Abstract
Exposure to natural environments has consistently been shown to boost human health. However, population-level benefit is constrained by both inequitable access to high-quality natural spaces and the lack of medical prescriptions for nature-based therapy. Addressing these challenges requires an improved understanding of the mechanisms linking environmental attributes to positive health outcomes. A systematic, standardised experimental approach is needed to support this effort. This manuscript presents two complementary experiments—a randomised controlled trial (n = 100) and a counterbalanced crossover trial (n = 30)—designed to assess the effect of a 30-min exposure to forest and industrial acoustic environments on selected biomarkers. This is the first in a series of laboratory experiments which isolate and expose individual senses to natural and industrial stimuli, while measuring biological parameters previously shown to respond positively to whole-body, real-world, nature immersion. Forest acoustics (recorded in a UK temperate rainforest, featuring bird song, running water, wind and rainfall) significantly improved biomarkers of mood, restoration and cognition, relative to industrial acoustics (recorded in Liverpool and London city centre), but not heart rate, blood pressure, heart rate variability, salivary cortisol or secretory Immunoglobulin A. These findings suggest that acoustic elements of forest environments play a role in mediating enhanced psychological state and cognition but do not appear to influence physiological stress or immunological parameters. This work advances understanding of how nature influences human biology and takes steps towards addressing existing challenges to nature-based therapy. In the short-term, these findings highlight the potential of acoustic interventions for individuals with limited access to nature.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-11469-x.
Subject terms: Anthropology, Biological anthropology, Public health
Introduction
Health benefits associated with exposure to nature
Over the past two decades, research has increasingly highlighted the health benefits associated with exposure to natural environments. Central to this understanding is the growing body of experimental evidence that has consistently demonstrated reductions in physiological and psychological stress after spending time in nature. Early laboratory studies demonstrated enhanced stress recovery when participants viewed natural rather than urban environments1. Subsequent real-world studies reinforced these findings. Nature-induced reduction in stress (relative to urban control environments) have been found across diverse populations, reflected by changes in biomarkers of hypothalamic-pituitary-adrenal and sympatho-adrenal-medullary axis activity2–5. Stress reduction is associated with improved health outcomes, including lower hypertension (a leading global disease risk factor6) and reduced prevalence of chronic conditions such as heart disease, diabetes, and cancer2–4. Nature exposure has also been shown to benefit psychological wellbeing by improving mood, reducing depression and anxiety and enhancing feelings of revitalisation7–9. Collectively, these health benefits are comparable to those conferred by regular physical activity or higher socioeconomic status5. From a psychological perspective, the diverse benefits of nature exposure have been attributed to its restorative potential, which refers to an environment’s capacity to (1) replenish cognitive resources depleted by the attentional demands of everyday life, and (2) reduce stress levels elevated by the pressures of modern living10.
Beyond reducing stress and improving mood, nature exposure has been experimentally shown to improve cognitive constructs such as directed attention, working memory, creativity, cognitive flexibility and problem solving11–13. One of the more compelling examples of this effect is a year-long study of ~ 2600 children in Barcelona, Spain, which found a positive association between neighbourhood greenness and executive function, independent of factors such as socioeconomic status14. Nature exposure has also been associated with immunological enhancement, as measured via increased salivary immunoglobulins A, G and M, anti-inflammatory cytokines (and decreased proinflammatory cytokines) and NK cell activity15–19. Despite these promising findings, questions surrounding study design have led to uncertainties regarding reproducibility of some immune-focused studies, emphasising the need for robust experimental designs20,21.
Challenges facing the widespread application of nature-based therapeutic interventions
Increased understanding of the health benefits of nature exposure—particularly for alleviating symptoms of stress—has coincided with the emergence of chronic stress as a prominent threat to public health22. Nature exposure therefore holds significant promise as a population-level intervention to enhance health and wellbeing. However, two key barriers currently limit widespread access to its benefits.
First, access to natural environments is not equally distributed. It is shaped by factors such as socioeconomic status, which influences both proximity of high-quality natural areas and the ability to travel to them. Mobility is another constraint; individuals with disabilities often face accessibility challenges that limit their engagement with nature23,24. These disparities highlight the need for innovative approaches that can deliver the health benefits of nature without requiring physical presence in natural settings. Research into the health benefits of more accessible aspects of natural environments—such as natural acoustic features—is therefore valuable in promoting equitable access to nature’s benefits.
Secondly, nature exposure has yet to be widely adopted as a formal therapeutic recommendation. While healthcare systems in countries such as Japan, Finland and South Korea encourage spending time in nature to manage stress-related conditions2–4, uptake in other countries remains limited and inconsistent25. This hesitancy is largely due to a lack of rigorous clinical trials and mechanistic research designed to identify which features of nature are responsible for the observed health benefits25,26. Despite repeated calls to address this crucial gap in knowledge (e.g.,3), current understanding remains incomplete.
This lack of mechanistic insight stems from methodological limitations in both field and laboratory-based experimental investigations. Field-based studies, which comprise most of the work in this area, rarely include direct environmental measures, making it difficult to link specific environmental factors to effects on human biology. This key limitation is discussed in greater detail in27–29. Laboratory-based studies, by contrast, provide the opportunity to control, or even isolate, aspects of the natural environment and experimentally assess their influence on biomarkers of health. Indeed, a number of investigators have adopted a sense-based approach to examine the effects of acoustic30–33, visual34–36, tactile37,38 and olfactory39,40 elements of natural environments on health biomarkers previously shown to respond positively to whole-body nature exposure (see41 for a review). This methodological approach is valuable because it reduces input complexity by isolating individual sensory inputs, allowing researchers to assess their specific contributions to observed health outcomes.
However, the ability to draw broader conclusions from this body of research is currently constrained. First, substantial variation in study design and outcome measures across different sensory modalities limit direct comparison between studies and prevent meaningful insight into the relative contribution of each sense to the overall health effects of nature exposure. Second, findings within individual modalities are often inconsistent. For example, while some studies report beneficial effects of natural sounds—such as reduced heart rate and increased parasympathetic nervous system activity relative to urban sound exposure31,33,42—others fail to detect significant differences32. Together, these limitations highlight the need for more standardised and systematic approaches to disentangle the specific sensory mechanisms underlying nature’s health effects.
The current study
Here, a rigorous experimental approach is applied to address this important knowledge gap. We do so by (a) adopting a dual experiment protocol (reporting the results of (i) an investigator-blinded randomised controlled trial and (ii) a counterbalanced crossover trial) to determine the influence of natural (specifically, forest) and industrial acoustic environments on human biology, (b) including large sample sizes (n = 100 and n = 30, respectively) to increase statistical power and facilitate the detection of subtle effects, (c) assessing a broad range of human biomarkers (encompassing psychological, cognitive, physiological and immune measures) that are typically examined in isolation and (d) using authentic 30-minute sound recordings captured specifically for this study.
Furthermore, this study represents the first in a series of standardised, laboratory-based, experiments that systematically apply a consistent methodology to isolate and expose each sensory modality to both natural and industrial stimuli, while assessing their effects on biomarkers previously shown to be influenced by whole-body nature exposure. Collectively, this series of experiments will help to clarify the relative contribution of each sensory modality on the observed benefits of whole-body nature exposure. The resulting insights will provide a foundational step toward identifying the environmental variables that drive specific biological responses in humans.
