Abstract
The development of rapid and specific diagnostic tests to identify individuals infected with malaria is of paramount importance in efforts to control the severe public health impact of this disease. This study evaluated the ability of a newly developed rapid malaria diagnostic test, OptiMAL (Flow Inc., Portland, Oreg.), to detect Plasmodium vivax and Plasmodium falciparum malaria during an outbreak in Honduras. OptiMAL is a rapid (10-min) malaria detection test which utilizes a dipstick coated with monoclonal antibodies against the intracellular metabolic enzyme parasite lactate dehydrogenase (pLDH). Differentiation of malaria parasites is based on antigenic differences between the pLDH isoforms. Since pLDH is produced only by live Plasmodium parasites, this test has the ability to differentiate live from dead organisms. Results from the OptiMAL test were compared to those obtained by reading 100 fields of traditional Giemsa-stained thick-smear blood films. Whole-blood samples were obtained from 202 patients suspected of having malaria. A total of 96 samples (48%) were positive by blood films, while 91 (45%) were positive by the OptiMAL test. The blood films indicated that 82% (79 of 96) of the patients were positive for P. vivax and 18% (17 of 96) were infected with P. falciparum. The OptiMAL test showed that 81% (74 of 91) were positive for P. vivax and 19% (17 of 91) were positive for P. falciparum. These results demonstrated that the OptiMAL test had sensitivities of 94 and 88% and specificities of 100 and 99%, respectively, when compared to traditional blood films for the detection of P. vivax and P. falciparum malaria. Blood samples not identified by OptiMAL as malaria positive normally contained parasites at concentrations of less than 100/μl of blood. Samples found to contain P. falciparum were further tested by two other commercially available rapid malaria diagnostic tests, ParaSight-F (Becton Dickinson, Cockeysville, Md.) and ICT Malaria P.f. (ICT Diagnostics, Sydney, Australia), both of which detect only P. falciparum. Only 11 of the 17 (65%) P. falciparum-positive blood samples were identified by the ICT and ParaSight-F tests. Thus, OptiMAL correctly identified P. falciparum malaria parasites in patient blood samples more often than did the other two commercially available diagnostic tests and showed an excellent correlation with traditional blood films in the identification of both P. vivax malaria and P. falciparum malaria. We conclude that the OptiMAL test is an effective tool for the rapid diagnosis of malaria.
Malaria has had a resurgence in many tropical areas (6). The disease now occurs in more than 90 countries worldwide, and it is estimated that there are over 500 million clinical cases and 2.7 million malaria-caused deaths per year (8). A multitude of factors have contributed to the reemergence of malaria, including (i) insecticide resistance in the Anopheles mosquito, (ii) social instability resulting in movements of unexposed nonimmune individuals into areas where malaria is endemic, and (iii) the failure to develop an effective malaria vaccine (6, 15). Compounding the problems of malaria’s geographical expansion and of increasing morbidity and mortality are the emergence and rapid spread of antimalarial-drug resistance (2, 7), which necessitate the use of more expensive and sometimes toxic antimalarial drugs and longer treatment courses (9). In addition, the cyclic recurrence of malaria epidemics has a tremendous impact on the health infrastructure in developing countries and adversely affects local economies, since infected individuals are often too debilitated to work (6).
One of the most pronounced problems in controlling the morbidity and mortality caused by malaria is limited access to effective diagnosis and treatment in areas where malaria is endemic (10). Clinical diagnosis of infection with the malaria parasite requires microscopic observation of parasites on a Giemsa-stained blood smear. This technique has undergone little improvement since its development at the beginning of this century. Diagnosis of malaria by this method can be problematic, since it requires up to 60 min of preparation time, is labor-intensive, and requires considerable expertise for its interpretation, particularly at low levels of parasitemia (3). In addition, in patients with Plasmodium falciparum malaria, the parasites can be sequestered and are not always present in peripheral blood. Thus, a P. falciparum infection could be easily missed due to the absence of the parasite in a blood film.
Recently, a new rapid malaria detection test, OptiMAL (Flow Inc., Portland, Oreg.), was introduced. This test is based on detection of an enzyme produced by live parasites, parasite lactate dehydrogenase (pLDH), and has the ability to differentiate the four major Plasmodium species associated with human malaria (Plasmodium vivax, P. falciparum, Plasmodium ovale, and Plasmodium malariae) in under 10 min. A trial evaluation of the OptiMAL test was designed to assess its effectiveness in differentiating malaria parasites in patient blood samples during a malaria outbreak in Honduras.
MATERIALS AND METHODS
Study site.
