Abstract
A rapid (<7-min) immunochromatographic test for immunoglobulin M (IgM) and IgG antibodies to dengue viruses was evaluated by using hospital admission and discharge sera from 124 patients. The reference laboratory diagnosis was based on the results of virus isolation, hemagglutination-inhibition assay (HAI), and enzyme immunoassay (EIA). By the standard assays, patients experienced primary dengue virus infection (n = 30), secondary dengue virus infection (n = 48), Japanese encephalitis (JE) virus infection (n = 20), or no flavivirus infection (n = 26). The rapid test demonstrated 100% sensitivity in the diagnosis of dengue virus infection and was able to distinguish between primary and secondary dengue virus infections through the separate determinations of IgM and IgG. For all patients with primary dengue virus infection a positive test for IgM to dengue virus and a negative test for IgG to dengue virus were obtained, whereas for 46 of 48 patients (96%) with secondary dengue virus infection, a positive test for IgG to dengue virus with or without a positive test for IgM to dengue virus was obtained. The remaining two patients with secondary dengue virus infection had positive IgM test results and negative IgG test results. Furthermore, the rapid test was positive for patients confirmed to be infected with different dengue virus serotypes (12 infected with dengue virus serotype 1, 4 infected with dengue virus serotype 2, 3 infected with dengue virus serotype 3, and 2 infected with dengue virus serotype 4). The specificity of the test for nonflavivirus infections was 88% (3 of 26 positive), while for JE virus infections the specificity of the test was only 50% (10 of 20). However, most patients with secondary dengue virus infection were positive for both IgM and IgG antibodies to dengue virus, while no patients with JE virus infection had this profile, so cross-reactivity was only a concern for a small proportion of patients with secondary dengue infections. The rapid test demonstrated a good correlation with the reference EIA and HAI and should be useful for the rapid diagnosis of dengue virus infections.
Dengue viruses (family Flaviviridae, genus Flavivirus) are found in many areas of the tropics and subtropics. The four dengue virus serotypes (dengue virus types 1 to 4) are closely related yet antigenically distinct (28). The viruses cause disease in humans and are transmitted by mosquito, principally Aedes aegypti. In terms of morbidity, mortality, and economic costs, dengue virus infection is the most important mosquito-borne virus disease in the world, with an estimated 100 million cases per year. Furthermore, the incidence and spread of the disease are increasing (21).
Infection with a dengue virus may be clinically inapparent or may be present as a nonspecific febrile illness, classic dengue fever, or dengue hemorrhagic fever (DHF) (2). Classic dengue fever is characterized by fever, malaise, headache, arthralgia, myalgia, and rash. In the early febrile phase, DHF is indistinguishable from dengue fever; as fever remits, DHF is distinguished from dengue fever by the onset of plasma leakage, marked thrombocytopenia, and a bleeding diathesis. Severe plasma leakage can lead to shock (dengue shock syndrome), with the mortality rate for untreated patients being in excess of 10%. Proper fluid management can be lifesaving (11, 22).
Traditionally, the hemagglutination-inhibition assay (HAI) has been used to classify dengue virus infections as a first or primary infection (gradual increase to a moderate titer) versus a sequential or secondary infection (rapid increase to a high titer) (26). A primary antibody response suggests a first flavivirus infection for the individual. Secondary infections have been associated with more severe disease in areas where dengue virus infection is endemic (8). This serologic definition depends upon an assay with paired serum specimens, with the second specimen collected at least 7 days into the illness, although any acute-phase specimen with a hemagglutination-inhibition titer of ≥1:2,560 is defined as coming from a patient experiencing a secondary dengue virus infection (29). However, the variable potency of hemagglutinins made in different laboratories has compromised the general applicability of this assay in the classification of dengue virus infections. More recently, the enzyme immunoassay (EIA) for immunoglobulin M (IgM) and IgG antibodies to dengue virus has been shown to distinguish primary from secondary dengue virus infections, but the test may require an overnight incubation (1, 5, 10, 14); a more rapid test would be advantageous. Separate dot blot assays for IgM and IgG antibodies to dengue virus have been developed, but their ability to distinguish antibody patterns (primary versus secondary) is poorly characterized (3, 4, 18).