This first manuscript focusses on hearing and considers the effect of a 30-min exposure to recordings captured in forest and/or industrial acoustic environments. The complementary, dual-experiment, design used here enhances the robustness of the findings, reduces the risk of type I error and allows for an assessment of the reproducibility of results. Each recording was composed of six unique five-minute segments. Each recording was purposefully recorded from areas where people tend to be. This provided a realistic and ecologically valid representation of how people experience the soundscape. The forest recording was captured from paths in in the ancient temperate rainforest of Cabilla Woods, Cornwall (UK), located within the Bodmin Moor area of the Cornwall Area of Outstanding Natural Beauty. This site, designated a Site of Special Scientific Interest due to its high biodiversity, provides a rare opportunity to record an isolated natural acoustic environment free from industrial interference. The five-minute segments including in the forest recording featured areas with bird song, a running stream, wind and rainfall. In contrast, to reflect the acoustic conditions experienced by the majority of the world’s population in the twenty-first century43, industrial recordings were made in the highly industrialised city centres of Liverpool and London. The 5-min segments included in the industrial environment featured ambient sounds from a pedestrianised city centre high street (featuring human voices), a highly frequented path near an urban highway, an underground metro station, an overground train station and from inside a train during a journey through the city centre (featuring human voices and train noises including automated announcements, doors opening and closing and engine sound). See Supplementary Information 1 for further details on both sets of recordings.
The juxtaposition of a forest soundscape with an industrial soundscape was purposeful. According to the United Nations, over half of the global population reside in urban areas, with Northern America (82%), Latin America and the Caribbean (81%), Europe (74%) Oceania (68%) and Asia (50%) being the most urbanised regions43. This proportion is projected to rise to 68% by 2050, further emphasising the need to determine the health implications of exposure to industrialised environments. In contrast, the forested soundscape was chosen because, throughout millions of years of hominin evolutionary history, natural environments provided the primary habitat for our species. It is these natural environments, such as forests, that provided the environmental parameters within which natural selection acted to shape our biology.
The overarching question considered in this manuscript is: ‘To what extent do the acoustic characteristics of forest and industrialised environments influence human biology?’.
One of three outcomes is possible:
No differences—indicating that forest and industrial acoustic environments have similar effects on human biology. This would suggest that sound alone is not sufficient to confer the health benefits associated with exposure to nature.
Consistent differences—indicating that one acoustic environment provides greater benefit compared to the other across all biological measures. This would suggest that acoustic elements from one environment play a role in mediating positive (or negative) health effects.
Inconsistent differences—indicating that the influence of each acoustic environment varies across the range of biological measures taken. This would highlight the complexity of sensory-environment interactions and suggest that tailored interventions may be needed depending on specific health goals.
Results
Pre-Trial and Post-Trial means and standard deviations, test statistics, confidence intervals and effect sizes for each variable can be found in Tables 2, 3, 4 and 5 in Supplementary Information.
Mood
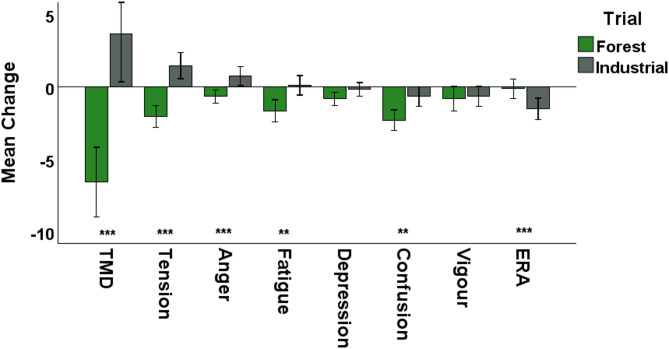
Exposure to Forest and Industrial acoustic environments had opposing effects on mood in both experiments; the Forest condition improved mood while the Industrial condition worsened mood (Table S1). In Experiment 1 (the randomised controlled trial), the Forest and Industrial conditions had significantly different effects on the composite measure of mood (Total Mood Disturbance, TMD, p < 0.001, d = 1.0), as well as on the mood subcomponents Tension (p < 0.001, d = 1.2), Anger (p < 0.001, d = 0.7), Fatigue (p = 0.006, d = 0.7), Confusion (p = 0.001, d = 0.7) and Esteem-Related Affect (ERA) (p = 0.006, d = 0.6). These results were consistent in both male and female participants. See Fig. 1 and Table S2.
Fig. 1.
Bar chart displaying the change in Total Mood Disturbance (TMD) and mood subcomponents following the Forest and Industrial Trials in Experiment 1 (the randomised controlled trial). Significantly different effects on mood following exposure to Forest and Industrial acoustics are represented by: ***p < 0.001; **p < 0.01; *p < 0.05. Error bars represent 95% confidence intervals. Note: a decrease in TMD represents an improvement in mood. ERA, Esteem related affect.
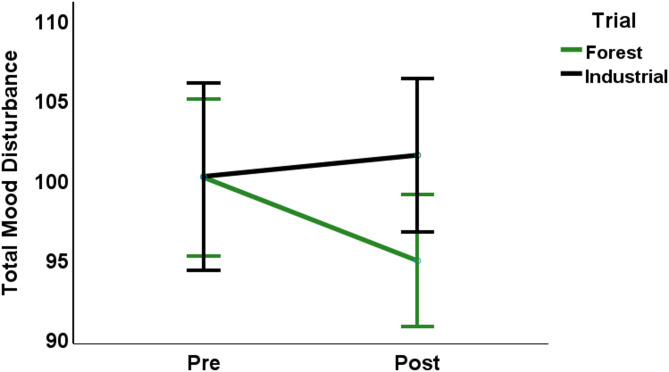
In Experiment 2 (the counterbalanced crossover trial), significant interaction effects of time and condition were identified for TMD (p < 0.05, ηp2 = 0.157, Fig. 2) as well as mood subcomponents Tension (p < 0.01, ηp2 = 0.230,) and Anger (p < 0.05, ηp2 = 0.174), whereby the Forest Condition improved mood and the Industrial Condition worsened mood.
Fig. 2.
Line chart displaying the significant Time × Condition interaction effect for Total Mood Disturbance (TMD) following the Forest and Industrial Trials. TMD significantly decreased Pre-to-Post Forest, and Post-Forest TMD was significantly lower than Post-Industrial TMD. Error bars represent 95% confidence intervals.
