Abstract
The Elek culture plate precipitin test is routinely used for the detection of exotoxin from toxigenic strains of Corynebacterium diphtheriae. Recently, the World Health Organization standardized this test to ensure accuracy, reliability, and reproducibility. In this study, we further modified the standard Elek test by using the antitoxin-in-membrane (AIM) and antitoxin-in-well (AIW) approaches. In the AIM tests, each strain was stabbed and streaked backwards and away from a point approximately 7 mm from the edge of a sterile cellulose acetate-cellulose nitrate filter membrane disk (pore size, 0.45 μm; diameter, 25 mm) containing 25 IU of diphtheria antitoxin. For AIW tests, a central well (diameter, 5 mm) containing 9 μl of antitoxin (4.5 IU) was surrounded by eight equidistant stab-streaks of each strain placed 10 mm from the well. In both methods, precipitin bands of identity typically were noted after 24- and 48-h incubations at 37°C. Both toxigenic and weak toxigenic strains gave clear and reproducible results. Compared with the standard Elek test, the AIM and AIW tests each use 50% less medium and 75 and 87% less antitoxin, respectively. AIM has the potential to test up to 14 isolates and AIW has the potential to test up to 24 isolates on the same plate. Furthermore, clearer positives were noted with weak toxigenic strains. In a blinded test of 209 verified C. diphtheriae isolates, a 99.5% agreement with the standard Elek test was obtained overall. Both modifications conserve reagents and medium, permit the simultaneous testing of a larger number of strains, and may be particularly suitable for reference laboratories or hospitals involved in diphtheria epidemic settings.
Significant outbreaks and cases of diphtheria have occurred recently in the New Independent States of the former Soviet Union, and additional cases have appeared in neighboring countries (1, 5, 8, 9). The diagnosis of diphtheria on clinical grounds is usually based on classic pathognomonic signs, which include pseudomembrane in the pharynx and the effects of the potent exotoxin on distant organs (6, 7, 10–12). Elek, in 1948, described a relatively simple and reliable in vitro test for diphtheria toxin detection (3, 4). For almost 50 years, this test has served as the standard test. The method and media have recently been standardized by the World Health Organization (2). In light of the current diphtheria epidemic, reference laboratories and some large hospitals and diagnostic centers may need to screen for the toxin among increased numbers of Corynebacterium diphtheriae isolates. We studied and tested various potential enhancements of the Elek assay, and we describe here the antitoxin-in-membrane (AIM) and antitoxin in-well (AIW) procedures, which provide for a better economy of time and resources.
(This work was presented in part at the 13th International Convocation of Immunology, State University of New York, Buffalo, N.Y., June 1996.)
MATERIALS AND METHODS
Strains.
A total of 209 C. diphtheriae strains were included in this study. All strains are part of the collection of the Diphtheria Research Project, National Center for Infectious Diseases, Centers for Disease Control and Prevention. Fresh cultures, grown at 37°C for 24 h on sheep blood agar (Carr-Scarborough Microbiologicals, Atlanta, Ga.), were used for all tests. The control tox+ strains used were 496, 510, 880, G4169, and G4177. The control weak tox+ strains used were 881 and G4179, and the control tox-negative strains used were 511, 916, and 917. All tests were done in duplicate.
Medium.
Elek agar was prepared by the addition of newborn bovine serum (ICN Pharmaceuticals Inc., Irvine, Calif.) to Elek basal medium agar (2) at a 1:5 ratio (vol/vol). For the AIM and AIW tests exactly half of the amount of medium used with the standard Elek test (9 versus 18 ml) was added to sterile 15- by 110-mm plastic petri dishes.
Antitoxin.
The two equine diphtheria antitoxins that were used were obtained from Wyeth Laboratories Inc. (Marietta, Pa.) and Connaught Laboratories (Swiftwater, Pa.). Unless otherwise specified, a sterile, water-diluted final concentration of both antitoxins at 500 IU/ml was used as the working solution for the standard Elek plate test and the AIM and AIW tests.
Incubation and plate readings.
All test plates were incubated at 37°C and observed at 24- and 48-h intervals for the appearance of precipitin bands. These bands developed in the areas of optimal proportions for antitoxin-toxin reaction and were seen best by reflected light against a dark background (3).
Number of tests and comparisons.
The AIM and AIW tests and the standard Elek toxin plate tests were performed with 209 different CDC strains to test and verify the toxigenicity of the strains.
Standard Elek assay.
The standard Elek test was done as described earlier (2). In this test, a weak tox+ strain and a tox+ strain were each tested for precipitin reactions after inoculation into medium by either a single stab inoculation or a stab and a backward surface streak. Both types of inoculation were compared at test distances of 5, 6, 7, 8, 9, 10, and 11 mm from the paper strip.
AIM test.