The PanBio Dengue Fever Rapid Test (Dengue Rapid Test) is an immunochromatographic test for the determination of IgM and IgG antibodies to dengue viruses. This test offers advantages over HAI and traditional EIAs for the serologic diagnosis of dengue virus infections since it provides standardized reagents which should reduce interlaboratory variation and test performance requires less than 7 min. In this study, the Dengue Rapid Test was compared to EIA and HAI by using paired serum specimens from patients with or without dengue virus infections.
MATERIALS AND METHODS
Case definitions.
In children experiencing a febrile illness consistent with dengue fever or DHF, dengue virus infections were defined as the isolation of a dengue virus, the detection of IgM to dengue virus (as opposed to IgM to Japanese encephalitis [JE] virus), or a sustained elevation (≥1:2,560) or a fourfold rise in dengue virus hemagglutination-inhibition titer. JE virus infection was defined as a febrile illness associated with a decrease in consciousness and the presence of IgM to JE virus in the cerebrospinal fluid. Dengue virus infection was categorized as primary or secondary according to the World Health Organization criteria (29) and the standard operating procedure for the reference EIA (10).
Serum samples.
Serum samples were collected from patients at the time of hospital admission and the time of discharge at either the Queen Sirikit National Institute of Child Health (Bangkok Children’s Hospital); the Kamphaeng Phet Provincial Hospital, Thailand; or the Centre for Tropical Diseases, Ho Chi Minh City, Vietnam. The serum was frozen at −70°C prior to assay. In this study we used paired serum specimens from 124 patients, representing patients with primary dengue virus infection (n = 30), secondary dengue virus infection (n = 48), JE virus infection (n = 20), or no evidence of flavivirus infection (n = 26).
HAI.
Acetone-extracted sera were tested for antibodies by HAI as described previously (6), except that the assay was modified to a microtiter plate format. Dengue virus types 1 to 4 and JE virus (8 U each) were used. Antigens were produced by sucrose acetone extraction of the brains of suckling mice infected with the following prototype mouse-adapted virus strains: DEN-1 Hawaii, DEN-2 New Guinea C, DEN-3 H-87, DEN-4 H-241, and JE virus Nakayama. A fourfold increase was considered positive for acute flavivirus infection. The infection was diagnosed as a primary infection if the titers a week or more after the onset of illness were less than or equal to 1:1,280 or as a secondary infection if antibody titers were greater than 1:1,280.
Armed Forces Research Institute of Medical Science (AFRIMS) enzyme-linked immunosorbent assay (ELISA).
The in-house ELISA was performed as described previously (10). For single specimens, 40 U of IgM antibody to dengue virus (with the dengue virus IgM antibody titer being greater than the JE virus IgM antibody titer) was considered evidence of a dengue virus infection (30 U if for the paired sera the acute-phase specimen had less than 15 U of antibody). A dengue virus IgM:IgG ratio equal to or greater than 1.8:1 defined a primary dengue virus infection. A ratio of less than 1.8:1 defined a secondary dengue virus infection. By using serial specimens, a twofold increase in the IgG antibody titer to dengue virus with an absolute value of 100 U or greater indicated a secondary flavivirus infection in the absence of IgM antibody to dengue virus of 40 U or more.
Virus isolation.
Serologic diagnoses by HAI and ELISA were further confirmed by virus isolation for 21 of the 78 patients (27%) with dengue virus infection. Virus isolation was attempted by injecting approximately 0.34 μl of undiluted patient sera into 15 live Toxorrhyncites splendens mosquitoes (23, 24). After 14 days, approximately 10 surviving mosquitoes were tested for flavivirus antigen by indirect fluorescent-antibody assay of the head (12). Virus-positive mosquitoes were used to infect C6/36 cell cultures for identification of the virus type by using a panel of monoclonal antibodies (MAbs) against dengue virus and JE virus in an ELISA (13). Acute-phase sera from 9 of the 30 patients with primary dengue virus infection yielded virus (6 yielded DEN-1, 1 yielded DEN-2, and 2 yielded DEN-3). Virus was recovered from the sera of 12 of the 48 patients with antibody responses indicating secondary dengue virus infection (6 yielded DEN-1, 3 yielded DEN-2, 1 yielded DEN-3, and 2 yielded DEN-4). Virus was not recovered from the serum of patients with JE virus infection or those lacking antibody evidence of a recent flavivirus infection.