Physiological stress
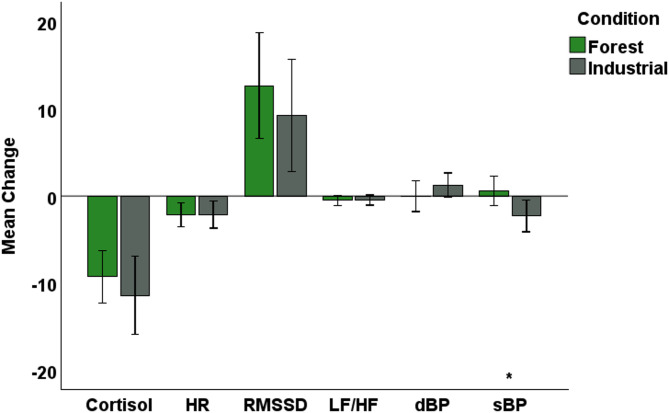
There were no differences in measures of physiological stress at baseline prior to listening to the Forest or Industrial acoustic environments, in either experiment. There were no consistent differences in the effect of exposure to Forest or Industrial acoustics on physiological biomarkers. While systolic blood pressure decreased following the Industrial condition relative to the Forest condition in Experiment 1 (p < 0.05, d = 0.5, but not in Experiment 2), there were no differential effects of acoustic condition on diastolic blood pressure, salivary cortisol, heart rate or heart rate variability (Root mean square of successive differences [RMSSD] and High Frequency [HF], markers of parasympathetic nervous system activity) in either experiment (Fig. 3, Table S3).
Fig. 3.
Bar chart representing change in physiological biomarkers following exposure to Forest and Industrial acoustics in Experiment 1. The two conditions had a significantly different effect on only systolic blood pressure (sBP). Significantly different effects on physiological biomarkers following exposure to Forest and Industrial acoustics are represented by: ***p < 0.001; **p < 0.01; *p < 0.05. Error bars represent 95% confidence intervals. dBP: diastolic blood pressure.
Self-reported restorative measures and mental bandwidth
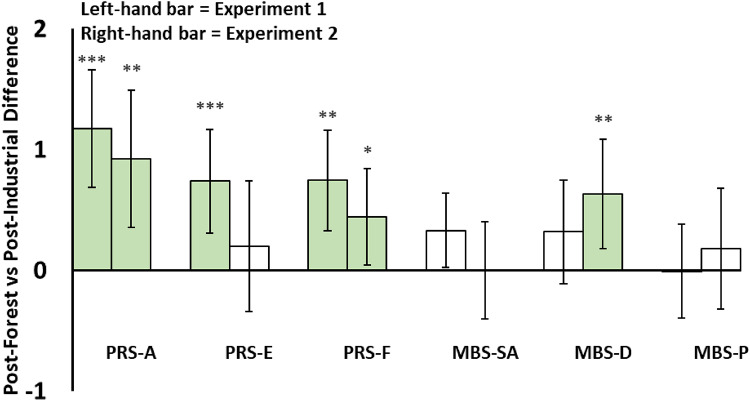
Perceived Restorativeness and Mental Bandwidth were measured after (but not before) exposure to the Forest and Industrial acoustics. These scales were presented after the soundscape exposure because both scales required participants to provide answers based on what they had experienced during the trial. Perceived Restorativeness was greater Post-Forest than Post-Industrial for five of six measurements (PRS-Away—Experiment 1: p < 0.001, d = 1.0, Experiment 2: p = 0.001, d = 0.6; PRS-Extent—Experiment 1: p < 0.001, d = 0.7; PRS-Fascination—Experiment 1: p = 0.001, d = 0.7, Experiment 2: p < 0.05, d = 0.4). By contrast, measures of Mental Bandwidth did not differ following exposure to Forest and Industrial acoustics (only one of six measurements was greater Post-Forest compared against Post-Industrial (MBS-Self-Awareness—Experiment 1: p = 0.061, d = 0.4; MBS-Daydreaming—Experiment 2: p < 0.01, d = 0.5). See Fig. 4 and Table S4.
Fig. 4.
Bar chart showing differences in self-reported restorative measures and mental bandwidth scales, Post-Forest vs. Post-Industrial. Perceived Restorativeness (PRS-Away/Extent/Fascination) was greater Post-Forest than Post-Industrial for five of six measurements, while measures of Mental Bandwidth (MBS-Self Awareness / Daydreaming / Planning) did not differ following exposure to the Forest and Industrial acoustics. Positive values signify Post-Forest > Post-Industrial, and the bars within each two-bar cluster represent the results from Experiment 1 (left) and Experiment 2 (right) respectively. Error bars represent 95% confidence intervals. Statistically significant differences are denoted by light green bars and by ***p < 0.001; **p < 0.01; *p < 0.05.
Cognitive function
The Forest and Industrial acoustic conditions did not differentially affect measures of cognitive function in Experiment 1. Cognitive function increased following exposure to both the Forest and Industrial acoustic conditions, as indicated by significant increases in performance (DPrime Forest p < 0.01, d = 0.5; Industrial p < 0.001, d = 0.7) and the proportion of successful selections (Hit Prop Forest p < 0.001, d = 0.5; Industrial p < 0.001, d = 0.8). In Experiment 2, however, for which the protocol of the cognitive test was modified to counter the learning effect that likely influenced the results of Experiment 1 (see Methods), a significant interaction effect of time and condition (p < 0.05, ηp2 = 0.178) was observed whereby the Forest condition led to a greater DPrime increase than the Industrial condition (DPrime significantly increased following each condition). For Experiment 2, there were no interaction effects for Hit Prop or FA Prop. See Table S5.
Immune function
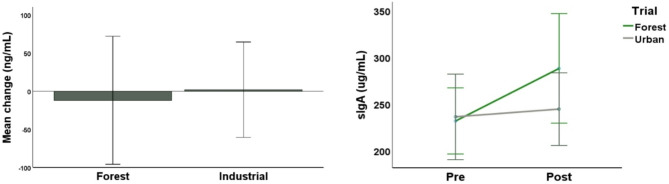
The Forest and Industrial conditions did not have differential effects on salivary secretory IgA (sIgA) in either experiment. See Fig. 5 and Table S5 below.
Fig. 5.
Bar and line charts displaying the lack of significant change of sIgA in Experiments 1 (left) and 2 (right). Error bars represent 95% confidence intervals.
Discussion
The two experiments reported here demonstrate that exposure to forest acoustic environments benefit human biology relative to industrial acoustic environments. First, both experiments observed opposing effects on mood following exposure to forest and industrial acoustics. In Experiment 1 (randomised control trial), exposure to forest acoustics significantly enhanced overall mood (as indicated by a decrease in Total Mood Disturbance, TMD) and improved five of the seven mood subcomponents. Conversely, exposure to industrial acoustics worsened overall mood (TMD increased) and negatively affected four of the seven mood subcomponents. Similarly, in Experiment 2 (counterbalanced cross-over trial), exposure to forest acoustics significantly improved overall mood, as well as the Tension and Anger subcomponents, relative to industrial acoustics. These results are consistent with previous work31,33,44–46 and suggest that the auditory pathway is at least partially responsible for nature-induced improvements in mood2–4,46.
There was also evidence of forest-induced improvements in directed attention, a measure of cognitive function. In Experiment 1, performance (DPrime) improved across both conditions, likely reflecting the results of the well-documented practice effect47. However, Experiment 2—which incorporated two familiarisation tests designed to attenuate learning effects—revealed a significant interaction between time and condition. Specifically, forest acoustics led to a greater improvement in attentiveness than industrial acoustics (although attentiveness did increase following both trials).
