Abstract
Equine influenza is a cause of epizootic respiratory disease of the equine. The detection of equine influenza virus using real-time Light Cycler reverse transcription (RT)-PCR technology was evaluated over two influenza seasons with the analysis of 171 samples submitted for viral respiratory disease. Increased sensitivity was found in overall viral detection with this system compared to Directigen Flu A and virus isolation, which were 40% and 23%, respectively, that of the RT-PCR. The assay was also evaluated as a viable replacement for the more traditional methods of quantifying equine influenza virus, 50% egg infectious dose and 50% tissue culture infectious dose. There was a significant positive correlation (P < 0.05) between the quantitative RT-PCR and both of these assays.
Equine influenza is considered the most economically important respiratory disease of the equine in countries with substantial breeding and racing industries. Equine influenza virus belongs to the Orthomyxoviridae family and there are two distinct subtypes, H7N7 (11) and H3N8 (13). It is generally accepted that the former is no longer in circulation as the last confirmed outbreak caused by this virus was in 1978 (14), however, outbreaks caused by H3N8 viruses occur annually. A study conducted in Colorado in 1998 showed that the pathogen was responsible for two-thirds of equine viral respiratory infections (7).
It is imperative to have specific and sensitive virus detection systems available for rapid diagnosis of equine influenza. Traditionally, the gold standard has been virus isolation from nasopharyngeal swabs using embryonated hen eggs. Other diagnostic tests employed include virus culture in Madin Darby canine kidney cells or measurement of a rise in antibody titer in paired sera by hemagglutination inhibition assay (9). These established techniques are time consuming and laborious. Furthermore, the sensitivity of virus isolation is dependant on the presence of infectious particles and some virus strains are difficult to isolate (8). Serology provides only retrospective data which do not facilitate prompt intervention and appropriate case management. In recent years enzyme-linked immunosorbent assays such as Directigen FluA (Becton Dickson) and reverse transcription (RT)-PCR have been introduced in some laboratories to provide more timely results although the sensitivity of the former has been shown to be quite variable (6, 10, 12).
The Light Cycler PCR system from Roche Molecular Biochemicals, allows for high-speed thermal cycling and quantitative RT-PCR (qRT-PCR) using SYBR Green I dye which binds specifically to double-stranded DNA. The aim of this study was to transfer our most sensitive tool for equine influenza virus detection (10) to a more rapid, real-time RT-PCR system suitable for routine diagnostics using the Light Cycler technology. The advantages of this system include faster result turnaround time, a closed system less prone to contamination and the elimination of gel electrophoresis. We also sought to examine real-time RT-PCR as a viable replacement for the more traditional methods of quantifying equine influenza virus, i.e., the determination of 50% egg infectious dose (EID50) or tissue culture infectious dose (TCID50).
MATERIALS AND METHODS
Samples.
A total of 171 nasopharyngeal swabs collected from suspect cases of viral respiratory disease during a 2-year period were tested for the presence of equine influenza virus. Some of these diagnostic samples were accompanied by sera for hemagglutination inhibition testing. The second set of samples used to validate the qRT-PCR system were previously described (10). Briefly, nasopharyngeal swabs from four seronegative foals exposed to an aerosol of influenza strain A/Equi/Kildare/89 at 106 EID50/ml were collected for 10 consecutive days postchallenge.
Virus isolation, Directigen Flu A, and hemagglutination inhibition.
Virus isolation in embryonated eggs was carried out as previously described (10). The immunoassay Directigen Flu A for the detection of nucleoprotein was carried out in accordance with the manufacturer's instructions (10). The hemagglutination inhibition assay for the measurement of antibodies to type 1 and type 2 equine influenza viruses was adapted from the Office International des Epizooties (OIE) Manual of Standards for Diagnostic Tests and Vaccines (9). Seroconversion was defined as a fourfold or greater rise in antibody titer.
PCR primers.
Primers M52C (5′-CTTCTAACCGAGGTCGAAACG-3′) and M253R (5′-AGGGCATTTTGGACAAAGCGTCTA-3′) designed by Fouchier et al. (4) were used to amplify a 244-bp amplicon from nucleotides 32 to 276 of the matrix gene. A second primer set was designed to confirm the samples positive only by RT-PCR. A 373-bp region of the hemagglutinin gene (nucleotides 566 to 938) was amplified using primer HA3F (5′-GAATGTGACAATGCCTAAC-3′) and primer HA3R (5′-GATGCTTCCATTTGGTGTA-3′).