This study was conducted in the Trujillo area of northern Honduras during a malaria outbreak in January 1997. Permission to conduct blood testing was obtained from the Honduran Ministry of Health. The majority of the blood samples were collected from individuals living in rural areas and small villages. A total of 202 whole-blood samples were collected from patients with malaria-like symptoms, including fever and/or chills, of several days’ duration.
Sample collection.
Informed consent was obtained from all patients. Five milliliters of venous blood was drawn into EDTA-coated syringes, distributed into sterile test tubes, and placed immediately on ice. Thin- and thick-smear blood films were made on-site at the time of specimen collection. Whole-blood samples plus the thin- and thick-smear microscope slides were transported the next day to the University of Miami School of Medicine laboratories for analysis. According to the local treatment guidelines for malaria, symptomatic patients were treated immediately with chloroquine (500 mg daily for 4 days) by local health care staff prior to diagnosis. Due to the lengthy ongoing malaria outbreak, this was the only antimalarial medication available at that time. Patients were instructed to return in 4 days for additional chloroquine if clinical signs persisted.
Malaria diagnosis with thin- and thick-smear films.
Thin- and thick-smear blood films were stained with 2% Giemsa stain in phosphate-buffered saline (pH 7.2) and analyzed under the microscope at ×1,000 magnification for the presence of malaria parasites. The average time spent per slide varied depending on the number of parasites present in the sample. The study was blinded, since results from microscope slides were not shared with the individuals performing the OptiMAL test until all samples were processed. Slides were read independently by two experts. Parasitemia levels were calculated with results from thick-smear films. Parasites were counted in 100 consecutive fields. Parasite densities were calculated by assuming 0.2 μl of blood per thick smear and that each microliter of blood contained 10,000 leukocytes (11, 14).
Malaria diagnosis with OptiMAL.
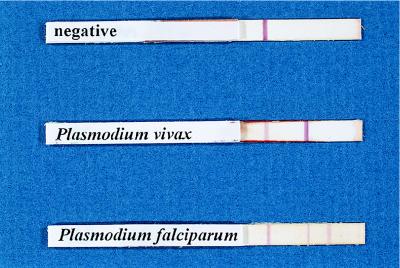
All whole-blood samples were tested with the OptiMAL assay. This test utilizes a dipstick coated with monoclonal antibodies against the intracellular metabolic enzyme pLDH. The pLDH antigen is present in and released from parasite-infected erythrocytes. Differentiation of malaria parasites is based on antigenic differences between the pLDH isoforms. Since pLDH is produced only by live Plasmodium parasites, this test has the ability to differentiate live from dead organisms. The test uses two reagents (A and B) included in the kit. Normally, the test is performed in the field by obtaining blood from a patient by a finger stick. The test was not yet available when we left for Honduras, but we obtained and evaluated it upon our return. Briefly, 1 drop of blood was mixed with 2 drops of reagent A, and the sample was allowed to migrate to the top of the OptiMAL dipstick. After 8 min, the OptiMAL strip was cleared by adding 2 drops of reagent B. The appearance of a dark band on the strip indicates a positive reaction for any one of the four major malarial species infecting humans. The monoclonal antibody attached at this area of the strip is against an enzyme common to the four target Plasmodium species. If P. falciparum was present in the test sample, a second band appeared on the strip. The monoclonal antibody at this site is specific for P. falciparum only. A mixed infection with P. falciparum and another Plasmodium species is indicated when both genus- and species-specific bands appear and the genus-specific band is much darker and more intense than the species-specific band. The darker band is present with mixed infections, since all stages of the parasite are present in the blood during P. vivax infections, while only two stages (ring and gametocyte) are present in the peripheral blood during P. falciparum infections. Since the pLDH enzyme is present in all stages and since there are more stages present during P. vivax infections, there is a stronger response with the OptiMAL stick. A positive control band appears on each strip as an indicator that the test is working correctly (Fig. 1).
FIG. 1.
OptiMAL sticks showing detection of P. vivax and P. falciparum and a negative serum sample.
P. falciparum malaria diagnosis with the ParaSight-F and ICT malaria tests.
Two commercially available tests, ParaSight-F (Becton Dickinson, Cockeysville, Md.) and ICT Malaria P.f. (ICT Diagnostics, Sydney, Australia), were tested on blood samples that were positive for P. falciparum to determine the level of agreement with the OptiMAL test and blood smears. The manufacturer’s instructions were followed.
RESULTS
Comparison of OptiMAL and blood film methods.
