody against toxin B was detectable by crossed immunoelectrophoresis, though it was weak. The weak reactions likely resulted from lower levels of toxin, since the isolates have cytotoxic titers 10- to 100-fold lower than that of VPI 10463. The negative reaction of these isolates in the toxin A-specific EIAs did not appear to result from insufficient levels of toxin A, since other isolates with cytotoxic titers lower than either no. 457 or no. 23454 were highly reactive in toxin A-specific EIAs. These isolates appear to be phenotypically distinct from each other and from VPI 10463, VPI 11186, and CCUG 8864, based on their different protein profiles.
The identification of these isolates presents a new challenge in the diagnosis of C. difficile disease. The incidence of toxA−/toxB+ isolates in our study was low (0.2%), and most C. difficile isolates from symptomatic patients are toxA+/toxB+. However, the presence of a toxA−/toxB+ isolate in a stool specimen from a patient with a clinical history consistent with C. difficile disease suggests to us the possibility that toxA−/toxB+ isolates may spread through wards and remain undetected in hospitals that use toxin A-specific EIAs. The only toxA−/toxB+ isolate that has been carefully characterized at the molecular level is CCUG 8864, which carries a truncated toxA gene (5, 17, 27). Type F isolates from asymptomatic neonates and infants also have been reported to be toxA−/toxB+, but they have not been as carefully studied (7, 8). Our results, which showed two representative serogroup type F strains to be negative in the Tox-A Test but positive in the TOX A/B TEST and the Tox-B Test, support the possibility that serogroup type F strains are toxA−/toxB+. The toxigenic phenotype of these isolates is still unclear, however, because Von Eichel-Streiber et al. showed that under certain conditions, the serogroup type F reference strain (strain 1470) produces both toxins in vitro (29). The ability of type F strains to cause disease also remains unclear. Previous studies (7, 8) described type F strains from asymptomatic infants, but recent results by Kato et al. (14) showed that they are present in C. difficile disease patients in Japan. Therefore, additional studies are needed to further characterize these isolates and their role in disease. Interestingly, the toxA−/toxB+ phenotype also has been reported with toxigenic C. sordellii, which produces a toxin A-like enterotoxin (HT) and a toxin B-like cytotoxin (LT) (12). Like CCUG 8864, this isolate lacks the repeating units of the enterotoxin gene. Therefore, there may be a specific genetic mechanism by which these strains arise.
How toxA−/toxB+ isolates are involved in C. difficile disease is not clear. Current data support the idea that toxin A causes most of the clinical symptoms associated with C. difficile disease. This is based on the potent enterotoxic activity of toxin A, the lack of activity of toxin B in the intestine, and the ability of toxin A antibodies to protect experimental animals against C. difficile disease (3, 16, 18, 20, 28). However, toxA−/toxB+ isolates may cause disease due to a more active toxin B. This possibility is based on our findings that toxin B from CCUG 8864 is 10-fold more cytotoxic and lethal than toxin B from toxA+/toxB+ isolates (17). In addition, unlike toxin B from toxA+/toxB+ isolates, CCUG 8864 toxin B is weakly enterotoxic. There also is the possible role of the truncated toxA gene product. Additional studies are needed to characterize the toxins from these atypical isolates at the molecular level and to continue surveillance and identification of new C. difficile isolates that produce altered toxins. It is important, however, that these types of studies are carefully controlled. Improper assay conditions, such as incomplete washing of EIA plates, may lead to misidentification of strains as toxA−/toxB+ or toxA+/toxB−. DNA probes or PCR primers used to characterize these isolates also must be selected carefully. For example, DNA probes against the repeating units of toxin A would indicate the absence of the toxA gene in CCUG 8864 when, in fact, this strain carries the upstream portion of the gene. In addition, the interpretation of toxA−/toxB+ results may be more complicated than just determining the presence or absence of the toxA gene, as suggested by results with type F strains that appear to carry the toxA and toxB genes but for which only toxin B is detectable in stool specimens. Concomitant expression of toxins A and B, for example, may exhibit strain-to-strain variation. Characterization of these isolates will be challenging, but the results will lead to improved diagnostic testing for C. difficile disease and new information on how the toxins damage the intestine.
In our clinical evaluations, we identified several specimens that gave results in the tissue culture test not typical of the C. difficile toxins. Three specimens (0.2% of those tested) caused cell rounding that was not neutralized by C. difficile antitoxin. Even at higher dilutions, the specimens continued to cause cell rounding that was not neutralized with antitoxin. We were unable to isolate toxigenic C. difficile from these specimens, indicating that these likely were not true C. difficile positives. There also were seven specimens (0.6% of those tested) that caused cell stretching, all of which were neutralized by C. difficile antitoxin (Fig. 4). Although cell stretching is not typical of toxigenic C. difficile, neutralization by antitoxin resulted in confusion in the interpretation of test results. Upon further examination we found that the activity was neutralized by normal goat serum, and we were unable to isolate toxigenic C. difficile from the specimens. The incidence of these unusual specimens was low and, for the purposes of our study, these specimens were considered C. difficile-negative. These findings illustrate, however, that caution should be taken to avoid “overinterpretation” of test results with specimens that cause atypical stretching or cell rounding that is not neutralized by C. difficile antitoxin.