RT-PCR.
One-step RT-PCR was performed using the Light Cycler RNA amplification kit, SYBR Green I (Roche). RNA was extracted and purified from 100 μl of nasal secretions using the RNAgents Total RNA isolation system (Promega Corporation, Madison, Wisconsin) according to the manufacturer's recommendations. A 10 μl RT-PCR mix consisted of 6.0 mM MgCl2, 2.0 μl of 5X reaction mix, 0.2 μl enzyme mix, and 1.5 μl of RNA. Primers were used at a final concentration of 0.5 μM. Reverse transcription was carried out at 55°C for 10 min, followed by an initial denaturation step at 95°C for 30 seconds. cDNA was then amplified with 40 to 50 cycles of 95°C held for 0 seconds, 65°C for 5 seconds (secondary target temperature 55°C), and 72°C for 10 seconds. Fluorescence data were acquired at the end of each cycle in a single step. Once the plateau phase of the PCR had been reached, amplification was stopped and a standard melting curve analysis was performed (95°C held for 0 seconds, 65°C for 10 seconds, and a 0.1°C/second rise to 95°C) with continual fluorescence measurement. A second independent RT-PCR protocol was carried out with hemagglutinin (HA) gene primers for validation purposes. RT-PCR conditions used with the second primer set were the same as those used with the Matrix primers except the 65°C annealing temperature was held for 17 seconds.
qRT-PCR.
For quantification of virus shedding, purified viral genomic RNA was used as a standard. The concentration of the standard nucleic acid was determined by measuring the absorbance at 260 nm. Serial log dilutions of the RNA were used to generate a standard curve ranging from 5 × 107 to 500 genome copies/μl of viral RNA. The standard curve is the linear regression line through the data points on a plot of Ct (threshold cycle) versus logarithm of standard sample concentration. The crossing points of unknown samples were determined from the regression line and the corresponding concentrations calculated. The genome copy number was then calculated.
Statistical analysis.
Microsoft SPSS 12.0.1 for Windows was used for formal statistical testing, and the significance level was set at 0.05. The bivariate correlations function was used to compute Pearson's correlation coefficient for comparing the quantitative tests.
RESULTS AND DISCUSSION
One hundred and seventy-one nasopharyngeal swabs submitted over a two year period from cases of suspected viral respiratory disease were tested for the presence of equine influenza virus (Table 1). Virus was isolated from eight horses and antigen was detected by Directigen Flu A in 14 swabs. Acute and convalescent-phase sera were only available for seven horses of which six on five different premises seroconverted to H3N8 equine influenza. Thirteen of the 23 unpaired serum samples submitted to the laboratory and the acute sample from the horse that did not seroconvert had high antibody titers to H3N8 equine influenza virus but no detectable antibody titers against H7N7. As all of the vaccines available during the study period contained both H3N8 and H7N7 viruses, these results were considered to suggest exposure to virus by natural infection. However, while such serological results may corroborate a diagnosis of influenza, they are not confirmatory.
TABLE 1.
Results for clinical samples
| Premise | Horse no. | Resulta
|
||||
|---|---|---|---|---|---|---|
| RT-PCR | VI | DFA | Serology
|
|||
| Paired | Acute | |||||
| 1 | 1 | + | + | + | + | − |
| 2 | + | − | − | NA | S | |
| 3 | + | − | − | + | S | |
| 4 | + | − | − | NA | S | |
| 2 | 5 | + | − | − | − | S |
| 6 | + | − | + | + | − | |
| 7 | + | + | + | NA | − | |
| 8 | + | − | − | NA | S | |
| 3 | 9 | + | − | − | NA | S |
| 10 | + | − | + | NA | NA | |
| 4 | 11 | + | − | + | + | − |
| 5 | 12 | + | − | + | + | − |
| 6 | 13 | + | + | + | + | V |
| 7 | 14 | + | + | + | NA | − |
| 8 | 15 | + | + | + | NA | − |
| 16 | + | − | − | NA | S | |
| 9 | 17 | + | − | − | NA | S |
| 18 | + | − | − | NA | S | |
| 10 | 19 | + | − | − | NA | S |
| 20 | + | − | − | NA | V | |
| 21 | + | − | − | NA | S | |
| 11 | 22 | + | − | + | NA | − |
| 23 | + | − | + | NA | − | |
| 12 | 24 | + | − | − | NA | S |
| 13 | 25 | + | + | + | NA | − |
| 26 | + | − | − | NA | NA | |
| 14 | 27 | + | − | − | NA | NA |
| 28 | + | − | − | NA | NA | |
| 15 | 29 | + | − | − | NA | V |
| 30 | + | − | − | NA | V | |
| 16 | 31 | + | − | − | NA | NA |
| 17 | 32 | + | − | + | NA | S |
| 18 | 33 | + | + | + | NA | V |
| 34 | + | + | − | NA | S | |
| 19 | 35 | + | − | − | NA | S |
VI, virus isolation; S, suspected exposure to H3N8 equine influenza by natural infection; NA, not available; V, antibodies to H7N7 and H3N8 viruses consistent with vaccination; DFA, Directigen Flu A.