The general improvement in attentiveness in both the forest and industrial conditions may reflect the beneficial effects of sitting quietly, away from the pressures of daily life. While it is possible that increased arousal may have contributed to improved cognitive performance48 in the industrial condition, no differences in autonomic nervous system activity were observed to indicate that this was the case. The relative improvement in attentiveness after listening to the forest acoustics in Experiment 2, compared to industrial acoustics, aligns with previous observations of improved cognition after listening to auditory depictions of natural versus industrial environments30. This effect may be mediated by participants’ improved mood, which is known to enhance cognitive performance49,50. In parallel, Attention Restoration Theory (ART) posits that exposure to natural environments restores cognitive function by alleviating mental fatigue and replenishing attentional resources51,52. Nature sounds, as an integral component of these environments, may contribute to this process by providing “soft fascinations”—stimuli that gently engage attention without imposing cognitive demands or depleting cognitive resources. This shift from effortful, directed focus (typical requirements of industrial or task-oriented settings) to a relaxed, restorative state facilitates the recovery of cognitive resources. Consequently, exposure to natural sounds may reduce stress and mental fatigue, thereby improving attentiveness, focus, and overall cognitive performance through more efficient information processing.
Considering mood and cognition together, the observed relative positive effects of forest acoustics may result from the presence of beneficial auditory features (e.g., bird calls, flowing water) and the absence of harmful elements common in industrial soundscapes (e.g., traffic, construction noise), or potentially from a combination of the two. However, the study design employed here, which does not include a neutral condition (e.g., white noise), limits the ability to disentangle these effects.
The results reported here also suggest that the biological benefits associated with exposure to isolated forest acoustic environments are narrower than those reported following whole-body (all senses) nature exposure. While mood and cognitive function improved, exposure to forest and industrial acoustic stimuli did not differentially affect biomarkers of physiological stress or immune function. This indicates that auditory stimulation alone is insufficient to induce the changes in physiological stress and immune function previously observed following whole body exposure to natural environments2–4,20,21.
Considering physiological stress, the only difference observed was in Experiment 1, where systolic blood pressure was significantly lower following the Industrial condition, relative to the forest condition. There were no differential effects of exposure to the industrial or forest acoustic environments in Experiment 2. The decrease in heart rate observed following both forest and industrial acoustic conditions in each experiment might reflect the relaxing effect of sitting quietly for 30-min, potentially overriding any differential effects of the acoustic environments. To some degree, these results contrast with previous studies that have reported reductions in biomarkers of physiological stress after listening to nature sounds. The reasons for this are unclear, and future, more comprehensive, assessments of the stress response (using, for example, near-infrared spectroscopy or skin conductance level) may be illuminating. While decreased heart rate and increased parasympathetic nervous system activity (via analysis of heart rate variability) have been reported in several studies after listening to natural relative to urban sounds31,33,42, other studies failed to observe differential effects32. This overall inconsistency for the physiological measurements reported here compared to previous experiments may be due, in part, to variation in experimental design. For example, prior studies have used different biomarkers to assess physiological stress (e.g., functional near-infrared spectroscopy31,33, skin conductance level32 and respiratory measures42), varied the duration of listening periods (ranging from 1-min33 to over 13-min30) and differed in their use of experimentally induced stress (some studies induced stress31,32 while others did not30,33). Furthermore, the design of crossover investigations are variable – Experiment 2 in this study evaluated the effect of forest and industrial acoustics on different days, whereas previous work has assessed both within the same testing session30–33.
In contrast to the abundance of previous work that has measured the effect of nature exposure on physiological stress, the effect on immune function remains largely unexplored. It is generally understood, however, that immune function is sensitive to environmental noise53. Anthropogenic noise, such as that from traffic, has been shown to adversely affect immune function54 via mechanisms including sleep disruption, which suppresses antiviral responses and increases systemic inflammation55, and chronic stress, which impairs both innate and adaptive immunity54,56. The use of a more comprehensive assessment of immune biomarkers in this study could have increased sensitivity and improved the likelihood of detecting immune-related effects.
Key strengths of this study include the rigorous dual experimental design, the large sample size (n = 100 and n = 30, respectively), the authenticity of the sound recordings used, the simultaneous measurement of psychological, cognitive, physiological and immune variables and the blinding of the investigator team to the experimental condition. The consistency of results across both experimental protocols affords further confidence in the results. A primary limitation lies in the narrow age range, the geographical homogeneity of the participants and the lack of an initial baseline assessment of participants’ auditory sensitivity, all of which constrain the generalisability of the findings to broader populations. Additionally, as the experiments were conducted in a laboratory setting, participants were exposed to industrial visual, olfactory and tactile sensory inputs during both the forest and industrial acoustic conditions. These extraneous sensory inputs may have diminished the impact of forest acoustic stimuli on some outcome measures3,4, potentially resulting in an underestimation of the benefits conferred by forest acoustic environments.
The results of this study—the first in a series of standardised experimental investigations designed to systematically isolate and expose different senses to both natural and industrial stimuli—represent an important step toward identifying the specific aspects of the natural world that influence human biology and the underlying mechanisms that drive these relationships. An understanding of these mechanistic relationships is critical to overcoming the barriers currently hindering the widespread prescription of nature-based therapies3,25–27. More immediately, however, this work highlights the potential value of acoustic nature-based interventions for individuals with limited access to nature. The acoustic environment provides a wealth of sensory information to which humans appear to be quite sensitive57. For instance, human emotions systematically respond to changes in the acoustic environment, independent of the source of the sound, shaping how both environmental sounds and associated visual stimuli are perceived58. In addition, cognitive and immune function can be affected by the acoustic environment30,53,59. Natural and industrial acoustic environments differ significantly. Anthropogenic industrial noise, for example, has been linked with increased stress, reduced sleep quality, cardiovascular disease, endocrine defects and impaired cognition59–63. In contrast, and consistent with some of the results presented here, natural sounds have been shown to confer health benefits, alleviate physiological stress and enhance mood and cognition30,64.
The findings presented here reflect the potential for incorporating natural soundscapes into urban design, workplace environments and therapeutic interventions. Forest acoustic environments can enhance mood, restoration and directed attention relative to industrial noise. Thus, urban designs that buffer or eliminate industrial noises while enhancing natural sounds could help to attenuate the negative impacts of urban cityscapes on mental health. Similarly, workplace settings that incorporate nature-inspired acoustics may promote employee well-being, improve focus and boost productivity. Moreover, therapeutic interventions that utilize natural soundscapes could provide accessible, cost-effective, non-invasive approaches for individuals with limited access to natural environments.