FIG. 4.
Cell-rounding reaction caused in Chinese hamster ovary K-1 cells by C. difficile toxins A and B. (A) Cell-rounding activity typical of both toxins. Toxin B masks the activity of toxin A because of its high specific activity in the assay. (B) Neutralization of cell-rounding activity with specific C. difficile antitoxin. The activity is not neutralized with nonimmune serum. (C) Cell-stretching that may be confused with the cytotoxic activity of C. difficile toxins A and B. In our studies, the cell-stretching activity was neutralized by specific C. difficile antitoxin and by nonimmune serum.
In conclusion, the TOX A/B TEST is a new in vitro diagnostic test that can be used as an aid in the diagnosis of C. difficile disease. It is simple and rapid and exhibits high correlation with the tissue culture test. The TOX A/B TEST also detects C. difficile isolates that may be missed with toxin A-specific EIAs. Therefore, the test should be useful as a tool for identifying unusual isolates of C. difficile.
ACKNOWLEDGMENTS
We thank Mary Alice Woodburn and Earl Petzold for assistance in isolation and characterization of the isolates obtained in this study. We thank Carlyn Bruce for help in maintaining monolayer cells for the tissue culture test.
REFERENCES
- 1.Aktories K, Just I. Monoglucosylation of low-molecular-mass GTP-binding rho proteins by clostridial cytotoxins. Trends Cell Biol. 1995;5:441–443. doi: 10.1016/s0962-8924(00)89107-2. [DOI] [PubMed] [Google Scholar]
- 2.Barroso L A, Wang S Z, Phelps C J, Johnson J L, Wilkins T D. Nucleotide sequence of Clostridium difficile toxin B gene. Nucleic Acids Res. 1990;18:4004. doi: 10.1093/nar/18.13.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartlett J G. Antibiotic-associated diarrhea. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. Infection of the gastrointestinal tract. New York, N.Y: Raven Press; 1995. pp. 893–904. [Google Scholar]
- 4.Bender B S, Laughon B E, Gaydos C, Forman M S, Bennett R, Greenough III W B, Sears S D, Bartlett J G. Is Clostridium difficile endemic in chronic-care facilities? Lancet. 1986;i:11–13. doi: 10.1016/s0140-6736(86)92559-6. [DOI] [PubMed] [Google Scholar]
- 5.Borriello S P, Wren B W, Hyde S, Seddon S V, Sibbons P, Krishna M M, Tabaqchali S, Manek S, Price A B. Molecular, immunological, and biological characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile. Infect Immun. 1992;60:4192–4199. doi: 10.1128/iai.60.10.4192-4199.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clabots C R, Johnson S, Olson M M, Peterson L R, Gerding D N. Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection. J Infect Dis. 1992;166:561–567. doi: 10.1093/infdis/166.3.561. [DOI] [PubMed] [Google Scholar]
- 7.Delmee M, Verellen G, Avesani V, François G. Clostridium difficile in neonates: serogrouping and epidemiology. Eur J Pediatr. 1988;147:36–40. doi: 10.1007/BF00442608. [DOI] [PubMed] [Google Scholar]
- 8.Depitre C, Delmee M, Avesani V, L’Haridon R, Roels A, Popoff M, Corthier G. Serogroup F strains of Clostridium difficile produce toxin B but not toxin A. J Med Microbiol. 1993;38:434–441. doi: 10.1099/00222615-38-6-434. [DOI] [PubMed] [Google Scholar]
- 9.Dove C H, Wang S Z, Price S B, Phelps C J, Lyerly D M, Wilkins T D, Johnson J L. Molecular characterization of the Clostridium difficile toxin A gene. Infect Immun. 1990;58:480–488. doi: 10.1128/iai.58.2.480-488.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frey S M, Wilkins T D. Localization of two epitopes recognized by monoclonal antibody PCG-4 on Clostridium difficile toxin A. Infect Immun. 1992;60:2488–2492. doi: 10.1128/iai.60.6.2488-2492.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George W L, Sutter V L, Citron D, Finegold S M. Selective and differential medium for isolation of Clostridium difficile. J Clin Microbiol. 1979;9:214–219. doi: 10.1128/jcm.9.2.214-219.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green G A, Schue V, Giarardot R, Monteil H. Characterization of an enterotoxin-negative, cytotoxin-positive strain of Clostridium sordellii. J Med Microbiol. 1996;44:60–64. doi: 10.1099/00222615-44-1-60. [DOI] [PubMed] [Google Scholar]