A total of 202 blood samples were tested for malaria parasites by the OptiMAL method, and the results were compared to results obtained from reading thin- and thick-smear blood films. The blood film results indicated that 48% (96 of 202) of the patients were infected with malaria, based on the morphologies of the parasite stages. Among the positive patient samples, P. vivax was present in 82% (79 of 96) while P. falciparum was present in 18% (17 of 96). Correspondingly, the OptiMAL test results indicated that 45% (91 of 202) of the patient samples were positive for malaria parasites. Infections with P. vivax accounted for 81% (74 of 91) of the positive samples, while infections with P. falciparum accounted for 19% (17 of 91) of the total malaria cases. Both methods identified one patient with a mixed infection of P. falciparum and P. vivax. The mixed infection has been tabulated with the P. falciparum infection numbers for ease of analysis.
The blood films identified five P. vivax-positive samples that were not identified by the OptiMAL test; however, there was 100% agreement between blood film results and OptiMAL results for the other 74 samples containing P. vivax. Although both methods detected 17 cases of P. falciparum infection, there were 2 cases detected by OptiMAL that were not detected by the blood films and 2 cases detected by blood film that were not detected by the OptiMAL method. OptiMAL had sensitivities of 94% (95% confidence interval [CI], 85.2 to 97.6%) and 88% (95% CI, 62.3 to 97.9%) and specificities of 100% (95% CI, 96.2 to 100.01%) and 99% (95% CI, 95.5 to 99.8%), respectively, when compared to traditional blood films for the detection of P. vivax and P. falciparum infections (Table 1). Positive and negative predictive values were 100% (95% CI, 93.9 to 100.0%) and 96% (95% CI, 90.7 to 98.6%), respectively, for P. vivax and 88% (95% CI, 62.3 to 97.9%) and 99% (95% CI, 95.7 to 99.8%), respectively, for P. falciparum.
TABLE 1.
Malaria parasite detection by blood films and by OptiMAL
| Species | OptiMAL result | Blood film result
|
||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| P. vivax | Positive | 74 | 0 | 74 |
| Negative | 5 | 123 | 128 | |
| Total | 79 | 123 | 202 | |
| P. falciparum | Positive | 15 | 2 | 17 |
| Negative | 2 | 183 | 185 | |
| Total | 17 | 185 | 202 | |
Parasitemia.
Parasitemia levels ranged from 0.001 to 12% in the patient samples. This translated into 50 to 600,000 parasites/ml of blood. Patients with P. vivax present in the blood film and who were diagnosed as negative by the OptiMAL method had parasitemia values ranging from 0.001 to 0.008%. The two patients with P. falciparum present in blood films that was not detected by OptiMAL had parasitemia values of 0.001 and 0.004%. However, parasitemia levels of 0.001% in other blood samples were detected by OptiMAL, indicating the level of error that is inherent in the enumeration of organisms on blood films.
The OptiMAL test proved more sensitive at higher levels of parasitemia (Table 2). Sensitivity in detecting P. vivax was 40% when parasites were present at less than 100/μl of blood and rose to 100% when parasites were present at more than 100/μl. Two samples containing P. vivax at more than 100/μl were missed by OptiMAL. Sensitivity of detection for P. falciparum was 67% when parasites were present at less than 100/μl of blood, and one P. falciparum-positive blood sample was missed by OptiMAL at parasite levels greater than 100/μl of blood.
TABLE 2.
Parasite levels detected by blood films and by OptiMAL
| No. of parasites/μl of blood | Species | No. of positive blood films | No. of OptiMAL results
|
Sensitivity (%) | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| 1–99 | P. vivax | 5 | 2 | 3 | 40 |
| P. falciparum | 3 | 2 | 1 | 67 | |
| 100–199 | P. vivax | 8 | 8 | 0 | 100 |
| P. falciparum | 1 | 1 | 0 | 100 | |
| 200–499 | P. vivax | 7 | 6 | 1 | 86 |
| P. falciparum | 0 | 0 | 0 | NAa | |
| 500–999 | P. vivax | 3 | 3 | 0 | 100 |
| P. falciparum | 1 | 1 | 0 | 100 | |
| 1,000–1,999 | P. vivax | 4 | 4 | 0 | 100 |
| P. falciparum | 4 | 3 | 1 | 75 | |
| >2,000 | P. vivax | 52 | 51 | 1 | 98 |
| P. falciparum | 8 | 8 | 0 | 100 | |
NA, not applicable.
Comparison of OptiMAL, ParaSight-F, and ICT Malaria P.f. for the detection of P. falciparum.