PanBio Dengue Rapid Test.
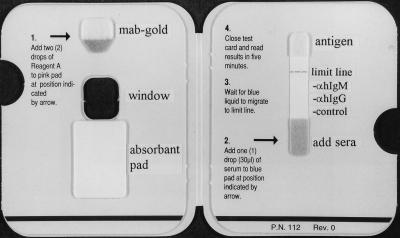
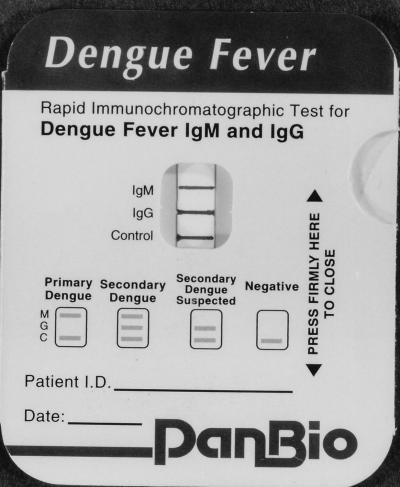
In the Dengue Rapid Test (PanBio, Brisbane, Australia), antibodies to dengue virus were determined by a rapid colloidal gold-based immunochromatographic test for the separate determination of IgM and IgG antibodies in a capture assay format (Fig. 1). Specimens were run blind, and the results were read without knowledge of the results of the other tests or the diagnosis. A drop of serum added to the blue pad for serum migrated along the nitrocellulose membrane, whereupon IgG and IgM were captured by lines of either anti-human IgG antibody and anti-human IgM antibody, respectively, striped onto the membrane. At the same time gold-labelled anti-dengue MAb was rehydrated by the addition of two drops of buffer to the pink pad. After the serum reached the limit line (<2 min), the card was closed. This allowed the rehydrated gold-labelled anti-dengue MAb to complex with dengue antigens stabilized in a pad at the top of the nitrocellulose membrane. In addition, closure of the pad caused visible gold-complexed antigen to flow down the nitrocellulose membrane into the large absorbent pad, whereupon it could bind to and reveal captured IgM or IgG antibody that was reactive with dengue virus. After 5 min, the assay result was visible through the window on the front panel of the card (Fig. 2). Captured gold-labelled antigen-antibody complexes appeared as maroon lines. The intensities of the lines observed in the rapid test were scored 0 (no reactivity), 0.5 (faint), 1 (distinct), or 2 (strong), depending on the intensity of the positive reaction. In addition to the anti-IgG and anti-IgM lines, a control line was also included to ensure that the test result is valid. The results were interpreted as shown on the front of the device (Fig. 2). Since the IgG cutoff was set to detect secondary and not primary dengue virus infection, primary dengue virus infection was defined by a visible IgM line without a visible IgG line, while secondary dengue virus infection was defined as a visible IgG line with or without a positive IgM line. A negative result was defined by the absence of both IgM and IgG lines (only the control line was visible).
FIG. 1.
Inside view of PanBio Dengue Rapid Test device showing general instructions for use. The locations of the antigen pad, gold conjugate pad, and absorbent pad are indicated, as are the anti-human IgG line, the anti-human IgM line, the control line, and the limit line.
FIG. 2.
PanBio Dengue Rapid Test device showing the result obtained with serum taken from a patient with secondary dengue virus infection (the IgM, IgG, and control lines are visible). The interpretation criteria for the test are also printed on the front of the device.
Data analysis.
Fisher’s exact test was performed to compare sensitivities and specificities of the rapid test. Analysis of variance (ANOVA) and the Tukey-Kramer multiple comparison test were used to compare the mean EIA ratios or hemagglutination-inhibition titers with the different rapid test scores. Statistical analyses were performed by using SPSS for Windows, version 7.5 (SPSS, Inc., Chicago, Ill.) and Instat software (Graphpad Software Inc., San Diego, Calif.).
RESULTS
Sensitivity and specificity of the rapid test.