- 13.Just I, Fritz G, Aktories K, Giry M, Popoff M R, Boquet P, Hegenbarth S, Von Eichel-Streiber C. Clostridium difficile toxin B acts on the GTP-binding protein rho. J Biol Chem. 1994;269:10706–10712. [PubMed] [Google Scholar]
- 14.Kato H, Kato N, Fukui K, Ohara A, Watanabe K. High prevalence of toxin A-negative/toxin B-positive Clostridium difficile strains among adult inpatients. J Clin Microbiol Infect. 1997;3:220. [Google Scholar]
- 15.Krivan H C, Clark G F, Smith D F, Wilkins T D. Cell surface binding site for Clostridium difficile enterotoxin: evidence for a glycoconjugate containing the sequence Galα1-3Galβ1-4GlcNAc. Infect Immun. 1986;53:573–581. doi: 10.1128/iai.53.3.573-581.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lima A A M, Lyerly D M, Wilkins T D, Innes D J, Guerrant R L. Effects of Clostridium difficile toxins A and B in rabbit small and large intestine in vivo and on cultured cells in vitro. Infect Immun. 1988;56:582–588. doi: 10.1128/iai.56.3.582-588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyerly D M, Barroso L A, Wilkins T D, Depitre C, Corthier G. Characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile. Infect Immun. 1992;60:4633–4639. doi: 10.1128/iai.60.11.4633-4639.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyerly D M, Johnson J L, Wilkins T D. Characterization of the binding portion of Clostridium difficile toxin A. Microecol Ther. 1989;19:233–237. [Google Scholar]
- 19.Lyerly D M, Roberts M D, Phelps C J, Wilkins T D. Properties of toxins A and B of Clostridium difficile. FEMS Microbiol Lett. 1986;33:31–35. [Google Scholar]
- 20.Lyerly D M, Saum K E, MacDonald D K, Wilkins T D. Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun. 1985;47:349–352. doi: 10.1128/iai.47.2.349-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyerly D M, Wilkins T D. Clostridium difficile. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. Infection of the gastrointestinal tract. New York, N.Y: Raven Press; 1995. pp. 867–891. [Google Scholar]
- 21a.Lyerly, D. M., and T. D. Wilkins. Unpublished data.
- 22.Martinez R D, Wilkins T D. Purification and characterization of Clostridium sordellii hemorrhagic toxin and cross-reactivity with Clostridium difficile toxin A (enterotoxin) Infect Immun. 1988;56:1215–1221. doi: 10.1128/iai.56.5.1215-1221.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez R D, Wilkins T D. Comparison of Clostridium sordellii toxins HT and LT with toxins A and B of C. difficile. J Med Microbiol. 1992;36:30–36. doi: 10.1099/00222615-36-1-30. [DOI] [PubMed] [Google Scholar]
- 24.McFarland L V, Mulligan M E, Kwok R Y Y, Stamm W E. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320:204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 25.Moore L V H, Bourne D M, Moore W E C. Comparative distribution and taxonomic value of cellular fatty acids in thirty-three genera of anaerobic gram-negative bacilli. Int J Syst Bacteriol. 1994;44:338–347. doi: 10.1099/00207713-44-2-338. [DOI] [PubMed] [Google Scholar]
- 26.Popoff M R. Purification and characterization of Clostridium sordellii lethal toxin and cross-reactivity with Clostridium difficile cytotoxin. Infect Immun. 1987;55:35–43. doi: 10.1128/iai.55.1.35-43.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torres J F. Purification and characterization of toxin B from a strain of Clostridium difficile that does not produce toxin A. J Med Microbiol. 1991;35:40–44. doi: 10.1099/00222615-35-1-40. [DOI] [PubMed] [Google Scholar]
- 28.Triadafilopoulos G, Pothoulakis C, O’Brien M J, LaMont J T. Differential effects of Clostridium difficile toxins A and B on rabbit ileum. Gastroenterology. 1987;93:273–279. doi: 10.1016/0016-5085(87)91014-6. [DOI] [PubMed] [Google Scholar]
- 29.Von Eichel-Streiber C, Heringdorf D, Habermann E, Sartingen S. Closing in on the toxic domain through analysis of a variant Clostridium difficile cytotoxin B. Mol Microbiol. 1995;17:313–321. doi: 10.1111/j.1365-2958.1995.mmi_17020313.x. [DOI] [PubMed] [Google Scholar]
- 30.Von Eichel-Streiber C, Laufenberg-Feldman R, Sartingen S, Schulze J, Sauerborn M. Comparative sequence analysis of the Clostridium difficile toxins A and B. Mol Gen Genet. 1992;233:260–268. doi: 10.1007/BF00587587. [DOI] [PubMed] [Google Scholar]