Various synthetic filter membrane disks were tested: cellulose nitrate and polycarbonate disks (VWR Scientific, Atlanta, Ga.); tuffryn, polyvinylidene, and nylon disks (Gelman Sciences Inc., Ann Arbor, Mich.); and cellulose acetate-cellulose nitrate disks (Millipore Corp., Bedford, Mass.). All disks had pore sizes of 0.45 μm, except for the VWR polycarbonate membrane filter, which had a pore size of 0.40 μm. One hour after the agar had solidified, cooled, and dried on the surface, a sterile, 0.45-μm-pore-size filter membrane disk (diameter, 25 mm), containing 0.05 ml of antitoxin (25 IU total), was placed on each Elek agar plate. Next, each strain was stabbed into the agar with a 0.001-ml loop and then surface streaked backwards for a few millimeters. In a small pilot study, the optimal distance between inoculum and disk was determined by stab inoculations, or stab-streak inoculations, which were done at distances of 5, 6, 7, 8, 9, 10, 11, and 15 mm from the edge of the filter membranes. These initial tests were done with six control strains: three tox+ strains, two tox-negative strains, and one weak tox+ strain. Up to 14 isolates could be tested with a single disk containing the antitoxin.
AIW test.
A central, 5-mm-diameter well was prepared with a sterile, stainless-steel cutter followed by aspiration of the cut agar. The well was filled with 9 μl of the antitoxin (4.5 IU) and was surrounded by eight 0.001-ml loops of individual strains of C. diphtheriae which were then stabbed into the agar at a distance of 10 or 15 mm from the edge of the center well and surface-streaked backwards for a few millimeters. Up to three sets of this arrangement could be done on one plate (24 isolates/plate).
RESULTS
Standard Elek plate tests.
The standard Elek plate tests produced typical precipitin bands within 48 h following incubation at 37°C. There was no detection of the weak tox+ strain at either 24 or 48 h for distances beyond 8 mm. At 24 h, no precipitin bands were produced by or detected for either the weak or tox+ strain, with the exception that the tox+ strain produced precipitation bands at 7 mm. The tox+ strain produced precipitin bands by 48 h at all tested distances (5, 6, 7, 8, 9, 10, and 11 mm).
AIM test.
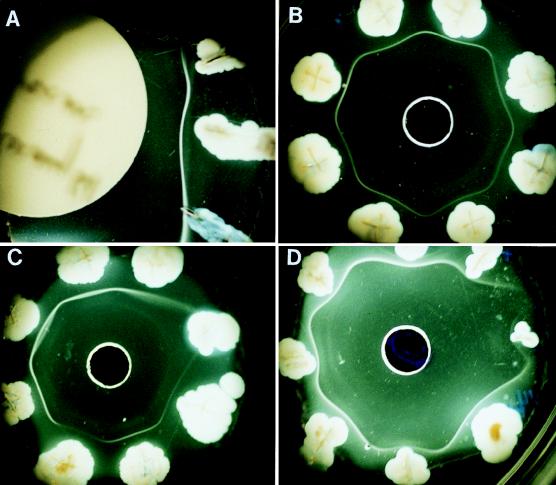
Up to 14 isolates could be tested on a single petri dish with one AIM test (Fig. 1A). One weak tox+ (881) and four tox+ (496, 510, 880, and G4169) strains gave clear and reproducible results in our initial, quadruplicated experiments with both the AIM and standard Elek tests. The inoculation of strains by a combined stab into agar with a surface streak backward for 5 mm was better than a simple stab alone. By the stab-and-surface-streak-backward method, precipitin bands tended to appear earlier and were more intense and curved around the positive culture. Furthermore, these bands were seen more consistently as positive by 48 h with the stab-streak method than were the simple stab inoculations. The weak tox+ strain, which was negative at 48 h by the stab-only method, was positive by 48 h with the stab-streak method.
FIG. 1.
(A) Segment of an Elek agar plate showing AIM test with three different toxigenic C. diphtheriae isolates and their precipitin bands. (B) AIW test with a 5-mm-diameter center well containing 9 μl of antiserum surrounded by eight different, toxigenic C. diphtheriae isolates inoculated originally at a distance of 10 mm from the center well. Precipitin bands of identity are shown. (C) AIW test with seven toxigenic and one nontoxigenic isolate. (D) AIW test with seven toxigenic and one weakly toxigenic strain.
Inoculum distances.
An AIM separation distance of 15 mm resulted in fewer bands after 24 h than did closer placements between the stab-streak cultures and the disks. Precipitin bands usually formed clearly and quickly after a 24-h incubation at 5- to 10-mm separations. The weak tox+ strain was positive by 24 h at a 7-mm distance but not at 10 mm. However, by 48 h, this same strain was positive at the 10-mm separation distance. Based on these data, 7 mm was chosen as the best membrane-to-inoculum distance for all subsequent AIM tests. Precipitin bands of identity were typically noted as early as 24 h, and no later than 48 h, at 37°C.