The implications of research seeking to explain how and why natural environments influence human biology extend beyond the development of public health interventions. First, a deeper understanding of the differential effects of natural and industrial environments on human biology has the potential to enhance understanding of human evolution. Since the emergence of anatomically modern Homo sapiens approximately 300,000 years ago65, nature’s forests, plains, coastlines, and mountains provided the primary environmental parameters within which natural selection acted to shape our biology. As a result, selective pressures associated with those natural ancestral habitats have likely led to a contemporary human biology primarily adapted to those natural environments. However, the last 200–300 years—a fraction of the human evolutionary timeline—has seen unprecedented industrialisation66, drastically altering our species’ primary habitat. Research examining the influence of contemporary natural environments (representative of ancestral habitats) on human biology relative to industrialised environments (humanity’s contemporary home) can therefore inform current understanding of the mechanisms that underpin Homo sapiens’ evolved ability to adapt to novel contexts. Investigations designed to identify which features of the natural environment are responsible for the beneficial effects on human biology could provide valuable insights into the selective mechanisms that drove the evolution of our species. The current study, for example, suggests the possibility that acoustic features in ancestral natural environments shaped human evolution, such that continued exposure and adaptation over time now underpins psychological wellbeing and cognitive functioning. Second, research in this area has the potential to increase the value society places on natural environments, thereby incentivising environmental conservation and regeneration27. Empirical evidence that highlights the importance of natural environments for human physiological and psychological health—along with essential functions such as cognition and immunity—may increase the value society places on natural environments. Such work could underscore the multifaceted benefits of contact with nature. By demonstrating the direct and indirect ways in which exposure to natural environments contributes to human health, this body of research could advocate for the conservation and regeneration of natural areas more broadly. Such initiatives are increasingly necessary, as the economic growth of industrialised nations over the past 200 years has led to the extensive degradation and exploitation of the Earth’s ecological and biogeochemical systems67. It is hoped that research designed to increase understanding of the link between nature exposure and human health will foster a broader cultural shift towards prioritising the preservation and restoration of natural environments.
In conclusion, this study demonstrates improvements in biomarkers of mood, restoration and cognitive function following exposure to a 30-min acoustic recording of a forest environment (an ancient UK temperate rainforest), relative to an industrialised environment (two major UK city centres). No differential effects on biomarkers of physiological stress or immune function were observed. These findings suggest that acoustic elements of forest environments contribute to the enhanced mood and cognition previously observed during real-world nature immersion but may not affect physiological or immunological parameters. This work advances our understanding of how nature influences human biology and takes steps toward addressing key barriers to the prescription of nature-based therapies. In the short term, this research highlights the potential for acoustic interventions for individuals with limited access to natural environments. Future research should build on these findings by systematically isolating and exposing additional senses using standardised laboratory experiments to assess the relative contributions of each sensory modality.
Methods
Male and female participants aged 18–25 were recruited from Leicestershire, UK. The experimental protocols were approved by the Loughborough University Ethics Review Sub-Committee (Application No: 2022-11128-1722) and all methods were carried out in accordance with the relevant guidelines and regulations. Testing was performed at Loughborough University, UK, and informed consent was obtained from all subjects.
Two experiments were conducted. In the first experiment, 100 participants (46 male, 54 female, mean age = 21.7 years, SD = 1.2 years) completed an investigator-blinded randomised controlled trial. In the second experiment, 30 participants (13 male, 17 female, mean age = 22.4 years, SD = 1.0 years) completed an investigator-blinded counterbalanced randomised crossover trial. All participants self-reported normal hearing.
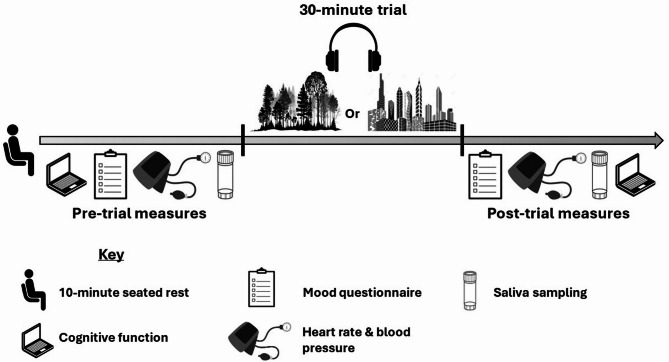
In each experiment, participants were asked to refrain from consuming alcohol or other recreational substances the evening before. They arrived at 08:15 and sat quietly in our closed, quiet laboratory for 10-min before baseline (‘pre-trial’) measures were collected, allowing participants to reach a common physiological baseline (see Fig. 6 and descriptions below). While seated, participants then listened to a 30-min recording of forest or industrial sounds played through Bose QuietComfort 20 Acoustic Noise Cancelling™ headphones (Bose Corporation, Framingham, MA, USA) connected to a laptop, while seated in a quiet room.
Fig. 6.
Experimental protocol. In Experiment 1 (randomised controlled trial), participants were randomly allocated to either the Forest Condition or the Industrial Condition. In Experiment 2 (counterbalanced crossover trial), participants completed both conditions in a randomised order on different days (with each session employing the same experimental protocol).
The same volume setting was used on both the laptop and headphones for all participants, ensuring consistency across trials. Although the actual perceived loudness differed between the forest and industrial recordings—reflecting inherent differences between the two environments—the volume setting was held constant. We intentionally did not normalise the sound pressure levels, as our aim was to present ecologically valid soundscapes representative of real-world acoustic environments. Industrial environments differ from natural ones in multiple ways, including increased sound intensity, the presence of mechanical noise (e.g., engines) and the masking of natural sounds such as birdsong. Normalising the sound levels would have removed a meaningful difference and compromised the ecological relevance of the stimuli.
The soundscapes were recorded specifically for this study in different areas of the UK (see Table 1 and Supplementary Information 1 for further details). A 30-min trial length was selected for its ecological validity, reflecting a realistic duration of exposure in daily life. Contemporary populations spend very little time outdoors. People in the UK, for example, report spending only 7% of their day outdoors on weekdays and 11% on weekends68, a pattern also observed in the United States, Canada and New Zealand69–72. All biomarkers assessed during the study reported in this manuscript have been previously shown to be responsive within a 30-min period73,74.
Table 1.
Summary of the acoustic properties of the forest and industrial soundscapes related to amplitude. Both the A-weighted and the integrated loudness measurements are reflect perceived loudness, as these values incorporate differences in sound sensitivity across the audible frequency range. Supplementary information 1 provides further details.
| Acoustic property | Forest soundscape | Industrial soundscape |
|---|---|---|
| Unweighted (dBFS) | − 43.54 | − 17.48 |
| A-Weighted (dBFS) | − 48.97 | − 24.04 |
| Integrated Loudness (LUFS) | − 41.28 | − 13.25 |
Immediately after listening to the recording, all measures were repeated to produce the ‘post-trial’ data.
Pre-trial and post-trial measurements included the following:
Physiological biomarkers
Resting heart rate and blood pressure were measured using a sphygmomanometer (M6, Omron, Kyoto, Japan) on the non-dominant upper arm while the participant was sitting upright. Saliva samples were collected via the passive drool method using Saliva Collection Aids (Salimetrics LLC., Ely, UK) and stored at − 80 °C until analysis. Salivary cortisol concentrations were assessed in duplicate according to the manufacturer’s instructions using a commercially available ELISA kit (Salimetrics, LLC., Ely, UK). Salivary cortisol samples with a coefficient of variation exceeding 10% were re-analysed.
Heart Rate Variability (HRV) was recorded during spontaneous breathing in an upright seated position using ambulatory devices (Polar H10) and the EliteHRV Smartphone app, both of which have been validated for research trials75. Participants remained in this seated position throughout the entirety of the seven minutes pre-trial and post-trial HRV measurements. HRV analysis included Root Mean Square of Successive Differences (RMSSD) and High Frequencies (HF), which reflect parasympathetic activity, and Low Frequencies (LF) and Low Frequencies/High-Frequencies ratio (LF: HF), which reflect sympathetic activity. RMSSD is thought to best represent parasympathetic activity and vagal tone76and was therefore used as the main HRV indicator of interest.