The performance of the rapid test with sera collected at the time of hospital discharge from patients with dengue virus infection, JE virus infection, or no flavivirus infection is presented in Table 1. The use of separate IgG and IgM results allowed the infections to be classified as primary or secondary dengue virus infection. The infection in all patients with primary dengue virus infection (n = 30) was correctly classified (IgM positivity only), while the infection in 46 of 48 patients (96%) with secondary dengue virus infection was also correctly classified (IgG positivity with or without IgM positivity). Furthermore, the two patients with secondary dengue virus infection but whose infections were missed were diagnosed as having primary infections (positive IgM result and negative IgG result), so the sensitivity for all dengue virus infections was 100%, with positive test results obtained for patients infected with all four dengue virus serotypes. Sera from only 3 of 26 patients who had no evidence of an acute flavivirus infection by EIA and HAI had a positive result by the rapid test (88% specificity). On the other hand, sera from half of the patients with JE virus infection (n = 20) showed a positive result by the rapid test.
TABLE 1.
Sensitivity and specificity of the Dengue Rapid Test with serum taken at the time of hospital discharge
| Diagnosis by AFRIMS ELISA (no. of specimens) | No. of specimens with the following Dengue Rapid Test result:
|
Sensitivity (%) | Specificity (%) | ||
|---|---|---|---|---|---|
| Negative | Primary dengue virus infection | Secondary dengue virus infection | |||
| Negative (26) | 23 | 2 | 1 | 88 | |
| Primary dengue virus infection (30) | 0 | 30 | 0 | 100 | |
| Secondary dengue virus infection (48) | 0 | 2 | 46 | 100 | |
| JE virus infection (20) | 10 | 5 | 5 | 50 | |
Comparison between Dengue Rapid Test and in-house EIA.
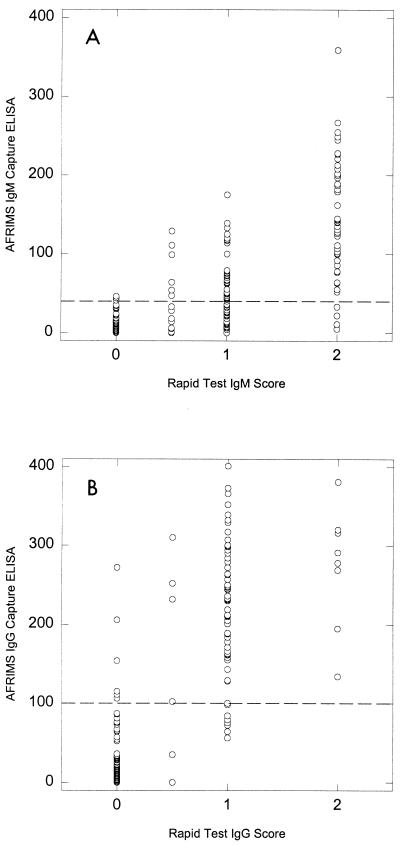
The relationship between the rapid test and an in-house EIA is presented in Fig. 3. There was a significant increase in mean EIA units with each increase in the rapid test score for both dengue virus-specific IgM and IgG (ANOVA, P < 0.0001). In addition, the percentage of patients whose sera showed elevated IgM or IgG antibody titers by EIA was significantly related to the rapid test score (Table 2) χ2 = 128.8 for IgM [P < 0.0001]; χ2 = 168.7 for IgG [P < 0.0001]).
FIG. 3.
Comparison of Dengue Rapid Test IgM score (0 = negative; 0.5 = faintly positive; 1 = distinctly positive; 2 = strongly positive) and dengue virus-specific IgM test result by EIA (A) or Dengue Rapid Test IgG score (the scoring system is the same as that for IgM) versus dengue virus-specific IgG test result by EIA (B). The cutoff in the in-house IgM EIA is 40 U, and the cutoff of the in-house IgG EIA is 100 U (shown by broken lines).
TABLE 2.