Comparison of standard Elek assay and AIM by testing 209 C. diphtheriae strains.
The AIM test was evaluated against 209 strains in a blind fashion. Of the 209 strains, the AIM test identified 181 (86.6%) as toxigenic and 28 (13.4%) as nontoxigenic. There was a 99.5% (208 of 209) concurrence between the AIM and the standard Elek methods. The single variable strain was a weak-toxin producer. When the strains were retested for reproducibility, there was complete (100%) agreement of the results for all 209 strains.
Membrane filters.
Six different membrane filter disks were evaluated and compared for precipitin immunodiffusion reactions (Table 1). With the six tester strains, bands were detected by 48 h for all positive strains, and no bands were detected for the negative strains. There were no false positives or negatives. Cellulose nitrate, nylon, polyvinylidene, tuffryn, and cellulose acetate-cellulose nitrate disks each gave bands with the tox+ strains after 24 h. Only the polycarbonate disk was negative at 24 h. Millipore cellulose acetate-cellulose nitrate disks formed the clearest and most distinct bands within 24 h. The cellulose nitrate, nylon, tuffryn, and polyvinylidene disks had distinct bands but none of these antitoxin disks consistently gave as strong or as clear precipitin bands as did the Millipore acetate-nitrate disk.
TABLE 1.
Properties of membrane filters and characteristic AIM precipitin reactions obtaineda
| Membrane filter (trade name) | Manufacturer | AIM test results |
|---|---|---|
| Cellulose acetate-cellulose nitrate | Millipore Corp. | Very clear, distinct precipitin bands by 24 h with all tox+ strains |
| Tuffrynb | Gelman | Bands detected as early as 24 h but not as clear or distinct as on Millipore filter |
| Polyvinylidene (Veficel)c | Gelman | Bands detected with strong and weak tox+ strains at 24 and 48 h respectively. Bands much fainter than on Millipore filter |
| Nylon (Nyloflan) | Gelman | Bands detected with strong and weak tox+ strains at 24 and 48 h. Bands much fainter than on Millipore filter |
| Polycarbonate | VWR | No precipitin bands detected at 24 h. All tox+ strains were detected at 48 h. |
| Cellulose nitrate | VWR (Nagle) | No bands detected at 24 h. All bands developed between 24 and 48 h. Distinct precipitin bands were nearly as strong as on Millipore filter |
All membrane pore sizes were 0.45 μm with the exception of the VWR polycarbonate filter, which had a pore size of 0.40 μm. All membrane filters were tested against four tox+, one weak tox+, and one tox-negative strain.
Hydrophilic membrane filter; extremely low-protein binding.
Hydrophilic membrane filter.
AIW test.
Precipitin bands formed in 9-ml Elek agar plates where toxigenic C. diphtheriae was stabbed at distances of either 10 or 15 mm from the central well containing 9 μl of antitoxin (Fig. 1B). Bands were detected earlier (24 versus 48 h) when the inoculum was placed at a distance of 10 mm from the central antibody well. There was 100% concurrence between the AIW test and the AIM test for the 209 C. diphtheriae strains and a 99.5% agreement between these tests and the standard Elek test.
DISCUSSION
In this study we modified the standard Elek test procedure to conserve reagents and medium and to permit the simultaneous testing of a greater number of strains for reference laboratories or hospitals involved in diphtheria epidemic settings. Elek suggested that test plates not be read after 48 h since false-positive precipitin bands may occur (4). We routinely followed this admonition. Our results also confirmed previous observations that it is essential that only fresh cultures be used for tests. Reliable results were always obtained with cultures that were 24-h old. The AIM and AIW tests and modifications of the medium, as presented here, were acceptable and reliable replacements for the standard Elek test. When appropriate medium, cultures, and techniques were used, highly reproducible and correlated results were obtained. There was a 100% concurrence between the AIW and the AIM tests and a 99.5% agreement between each test procedure and the standard Elek test. The AIW test demonstrated that precipitin bands can routinely be detected on the Elek medium if antibody-containing wells alone were used instead of filter strips or membranes. The precipitin bands were shown to develop in the areas of optimal proportions for antitoxin-toxin reactions, and when two neighboring and similar precipitin bands met, a band of identity formed between the two. Elek referred to this bending of the bands as “looping.” This looping is equivalent to what we term a bowing between the two bands, which can be used to identify a weak-toxigenic strain. Such a strain may not produce an immediately obvious precipitin band. Therefore, when bowing or looping is observed between an obvious strong toxigenic strain and an adjacent strain, it can be inferred that the adjacent strain is also positive. Obviously, this bowing will be seen only when the strain next to the weak toxigenic strain is toxigenic. With the AIM and AIW plate tests, a strong precipitin band will also show bowing when influenced by a weaker neighboring strain that often has not yet produced a precipitin band. Some weak toxigenic strains bow but never form a precipitin band. Of the 209 strains tested for toxigenicity, only one proved to be a problem in our blind study evaluation. It was initially diagnosed as negative but was a weak tox+ in the standard Elek test. Subsequently, it was weakly positive upon retest. Typically, this weak tox+ strain did not regularly form a precipitin band by 24 h. As with most weak tox+ strains, a precipitin band could be observed between 24 and 48 h. This weak tox+ strain was always diagnosed more easily when it was placed next to a known positive strain: a bowing was often observed, indicating toxigenicity. Finally, when searching for toxigenic strains of C. diphtheriae on clinical isolation plates, several suspect colonies should be tested and evaluated concurrently against a known, reliable tox+ reference strain.