-
(2)
Mood
Participants completed a printed version of the Abbreviated Profile of Mood States (POMS) questionnaire77,78. POMS assesses seven subcategories of mood (five negative: Tension, Anger, Fatigue, Depression, Confusion, and two positive: Vigour and Esteem Related Affect), which are aggregated to generate the Total Mood Disturbance (TMD) score.
-
(3)
Immune function
Immune function was measured via salivary secretory IgA (sIgA). sIgA plays a critical role in the immune defence of mucosal surfaces, the point of entry of respiratory pathogens such as SARS-CoV-2 and influenza virus79. Salivary IgA concentration was assessed in duplicate according to the manufacturer’s instructions using a commercially available ELISA (Salimetrics, LLC., Ely, UK). sIGA samples with a coefficient of variation exceeding 10% were re-analysed.
-
(4)
Cognitive function
The dual n-back task, originally developed by Jaeggi et al.80 is a cognitive measure that involves the simultaneous completion of an auditory n-back and a visual n-back task. Although the dual n-back has been discussed as a measure of working memory (e.g.,80,81). the construct validity of n-back assessments as working memory measures has been questioned82. Nevertheless, the dual n-back has been found to correlate moderately with measures of fluid intelligence83, and it has also been used in prior investigations of nature-based cognitive restoration30,84. For these reasons, we opted to use the dual n-back as our measure of cognitive performance.
In Experiment 1, each block contained 20 scored letter/square events (excluding the first n events). Each block contained 8 auditory targets and 8 visual targets (with 4 of these occurring on the same event). Participants completed a 1-back (monitoring the visual and auditory modalities for direct repeats) as a practice task, to familiarize themselves with the general task flow. Then, participants completed four blocks of a 2-back (monitoring the visual and auditory modalities for repeats from two positions previously) as their main assessment.
In Experiment 2, participants completed two online practice sessions prior to completing the main experiment. In the first practice session, participants completed a visual 1-back and auditory 1-back in separate blocks (40 trials each), In the second practice session, participants completed abridged versions of the separate visual and auditory 1-backs (20 trials each), followed by 2-back versions of the visual and auditory tasks (20 trials each), followed by the full DNB (2-back), combining both visual and auditory modalities (40 trials). The scored component of the DNB was also slightly modified relative to Experiment 1. Given the more comprehensive practice used in Experiment 2, the scored sessions of DNB did not contain the 1-back practice. Additionally, the length of the task was extended (from four to five blocks per session, and from 20 to 40 trials per block) to increase task difficulty.
-
(5)
Questionnaire
The Perceived Restoration Scale (PRS) and Mental Bandwidth Scale (MBS) were adapted from a previous study designed to examine how virtual walks through different environments facilitated psychological restoration85. The MBS is a seven-item scale designed to assess mental activities such as reflection and self-awareness during activities - in this case, listening to a soundscape. The scale contains three subcomponents (self-awareness, daydreaming and planning). Self-awareness and daydreaming were assessed with two questions each, whereas planning was assessed with three questions. Participants rated each item on a five-point Likert scale from 1 (‘not at all’) to 5 (‘extremely’). The PRS is a nine-item scale meant to assess the therapeutic potential of environments. The PRS consists of three subcomponents (being away, fascination and extent), which were assessed with three questions each. For the PRS, each item was also rated by participants using the same five-point item Likert scale.
Statistical analyses
All variables were checked for normality using the Shapiro–Wilk Test. For normally distributed variables, paired samples t-tests were performed to compare pre- and post-trial metrics, and independent samples t-tests were used to compare differences between male and female participants and between participants assigned to the forest and industrial conditions. For non-normally distributed variables, non-parametric equivalent tests were used (the Wilcoxon Signed-Rank Test and the Mann-Whitney U Test). In Experiment 2, two-way repeat measure ANOVAs were performed to evaluate the effects of Condition (Forest and Industrial) and Time (Pre-Trial and Post-Trial) on outcome variables. Analyses were conducted with the Statistical Package for the Social Sciences (SPSS) v.25 with significance set at < 0.05. We calculated a priori power in G*Power86 based on the two experimental designs and assumptions of a medium effect size (d = 0.4)87. To achieve a statistical power of 0.8, sample sizes of 78 and 18 would be required in Experiments 1 and 2, respectively. Following previous experience of participants dropping out (particularly from studies involving more than one session), additional participants were recruited.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank all participants for their time.
Author contributions
D.L. and C.N.S. conceived the work. D.L., C.N.S. and S.V.H. designed the experiments. E.G., C.H., B.H., T.K., J.R., R.S., M.S. and J.W. performed the experiments. D.L., C.N.S., S.V.H., M.N., M.S., V.P., K.M. and E.O.D. analysed samples, extracted data, analysed and interpreted the results. D.L. and C.N.S. wrote the paper. All authors reviewed the manuscript.