Relationship between Dengue Rapid Test score and EIA resulta
| Dengue IgM EIA
|
Dengue IgG EIA
|
||||
|---|---|---|---|---|---|
| Rapid test IgM scoreb | No. of specimens with EIA IgM of ≥40 U/total no. (%) | Mean IgM EIA U | Rapid test IgG scoreb | No. of specimens with IgG EIA ≥100 U/total no. (%) | Mean IgG EIA U |
| 0 | 3/118 (3) | 8.0 | 0 | 6/168 (4) | 19.4 |
| 0.5 | 6/14 (43) | 43.5 | 0.5 | 4/6 (67) | 155.2 |
| 1 | 33/68 (49) | 46.5 | 1 | 59/66 (89) | 220.5 |
| 2 | 44/48 (92) | 139.9 | 2 | 8/8 (100) | 273.0 |
By the chi-square test for independence, χ2 was 128.8 for IgM (P < 0.0001), and χ2 was 168.7 for IgG (P < 0.0001). Cutoff for IgM ELISA, ≥40 U; cutoff for IgG ELISA, ≥100 U.
0 = negative; 0.5 = faintly positive; 1 = distinctly positive; 2 = strongly positive.
Comparison between Dengue Rapid Test and HAI.
Since the IgG test is used to define secondary dengue virus infection, the intensity of the rapid test IgG score was compared to the hemagglutination-inhibition titer (Table 3). There was a significant relationship between the percentage of samples showing a hemagglutination-inhibition titer of ≥1:2,560 (the HAI cutoff value for secondary dengue virus infection) and the rapid test IgG score (χ2 = 169.9; P < 0.0001).
TABLE 3.
Relationship between rapid test IgG score and hemagglutination-inhibition titera
| Rapid test IgG score | No. of specimens with a hemagglutination-inhibition titer of ≥1:2,560/total no. tested (%) |
|---|---|
| 0 | 5/167 (3) |
| 0.5 | 3/6 (50) |
| 1 | 54/65 (83) |
| 2 | 8/8 (100) |
By the chi-square test for independence, χ2 was 169.9 (P < 0.0001).
Use of rapid test for the early detection of dengue virus infection.
The performance of the test with the first serum specimen of the pair was also investigated to determine the utility of this test for the early diagnosis of dengue virus infection (Table 4). A high proportion of dengue virus infections (71%) could be diagnosed through the use of the first serum specimen alone: 87% of primary infections and 60% of secondary infections. Furthermore, all 26 primary infections were correctly classified (positive IgM result, negative IgG result), while 24 of 29 (83%) secondary infections were correctly classified (positive IgG result), with the remaining infections being IgM positive and IgG negative. The first serum specimen from only 3 of 26 patients with no flavivirus infection were positive by the rapid test (specificity 88%), while half the patients with JE virus infection were positive by the rapid test.
TABLE 4.
Sensitivity and specificity of the Dengue Rapid Test with sera taken at the time of hospital admission
| Diagnosis by AFRIMS ELISA (no. of specimens)a | No. of specimens with the following rapid test result:
|
Sensitivity (%) | Specificity (%) | ||
|---|---|---|---|---|---|
| Negative | Primary dengue virus infection | Secondary dengue virus infection | |||
| Negative (26) | 23 | 3 | 0 | 88 | |
| Primary dengue virus infection (30) | 4 | 26 | 0 | 87 | |
| Secondary dengue virus infection (48) | 19 | 5 | 24 | 60 | |
| JE virus infection (20) | 10 | 6 | 4 | 50 | |
Using admission and discharge specimens.
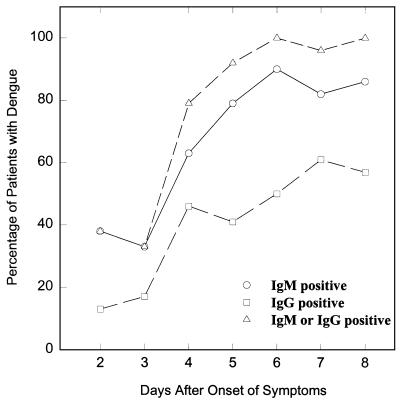
The rate of detection of IgM and IgG to dengue virus by the rapid test for up to 8 days after the onset of clinical symptoms was also investigated (Fig. 4). The combined use of IgG and IgM led to the earlier detection of dengue virus infection relative to the time to detection with the use of IgG or IgM alone. The infections in nearly 80% of patients with dengue virus infection were detected 4 days after the onset of symptoms, and this rose to over 90% by day 5.