Results from the ParaSight-F test and the ICT test did not always agree with results from the OptiMAL test in the diagnosis of P. falciparum malaria. Only 11 of the 17 (65%) P. falciparum-positive blood samples were identified by the other two tests. For these 11 positive samples, there were differences between the tests, since the ParaSight-F test identified one positive sample missed by the ICT test and the ICT test identified one positive sample missed by the ParaSight-F test. Five of the six samples that were not judged positive by the ParaSight-F and ICT tests were from patients with 50 P. falciparum organisms/μl of blood (0.001% parasitemia). The sixth was from a patient with 0.003% parasitemia.
DISCUSSION
This study compared the diagnosis of malaria by a new rapid test, OptiMAL, with malaria diagnosis by traditional microscopy and found that the two methods yielded comparable results. A total of 202 symptomatic patients were tested; blood films identified 48% of these as positive for malaria parasites, while the OptiMAL test identified 45% as malaria positive. That some malaria infections detected by blood films were not detected by the OptiMAL test may be explained by the fact that OptiMAL detects pLDH, which is produced only by living parasites. It is possible that some of the patients infected with malaria medicated themselves when malaria symptoms appeared during this outbreak and did not report this to the attending clinician. There are several possible explanations for discrepancies in test results obtained by blood film examination and by the OptiMAL test, including (i) insufficient detection of low parasitemia levels by OptiMAL, (ii) the fact that OptiMAL detects only live parasites producing pLDH, (iii) the sequestration of parasites, and (iv) false-positive reactions. Thus, the P. vivax and P. falciparum organisms observed in the seven blood film samples judged negative by OptiMAL may have been dead and not yet cleared from the host. The two patient blood samples in which OptiMAL detected P. falciparum and whose blood films were negative may be explained by the fact that P. falciparum can sequester and not be present in circulating blood. This suggests that the OptiMAL test may provide a more precise diagnosis of patients infected with P. falciparum malaria by detecting parasites that would be missed by traditional blood film screening. Another explanation could be that the patient blood samples contained parasites at concentrations below the OptiMAL test’s detection levels.
Currently there are two other commercially available rapid tests, the ParaSight-F and ICT tests, for diagnosis of P. falciparum malaria. While both of these tests offer diagnosis of malaria in under 15 min, there are differences between these two tests and the OptiMAL test evaluated in this study. The first difference is that the ICT and ParaSight-F tests are based on detection of parasite histidine-rich protein 2, which has been shown to remain in the blood at least 28 days after the initiation of antimalarial therapy (5, 13). Thus, parasite clearance after drug therapy may not be determined and patients who have cleared the parasite may be falsely diagnosed as malaria positive and given additional drug therapy. The second difference is that the ICT and ParaSight-F tests detect only one of the four Plasmodium species known to infect humans, P. falciparum. Since over half of the patient samples in this study contained P. vivax, the use of the ParaSight-F and ICT Malaria P.f. tests alone would have meant that the majority of the patients in this study would not have been diagnosed with malaria.
The sensitivities of the three tests were not similar in our study, since OptiMAL identified more P. falciparum-positive samples than did the ICT and ParaSight-F tests. There are few published studies on the ICT test. A report by Garcia et al. (4) indicated that the ICT test was able to detect P. falciparum malaria with parasite densities exceeding 80/μl but that it was unknown whether the test worked with densities of less than 80/μl. There are several reports in the literature on the ParaSight-F test. Its sensitivity has been reported by other researchers to range from 40 to 100% with parasite densities under 1,000/μl (1, 5, 12); however, those authors used different methods for the determination of parasite density. This is a common problem, since different laboratories base parasite densities on leukocyte counts which vary between 1,000 and 10,000/μl of blood. Other laboratories determine parasite density by using erythrocyte formulations. The use of various methods to determine levels of malaria parasitemia is turning into a major problem, as it makes comparisons of test results from different malaria researchers difficult.
There are compelling reasons to justify the implementation of a rapid malaria diagnostic test in the field. None of the rural clinics in this study had the ability to diagnose malaria on-site due to a lack of microscopes and trained technicians to evaluate blood films. Moreover, patient follow-up is difficult due to economic constraints. Many people cannot afford transportation, so they walk several hours, some carrying small children, to reach a local clinic; once they have been seen, they do not return. Diagnosis must therefore be immediate in order to provide proper treatment. This is of particular importance in areas such as Honduras, where dengue fever and other tropical diseases mimic each other’s symptoms (e.g., fever, chills, and headache). In this study, roughly half of the patients symptomatic for malaria were actually infected with malaria parasites. However, all received antimalarial medication, based on symptoms alone.