We evaluated AIM tests and the precipitin reactions obtained with membrane filters that had different chemical and physical properties. Our results showed that all filters are not created equally as far as the AIM tests are concerned. Of the six different types of filter, the Millipore cellulose acetate-cellulose nitrate filter gave the clearest and most reproducible banding patterns. The binding of antibody to the filter and the rates of its release and migration into the medium are obviously important considerations. Therefore, only certain membrane filters are suitable for the AIM test.
In conclusion, this study demonstrated that two new, simple, reliable tests (AIM and AIW) can conserve medium and antiserum and permit the evaluation of as many as 14 or 24 isolates per plate. The procedures should prove particularly useful to reference laboratories and hospitals and diagnostic centers involved in diphtheria epidemic settings, where numerous isolates must be tested in short periods of time.
ACKNOWLEDGMENT
This work was supported by a grant from the Georgia Immunization Program, Atlanta, Ga.
REFERENCES
- 1.Centers for Disease Control and Prevention. Diphtheria epidemic—New Independent States of the Former Soviet Union, 1990–1994. Morbid Mortal Weekly Rep. 1995;44:177–181. [PubMed] [Google Scholar]
- 2.Efstratiou A, Maple P A C. WHO manual for the laboratory diagnosis of diphtheria. Reference no. ICP-EPI 038(C). Geneva, Switzerland: World Health Organization; 1994. [Google Scholar]
- 3.Elek S D. The recognition of toxigenic bacterial strains in vitro. Br Med J. 1948;1:493–496. doi: 10.1136/bmj.1.4549.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elek S D. The plate virulence test for diphtheria. J Clin Pathol. 1949;2:250–258. doi: 10.1136/jcp.2.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galazka A M, Robertson S E, Oblapenko G P. Resurgence of diphtheria. Eur J Epidemiol. 1995;11:1–11. doi: 10.1007/BF01719954. [DOI] [PubMed] [Google Scholar]
- 6.Mims C A, Playfair J H L, Roitt I M, Wakelin D, Williams R, editors. Medical microbiology. London, United Kingdom: Mosby Year Book Europe Limited; 1993. [Google Scholar]
- 7.Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: American Society for Microbiology; 1995. [Google Scholar]
- 8.Nakao H, Pruckler J M, Mazurova I K, Narvskaia O V, Glushkevich T, Marijevski V F, Kravetz A N, Fields B S, Wachsmuth I K, Popovic T. Heterogeneity of diphtheria toxin gene, tox, and its regulatory element, dtxR, in Corynebacterium diphtheriae strains causing epidemic diphtheria in Russia and Ukraine. J Clin Microbiol. 1996;34:1711–1716. doi: 10.1128/jcm.34.7.1711-1716.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popovic T, Kombarova S Y, Reeves M W, Nakao H, Mazurova I K, Wharton M, Wachsmuth I K, Wenger J D. Molecular epidemiology of diphtheria in Russia, 1985–1994. J Infect Dis. 1996;174:1064–1071. doi: 10.1093/infdis/174.5.1064. [DOI] [PubMed] [Google Scholar]
- 10.Sartwell P E. Diphtheria. In: Sartwell P E, editor. Preventative medicine and public health. 10th ed. New York, N.Y: Appleton-Century Crofts; 1984. pp. 158–168. [Google Scholar]
- 11.Waldman R H. Corynebacteria. In: Waldman R H, Kluge R M, editors. Infectious diseases. New Hyde Park, N.Y: Medical Examination Publishing Co. Inc.; 1983. pp. 741–750. [Google Scholar]
- 12.Youmans G P. Diphtheria. In: Youmans G P, Paterson P Y, Sommers H M, editors. The biologic and clinical basis of infectious diseases. W. B. Philadelphia, Pa: Saunders Company; 1985. pp. 238–258. [Google Scholar]