FIG. 4.
Relationship between the sensitivity of the Dengue Rapid Test and the days after the onset of illness. The sensitivities with the use of IgM only (circles), the use of IgG only (squares), and the combined use of IgM and IgG (triangles) are shown.
DISCUSSION
A rapid and accurate method for the diagnosis of dengue fever is important for both the clinician and the patient. The commercially available Dengue Rapid Test described in this report is suitable for the detection of anti-dengue virus IgM and IgG antibodies, with results available in just 7 min. The utility of IgM capture and IgG capture in the diagnosis of dengue virus infection has been reported previously (1, 10, 14, 16, 17, 25), and the rapid test evaluated in this study showed an excellent correlation with a standard in-house EIA (10). In addition, a commercially available test will ensure reproducibility between different laboratories.
The combined use of IgM and IgG has been shown to increase sensitivity in the detection of dengue virus infection (10, 25). In this study, all patients with dengue virus infection were positive by the rapid test when paired serum specimens were used. Furthermore, the Dengue Rapid Test was able to detect 71% of cases of dengue virus infection through the use of the first serum specimen alone. Previous studies suggested that diagnosis based on the IgM antibody titer may take 5 to 7 days after the onset of illness (10, 25, 27). In this study, similar results were observed, with the majority of patients showing elevated IgM antibody titers by day 5 of illness. Secondary dengue virus infection is characterized by a high IgG response with or without an IgM response (15), and so the combined use of IgM and IgG in the rapid test led to the earlier detection of dengue virus infection, with most patients being positive by day 4 of illness. Furthermore, the test detected infection in patients infected with any of the four different dengue virus serotypes.
The Dengue Rapid Test showed good specificity (88%) for patients without flavivirus infections. Sera from half of the patients with JE showed cross-reactivity in this test. High levels of antibody cross-reactivity for patients with dengue virus and JE virus infections have been reported previously (10, 20). It was of interest that sera from none of the patients with JE had elevated dengue virus-specific IgM and IgG antibodies, while sera from the majority of patients with secondary dengue virus infection (71%) had this antibody profile. Caution should be used in interpreting tests that are positive for dengue virus IgM or IgG only in areas where dengue virus cocirculates with other flaviviruses. Most cases of JE can be differentiated from dengue virus infection on clinical grounds, although there may be unusual cases of dengue virus encephalopathy (9, 19).
Because secondary dengue virus infection is associated with the more serious form of the disease, the use of the Dengue Rapid Test to distinguish it from primary dengue virus infection was also investigated. Traditionally, HAI has been used to distinguish between primary and secondary dengue virus infections, with a titer of greater than 1:1,280 considered indicative of secondary dengue virus infection (29). The IgG result by the Dengue Rapid Test showed excellent agreement with the HAI result. The majority of specimens IgG positive by the rapid test showed hemagglutination-inhibition titers of ≥1:2,560, while the majority of specimens IgG negative by the rapid test showed hemagglutination-inhibition titers of <1:2,560. Consequently, this test could be used to distinguish between the primary and secondary forms of the disease. Sera from all patients with primary dengue virus infection had elevated IgM titers but not elevated IgG titers, while sera from 46 of 48 (96%) of patients with secondary dengue virus infection had elevated IgG titers, with or without elevated IgM titers. Furthermore, because the high hemagglutination-inhibition titers found in sera from patients with secondary dengue virus infection generally last for 30 to 40 days before declining to levels below 1:640 (7), the IgG response by the rapid test was also negative for the majority of patients with past dengue virus infections, as evidenced by the lack of reactivity for patients without dengue virus infection, many of whom would have had previous exposure to the virus due to the endemic nature of the disease in Thailand (27).
The commercially available EIA described in this study should be a valuable screening test for dengue fever and DHF in routine diagnostic laboratories. It is rapid, can easily be performed, has an extended shelf life (12 months at 4°C or 2 weeks at 37°C), and overcomes many of the limitations associated with HAI. Unlike HAI, no pretreatment of sera (e.g., acetone extraction) is required, and there is often no need for sera to be obtained after hospital discharge. Furthermore, differentiation between primary and secondary infection can be made through the use of a single dilution of serum rather than the series of dilutions needed in the HAI. The test also has the advantage of being able to be run at the point of care, where sophisticated laboratory equipment or experienced personnel may be unavailable.