The world health care community is in desperate need of rapid and precise diagnostic tools to assist in controlling malaria, monitoring drug therapy, and limiting mortality and suffering. Our data demonstrated that the OptiMAL test is an effective, sensitive, and rapid diagnostic test for malaria that could be invaluable in the fight to control malaria. Additional studies to evaluate the use of OptiMAL in monitoring drug therapy and in the detection of drug-resistant malarial strains are under way.
ACKNOWLEDGMENTS
This project was funded by NIH Fogarty grant 5D43TW00017-08.
We thank the Honduran Ministry of Health for allowing access to malaria patients and Honduran local health care staff for their assistance with this study. We are most grateful to Merita Aviles and Xiaofeng Liang for technical assistance.
REFERENCES
- 1.Beadle C, Long G W, Weiss W R, McElroy P D, Maret S, Oloo A J, Hoffman S L. Diagnosis of malaria by detection of Plasmodium falciparum HRP-2 antigen with a rapid dipstick antigen-capture assay. Lancet. 1994;343:564–568. doi: 10.1016/s0140-6736(94)91520-2. [DOI] [PubMed] [Google Scholar]
- 2.Collins W E, Jefferey G M. Primaquine resistance in Plasmodium vivax. Am J Trop Med Hyg. 1996;55:243–249. doi: 10.4269/ajtmh.1996.55.243. [DOI] [PubMed] [Google Scholar]
- 3.Dourado H, Abdon N, Martins S J. Falciparum malaria. Infect Dis Clin N Am. 1994;8:207–223. [PubMed] [Google Scholar]
- 4.Garcia M, Kirimoama S, Marlborough D, Leafasia J, Rieckmann K H. Immunochromatographic test for malaria diagnosis. Lancet. 1996;347:1549. doi: 10.1016/s0140-6736(96)90700-x. [DOI] [PubMed] [Google Scholar]
- 5.Humar A, Ohrt C, Harrington M A, Pillai D, Kain K C. ParaSight F test compared with the polymerase chain reaction and microscopy for the diagnosis of Plasmodium falciparum malaria in travelers. Am J Trop Med Hyg. 1997;56:44–48. doi: 10.4269/ajtmh.1997.56.44. [DOI] [PubMed] [Google Scholar]
- 6.Krogstad D J. Malaria as a reemerging disease. Epidemiol Rev. 1996;18:77–89. doi: 10.1093/oxfordjournals.epirev.a017918. [DOI] [PubMed] [Google Scholar]
- 7.Longworth D L. Drug-resistant malaria in children and travelers. Antimicrob Resistance Pediatr. 1995;42:649–664. doi: 10.1016/s0031-3955(16)38983-0. [DOI] [PubMed] [Google Scholar]
- 8.Nussenzweig R S, Zavala F. A malaria vaccine based on a sporozoite antigen. N Engl J Med. 1997;336:128–130. doi: 10.1056/NEJM199701093360210. [DOI] [PubMed] [Google Scholar]
- 9.Olliaro P, Cattani J, Wirth D. Malaria, the submerged disease. JAMA. 1996;275:230–233. [PubMed] [Google Scholar]
- 10.Pan American Health Organization. Malaria in the Americas. PAHO Bull. 1996;17:1–8. [Google Scholar]
- 11.Petersen E, Marbiah N T, New A, Gottschau A. Comparison of two methods for enumerating malaria parasites in thick blood films. Am J Trop Med Hyg. 1996;55:485–489. doi: 10.4269/ajtmh.1996.55.485. [DOI] [PubMed] [Google Scholar]
- 12.Shiff C J, Minjas J, Premji Z. The Parasight-F test: a simple rapid manual dipstick test to detect Plasmodium falciparum infection. Parasitol Today. 1994;10:494–495. doi: 10.1016/0169-4758(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 13.Verle P, Binh L N, Lieu T T, Yen P T, Coosemans M. ParaSight-F test to diagnose malaria in hypo-endemic and epidemic prone regions of Vietnam. Trop Med Int Health. 1996;1:794–796. doi: 10.1111/j.1365-3156.1996.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 14.Warhurst D C, Williams J E. Laboratory diagnosis of malaria. J Clin Pathol. 1996;49:533–538. doi: 10.1136/jcp.49.7.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wernsdorfer W H. Epidemiology of drug resistance in malaria. Acta Trop. 1994;56:143–156. doi: 10.1016/0001-706x(94)90060-4. [DOI] [PubMed] [Google Scholar]