Data availability
Data is available upon request from D.Longman@lboro.ac.uk.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ulrich, R. et al. Stress recovery during exposure to natural and urban environments. J. Environ. Psychol.11, 201–230 (1991). [Google Scholar]
- 2.Bratman, G. et al. Nature and mental health: an ecosystem service perspective. Sci. Adv.5, (2019). [DOI] [PMC free article] [PubMed]
- 3.Frumkin, H. et al. Nature contact and human health: A research agenda. Environ. Health Perspect.125, 1–18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen, M., Jones, R. & Tocchini, K. Shinrin-yoku (Forest bathing) and nature therapy: A state-of-the-art review. Int. J. Environ. Res. Public. Health.14, (2017). [DOI] [PMC free article] [PubMed]
- 5.White, M. et al. Spending at least 120 minutes a week in nature is associated with good health and wellbeing. Sci. Rep.9, 1–11. 10.1038/s41598-019-44097-3 (2019). [DOI] [PMC free article] [PubMed]
- 6.Lim, S. S. et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet. 380, 2224–2260 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bratman, G., Daily, G., Levy, B. & Gross, J. The benefits of nature experience: improved affect and cognition. Landsc. Urban Plan.138, 41–50 (2015). [Google Scholar]
- 8.Lee, J. et al. Influence of forest therapy on cardiovascular relaxation in young adults. Evidence-based Complement. Altern. Med.10.1155/2014/834360 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Todorova, Y. et al. Additional health benefits observed following a nature walk compared to a green urban walk in healthy females. Urban Sci.7, 1–13 (2023). [Google Scholar]
- 10.Berman, M. et al. Interacting with nature improves cognition and affect for individuals with depression. J. Affect. Disord. 140, 300–305 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berman, M., Jonides, J. & Kaplan, S. The cognitive benefits of interacting with nature. Psychol. Sci.19, 1207–1212 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Stevenson, M., Schilhab, T. & Bentsen, P. Attention restoration theory II: a systematic review to clarify attention processes affected by exposure to natural environments. J. Toxicol. Environ. Health Part. B: Crit. Rev.. 21, 227–268 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Dadvand, P. et al. Green spaces and cognitive development in primary school children. Proc. Natl. Acad. Sci.112, 7937–7942 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohira, H., Takagi, S., Masui, K., Oishi, M. & Obata, A. Effects on shinrin-yoku (forest-air bathing and walking) on mental and physical health. Bull. Tokai Women Univ.19, 217–232 (1999). [Google Scholar]
- 15.Li, Q. et al. Visiting a forest, but not a city, increases human natural killer activity and expression of Anti-Cancer proteins. Int. J. Immunopathol. Pharmacol.21, 117–127 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Li, Q. Effect of forest bathing trips on human immune function. Environ. Health Prev. Med.15, 9–17 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, Q., Miyazaki, Y. & Krensky, A. Phytoncides (wood essential oils) induce human natural killer cell activity. Immunol. Immunotoxicol.28, 319–333 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Li, Q. et al. Effect of phytoncide from trees on human natural killer cell function. Int. J. Immunopathol. Pharmacol.22, 951–959 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Chae, Y. et al. The effects of forest therapy on immune function. Int J. Environ. Res. Public. Health.18, (2021). [DOI] [PMC free article] [PubMed]
- 20.Andersen, L., Corazon, S. & Stigsdotter, U. Nature exposure and its effects on immune system functioning: A systematic review. Int. J. Environ. Res. Public. Health. 18, 1–42 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Connor, D., Thayer, J. & Vedhara, K. Stress and health: A review of psychobiological processes. Ann. Rev. Psychol.72, 663–688 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Hartig, T., Mitchell, R., de Vries, S. & Frumkin, H. Nature and health. iIn Annual Review of Public Health, vol. 35, 207–228 (Annual Reviews Inc., 2014). [DOI] [PubMed]
- 23.Robinson, J., Jorgensen, A., Cameron, R. & Brindley, P. Let nature be Thy medicine: A socioecological exploration of green prescribing in the UK. Int. J. Environ. Res. Public. Health. 17, 3460 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mears, M., Brindley, P., Maheswaran, R. & Jorgensen, A. Understanding the socioeconomic equity of publicly accessible greenspace distribution: the example of sheffield, UK. Geoforum. 103, 126–137 (2019). [Google Scholar]
- 25.Van den Berg, A. From green space to green prescriptions: challenges and opportunities for research and practice. Front. Psychol.8, 8–11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw, C. & Longman, D. Advancing nature therapy research via evolutionary theory. Under Rev.
- 27.Gobster, P., Schultz, C., Kruger, L. & Henderson, J. Forest therapy trails: A conceptual framework and scoping review of research. Forests. 13, 1–40 (2022). [Google Scholar]
- 28.Kang, J., Choi, J. & Lee, K. Development of an evaluation index for forest therapy environments. Int. J. Environ. Res. Public. Health21, (2024). [DOI] [PMC free article] [PubMed]
- 29.Van Hedger, S. et al. Of cricket chirps and car horns: the effect of nature sounds on cognitive performance. Psychon. Bull. Rev.26, 522–530 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Song, I., Baek, K., Kim, C. & Song, C. Effects of nature sounds on the attention and physiological and psychological relaxation. Urban For. Urban Green.86, 127987 (2023). [Google Scholar]
- 31.Alvarsson, J., Wiens, S. & Nilsson, M. Stress recovery during exposure to nature sound and environmental noise. Int. J. Environ. Res. Public Health. 7, 1036–1046 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jo, H. et al. Physiological and psychological effects of forest and urban sounds using high-resolution sound sources. Int. J. Environ. Res. Public. Health16, (2019). [DOI] [PMC free article] [PubMed]
- 33.Song, C., Ikei, H. & Miyazaki, Y. Physiological effects of visual stimulation with forest imagery. Int. J. Environ. Res. Public. Health15, (2018). [DOI] [PMC free article] [PubMed]
- 34.Jo, H., Song, C. & Miyazaki, Y. Physiological benefits of viewing nature: A systematic review of indoor experiments. Int. J. Environ. Res. Public. Health16, (2019). [DOI] [PMC free article] [PubMed]
- 35.Ulrich, R. View through a window may influence recovery from surgery. Science. 224, 420–421 (1984). [DOI] [PubMed] [Google Scholar]
- 36.Ikei, H., Song, C. & Miyazaki, Y. Physiological effects of touching the wood of Hinoki Cypress (Chamaecyparis obtusa) with the soles of the feet. Int. J. Environ. Res. Public. Health15, (2018). [DOI] [PMC free article] [PubMed]
- 37.Rickard, S. & White, M. Barefoot walking, nature connectedness and psychological restoration: the importance of stimulating the sense of touch for feeling closer to the natural world. Landsc. Res.46, 975–991 (2021). [Google Scholar]
- 38.Bentley, P. et al. Nature, smells, and human wellbeing. 1–14 (2023). [DOI] [PMC free article] [PubMed]
- 39.Weber, S. & Heuberger, E. The impact of natural odors on affective States in humans. Chem. Senses. 33, 441–447 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Franco, L., Shanahan, D. & Fuller, R. A. Review of the benefits of nature experiences: more than Meets the eye. Int. J. Environ. Res. Public Health. 14, 864 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, Z. & Kang, J. Sensitivity analysis of changes in human physiological indicators observed in soundscapes. Landsc. Urban Plann.190, 103593 (2019). [Google Scholar]
- 42.UN. 68% of the world population projected to live in urban areas by 2050, says UN. In United Nations Department Economic Social Affairs (2018).
- 43.Witten, E., Ryynanen, J., Wisdom, S., Tipp, C. & Chan, S. Effects of soothing images and soothing sounds on mood and well-being. Br. J. Clin. Psychol.62, 158–179 (2023). [DOI] [PubMed] [Google Scholar]
- 44.Ratcliffe, E. Sound and soundscape in restorative natural environments: A narrative literature review. Front. Psychol.12, (2021). [DOI] [PMC free article] [PubMed]
- 45.Yao, Zhang, X. & Gong, Q. The effect of exposure to the natural environment on stress reduction: A meta-analysis. Urban For. Urban Green.57, 126932 (2021). [Google Scholar]
- 46.Chrousos, G. Stress and disorders of the stress system. Nat. Rev. Endocrinol.5, 374–381 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Stenfors, C. et al. Positive effects of nature on cognitive performance across multiple experiments: test order but not affect modulates the cognitive effects. Front. Psychol.10, (2019). [DOI] [PMC free article] [PubMed]
- 48.Thompson, W., Schellenberg, E. & Husain, G. Arousal, mood, and the mozart effect. Psychol. Sci.12, 248–251 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Ashby, F., Valentin, V. & Turken, A. The effects of positive affect and arousal on working memory and executive attention: Neurobiology and computational models. In Emotional Cognition: From Brain to Behaviour, 245–287 (John Benjamins Publishing Company, 2008).
- 50.Ashby, F., Isen, A. & Turken, U. A neuropsychological theory of positive affect and its influence on cognition. Psychol. Rev.106, 519 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Kaplan, R. & Kaplan, S. The Experience of Nature: A Psychological Perspective (Cambridge University Press, 1989).