ACKNOWLEDGMENTS
This work was supported by the U.S. Army Medical Research and Materiel Command and PanBio Pty, Ltd. (Brisbane, Australia), through a cooperative research and development agreement.
We thank Panor Srisongkram for performing the Dengue Rapid Test and the reference EIA, Ming Choohong for performing the HAI, Rachel Kneen for specimen and data management, Tipawan Kungvanrattana for data entry, Sharone Green and Bruce L. Innis for critical readings of the manuscript, and the directors and staff of the Queen Sirikit National Institute of Child’s Health and the Centre for Tropical Diseases and Nicholas White for support.
REFERENCES
- 1.Bundo K, Igarashi A. Antibody-capture ELISA for detection of immunoglobulin M antibodies in sera from Japanese encephalitis and dengue hemorrhagic fever patients. J Virol Methods. 1985;11:15–22. doi: 10.1016/0166-0934(85)90120-x. [DOI] [PubMed] [Google Scholar]
- 2.Burke D S, Nisalak A, Johnson D E, Scott R M. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 3.Cardosa M J, Zuraini I. Comparison of an IgM capture ELISA with a dot enzyme immunoassay for laboratory diagnosis of dengue virus infections. Southeast Asian J Trop Med Public Health. 1991;22:337–340. [PubMed] [Google Scholar]
- 4.Cardosa M J, Tio P H. Dot enzyme immunoassay: an alternative diagnostic aid for dengue fever and dengue haemorrhagic fever. Bull W H O. 1991;69:741–745. [PMC free article] [PubMed] [Google Scholar]
- 5.Cardosa M J, Tio P H, Nimmannitya S, Nisalak A, Innis B L. IgM capture ELISA for detection of IgM antibodies to dengue virus: comparison of 2 formats using hemagglutinins and cell culture derived antigens. Southeast Asian J Trop Med Public Health. 1992;23:726–729. [PubMed] [Google Scholar]
- 6.Clarke D H, Casals J. Techniques for hemagglutination and hemagglutination inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 7.Gubler D J. Serological diagnosis of dengue/dengue haemorrhagic fever. Dengue Bull. 1996;20:20–23. [Google Scholar]
- 8.Halstead S B. Observations related to pathogenesis of dengue hemorrhagic fever. VI. Hypotheses and discussion. Yale J Biol Med. 1970;42:350–362. [PMC free article] [PubMed] [Google Scholar]
- 9.Hendarto S K, Hadinegoro S R. Dengue encephalopathy. Acta Paediatr Jpn. 1992;34:350–357. doi: 10.1111/j.1442-200x.1992.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 10.Innis B L, Nisalak A, Nimmannitya S, Kusalerdchariya S, Chongswasdi V, Suntayakorn S, Puttisri P, Hoke C H., Jr An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis cocirculate. Am J Trop Med Hyg. 1989;40:418–427. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- 11.Innis B L. Dengue and dengue hemorrhagic fever. In: Porterfield J S, editor. Kass handbook of infectious diseases. Exotic viral infections. 1st ed. London, United Kingdom: Chapman & Hall Medical; 1995. pp. 103–146. [Google Scholar]
- 12.Kuberski T T, Rosen L. A simple technique for the detection of dengue antigen in mosquitoes by immunofluorescence. Am J Trop Med Hyg. 1977;26:533–537. doi: 10.4269/ajtmh.1977.26.533. [DOI] [PubMed] [Google Scholar]
- 13.Kuno G, Gubler D J, Santiago de Weil N S. Antigen capture ELISA for the identification of dengue viruses. J Virol Methods. 1985;12:93–103. doi: 10.1016/0166-0934(85)90011-4. [DOI] [PubMed] [Google Scholar]
- 14.Kuno G, Gomez I, Gubler D J. An ELISA procedure for the diagnosis of dengue infections. J Virol Methods. 1991;33:101–113. doi: 10.1016/0166-0934(91)90011-n. [DOI] [PubMed] [Google Scholar]
- 15.Lam S K, Devi S, Pang T. Detection of specific IgM in dengue infection. Southeast Asian J Trop Med Public Health. 1987;18:532–538. [PubMed] [Google Scholar]
- 16.Lam S K. Rapid dengue diagnosis and interpretation. Malay J Pathol. 1993;15:9–12. [PubMed] [Google Scholar]
- 17.Lam S K. Application of rapid laboratory diagnosis in dengue control. Asia Pac J Mol Biol Biotech. 1995;3:351–355. [Google Scholar]
- 18.Lam S K, Fong M Y, Chungue E, Doraisingham S, Igarashi A, Khin M A, Kyaw Z T, Nisalak A, Roche C, Vaughn D W, Vorndam V. Multicentre evaluation of dengue IgM dot enzyme immunoassay. Clin Diagn Virol. 1996;7:93–98. doi: 10.1016/s0928-0197(96)00257-7. [DOI] [PubMed] [Google Scholar]
- 19.Lum L C, Lam S K, Choy Y S, George R, Harun F. Dengue encephalitis: a true entity? Am J Trop Med Hyg. 1996;54:256–259. doi: 10.4269/ajtmh.1996.54.256. [DOI] [PubMed] [Google Scholar]
- 20.Makino Y, Tadano M, Saito M, Maneekarn N, Sittisombut N, Sirisanthana V, Poneprasert B, Fukunaga T. Studies on serological cross-reaction in sequential flavivirus infections. Microbiol Immunol. 1994;38:951–955. doi: 10.1111/j.1348-0421.1994.tb02152.x. [DOI] [PubMed] [Google Scholar]
- 21.Monath T P. Dengue: the risk to developed and developing countries. Proc Natl Acad Sci USA. 1994;91:2395–2400. doi: 10.1073/pnas.91.7.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nimmannitya S. Management of dengue and dengue haemorrhagic fever. In: Thongcharoen P, editor. Monograph on dengue/dengue haemorrhagic fever. New Delhi, India: World Health Organization Regional Office for South-East Asia; 1993. pp. 55–61. [Google Scholar]
- 23.Rosen L, Gubler D J. The use of mosquitoes to detect and propagate dengue viruses. Am J Trop Med Hyg. 1974;23:1153–1160. doi: 10.4269/ajtmh.1974.23.1153. [DOI] [PubMed] [Google Scholar]
- 24.Rosen L, Shroyer D A. Comparative susceptibility of five species of Toxorhynchites mosquitoes to parenteral infection with dengue and other flaviviruses. Am J Trop Med Hyg. 1985;34:805–809. doi: 10.4269/ajtmh.1985.34.805. [DOI] [PubMed] [Google Scholar]
- 25.Ruechusatsawat K, Morita K, Tanaka M, Vongcheree S, Rojanasuphot S, Warachit P, Kanai K, Thongtradol P, Nimnakorn P, Kanungkid S, Igarashi A. Daily observation of antibody levels among dengue patients detected by enzyme-linked immunosorbent assay (ELISA) Jpn J Trop Med Hyg. 1994;22:9–12. [Google Scholar]
- 26.Russell P K, Udomsakdi S, Halstead S B. Antibody response in dengue and dengue hemorrhagic fever. Jpn J Med Sci Biol. 1967;20:103–108. [PubMed] [Google Scholar]
- 27.Vaughn D W, Green S, Kalayanarooj S, Innis B L, Nimmannitya S, Suntayakorn S, Rothman A L, Ennis F A, Nisalak A. Dengue in the early febrile phase: viremia and antibody responses. J Infect Dis. 1997;176:322–330. doi: 10.1086/514048. [DOI] [PubMed] [Google Scholar]
- 28.Westaway E G, Brinton M A, Gaidamovich S Y, Horzinek M C, Igarashi A, Kaariainen L, Lvov D K, Porterfield J S, Russell P K, Trent D W. Flaviviridae. Intervirology. 1985;24:183–192. doi: 10.1159/000149642. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Dengue haemorrhagic fever: diagnosis, treatment and control. Geneva, Switzerland: World Health Organization; 1986. [Google Scholar]