- 52.Kaplan, S. The restorative benefits of nature: toward an integrative framework. J. Environ. Psychol.15, 169–182 (1995). [Google Scholar]
- 53.Zhang, A. et al. The immune system can hear noise. Front. Immunol.11, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prasher, D. Is there evidence that environmental noise is immunotoxic? Noise Health. 11, 151–155 (2009). [DOI] [PubMed] [Google Scholar]
- 55.Irwin, M. Sleep and infectious disease risk. Sleep. 35, 1025–1026 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Recio, A., Linares, C., Banegas, J. & Díaz, J. Road traffic noise effects on cardiovascular, respiratory, and metabolic health: an integrative model of biological mechanisms. Environ. Res.146, 359–370 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Schulte-Fortkamp, B., Fiebig, A., Sisneros, J., Popper, A. & Fay, R. Soundscapes: Humans and their Acoustic Environment, vol. 76 (Springer Nature, 2023).
- 58.Ma, W. & Thompson, W. Human emotions track changes in the acoustic environment. Proc. Natl. Acad. Sci. U.S.A.112, 14563–14568 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wright, B., Peters, E., Ettinger, U., Kuipers, E. & Kumari, V. Understanding noise stress-induced cognitive impairment in healthy adults and its implications for schizophrenia. Noise Health. 16, 166–176 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Hammer, M., Swinburn, T. & Neitzel, R. Environmental noise pollution in the united states: developing an effective public health response. Environ. Health Perspect.122, 115–119 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mucci, N. et al. Urban noise and psychological distress: A systematic review. Int. J. Environ. Res. Public Health. 17, 1–22 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jakovljević, B., Belojević, G., Paunović, K. & Stojanov, V. Road traffic noise and sleep disturbances in an urban population: Cross-sectional study. Croat Med. J.47, 125–133 (2006). [PMC free article] [PubMed] [Google Scholar]
- 63.Moudon, A. Real noise from the urban environment. How ambient community noise affects health and what can be done about it. Am. J. Prev. Med.37, 167–171 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Buxton, R., Pearson, A., Allou, C., Fristrup, K. & Wittemyer, G. A synthesis of health benefits of natural sounds and their distribution in National parks. Proc. Natl. Acad. Sci. U. S. A. 118, 6–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hublin, J. et al. New fossils from Jebel irhoud, Morocco and the pan-African origin of Homo sapiens. Nature546, 289–292 (2017). [DOI] [PubMed] [Google Scholar]
- 66.White, M. The rise of cities in the 18th century. Br. Lib. (2009).
- 67.Whitmee, S. et al. Safeguarding human health in the anthropocene epoch: report of the rockefeller Foundation-Lancet commission on planetary health. Lancet. 386, 1973–2028 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Diffey, B. An overview analysis of the time people spend outdoors. Br. J. Dermatol.164, 848–854 (2011). [DOI] [PubMed] [Google Scholar]
- 69.Klepeis, N. et al. The National human activity pattern survey (NHAPS): a resource for assessing exposure to environmental pollutants. J. Expo Sci. Environ. Epidemiol.11, 231–252 (2001). [DOI] [PubMed] [Google Scholar]
- 70.Leech, J., Wilby, K., McMullen, E. & Laporte, K. The Canadian human activity pattern survey: report of methods and population surveyed. Chronic. Dis. Can.17, 118–123 (1996). [PubMed] [Google Scholar]
- 71.Matz, C. et al. Effects of age, season, gender and urban-rural status on time-activity: Canadian human activity pattern survey 2 (CHAPS 2). Int. J. Environ. Res. Public. Health. 11, 2108–2124 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khajehzadeh, I. & Vale, B. How new Zealanders distribute their daily time between home indoors, home outdoors and out of home. J. Social Sci. Online. 12, 17–31 (2017). [Google Scholar]
- 73.Keller, J. et al. SARS-CoV-2 specific sIgA in saliva increases after disease-related video stimulation. Sci. Rep.13, 1–10 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song, C., Ikei, H. & Miyazaki, Y. Physiological effects of nature therapy: A review of the research in Japan. Int J. Environ. Res. Public. Health13, (2016). [DOI] [PMC free article] [PubMed]
- 75.Moya-Ramon, M., Mateo-March, M., Peña-González, I., Zabala, M. & Javaloyes, A. Validity and reliability of different smartphones applications to measure HRV during short and ultra-short measurements in elite athletes. Comput. Methods Progr. Biomed.217, 106696 (2022). [DOI] [PubMed] [Google Scholar]
- 76.Kleiger, R., Stein, P. & Bigger, J. Jr Heart rate variability: measurement and clinical utility. Ann. Noninvasive Electrocardiol.10, 88–101 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grove, J. & Prapavessis, H. Preliminary evidence for the reliability and validity of an abbreviated profile of mood States. Int. J. Sport Psychol.23, 93–109 (1992). [Google Scholar]
- 78.McNair, D., Lorr, M. & Droppleman, L. Profile of mood states manual. (1992).
- 79.Chao, Y., Rötzschke, O. & Tan, E. The role of IgA in COVID-19. Brain Behav. Immun.87, 187–183 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jaeggi, S., Buschkuehl, M., Jonides, J. & Perrig, W. Improving fluid intelligence with training on working memory. Proc. Natl. Acad. Sci. U.S.A.105, 6829–6833 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salminen, T., Forlim, C., Schubert, T. & Kühn, S. Dual n-back training improves functional connectivity of the right inferior frontal gyrus at rest. Sci. Rep.10, 1–10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kane, M., Conway, A., Miura, T. & Colflesh, G. Working memory, attention control, and the N-back task: a question of construct validity. J. Exp. Psychol. Learn. Memory Cogn. 33, 615 (2007). [DOI] [PubMed] [Google Scholar]
- 83.Jaeggi, S. et al. The relationship between n-back performance and matrix reasoning—implications for training and transfer. Intelligence38, 625–635 (2010). [Google Scholar]
- 84.Stobbe, E., Forlim, C. & Kühn, S. Impact of exposure to natural versus urban soundscapes on brain functional connectivity, BOLD entropy and behavior. Environ. Res.244, 117788 (2024). [DOI] [PubMed] [Google Scholar]
- 85.Brancato, G., Van Hedger, K., Berman, M. & Van Hedger, S. Simulated nature walks improve psychological well-being along a natural to urban continuum. J. Environ. Psychol.81, (2022).
- 86.Caprara, G., Steca, P., Zelli, A. & Capanna, C. A new scale for measuring adults’ prosocialness. Eur. J. Psychol. Assess.21, 77–89 (2005). [Google Scholar]
- 87.Baumsteiger, R. & Siegel, J. Measuring prosociality: the development of a prosocial behavioral intentions scale. J. Pers. Assess.101, 305–314 (2019). [DOI] [PubMed] [Google Scholar]
- 89.Faul, F., Erdfelder, E., Lang, A. & Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 39, 175–191 (2007). [DOI] [PubMed] [Google Scholar]
- 90.Cohen, J. Statistical Power Analysis for the Behavioural Sciences (Lawrence Earlbaum Associates, 1988).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon request from D.Longman@lboro.ac.uk.