The RT-PCR proved to be the most sensitive of the assays used. Equine influenza virus nucleic acid was detected in the nasal swabs from 35 horses on 17 different premises, i.e., the overall detection rate was 20%, compared to 8% and 5% for Directigen Flu A and virus isolation, respectively. Of the 35 RT-PCR-positive swabs 27 were positive by one or more of the other diagnostic tests employed. All samples positive only by RT-PCR were also tested with the second primer set against the HA gene (data not shown) and there were no discrepancies.
This study demonstrates that RT-PCR is effective in the detection of virus shedding from seropositive horses. Virus was isolated from only 15% of seropositive horses that tested positive by RT-PCR. The detection of virus shedding in subclinically infected vaccinated horses is extremely important as the introduction of such animals into an immunologically naïve population can lead to explosive virus spread and high morbidity.
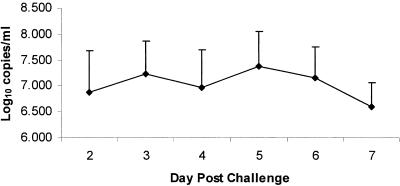
Vaccination remains the most effective control measure for equine influenza. Vaccines need to be safe and efficacious. Vaccine efficacy is measured by the reduction in clinical signs and the decrease in virus shedding. We examined whether qRT-PCR could viably replace the traditional methods of quantifying equine influenza virus shedding, i.e., EID50 and TCID50 analysis. Figure 1 shows the mean amount of virus shed by the four horses per day postchallenge by qRT-PCR analysis. We found a significant positive correlation (P < 0.05) between the traditional methods of viral quantification and the qRT-PCR results. RT-PCR was not only less labor intensive than the other two methods but was also far faster and 17% and 29% more sensitive than virus isolation in eggs and tissue culture, respectively.
FIG. 1.
Mean and standard error of the mean of viral RNA copies/ml (log10) in nasal secretions of four foals from days 2 to 7 postchallenge with equine influenza virus.
In conclusion, the Light Cycler RT-PCR protocol described here for the detection of equine influenza virus has been shown to be a more sensitive assay in the clinical diagnosis of influenza virus infection than standard techniques. The RT-PCR can also be reliably used for the quantification of equine influenza virus in vaccine efficacy studies Furthermore, the primers employed were designed to amplify a region of the matrix gene which is highly conserved across different subtypes of influenza A viruses (4). Thus, this RT-PCR detects influenza virus from multiple species. Given the propensity of influenza A viruses to cross the species (1, 15), 18 and their zoonotic potential (2, 3, 5) this assay can be used with relative ease and speed to aid virus surveillance that is advantageous not only to the equine industry but also to public health.
Acknowledgments
The initial investigations of outbreaks of respiratory disease were sponsored by Interchem. All other laboratory work was funded by the Department of Agriculture, Food and Rural Development, under the National Development Plan (Irish Equine Centre NDP\9\R\2). Michelle Quinlivan is a Ph.D. student funded by the Irish Research Council for Science, Engineering and Technology and Eugene Dempsey is a Ph.D. student aided by the Research Support Unit, Dublin Institute of Technology.
REFERENCES
- 1.Daly, J., T. Blunden, K. Smith, G. Dowd, N. Davis-Poynter, J. Miller, and S. MacRae. 2004. Suspected equine influenza infection in dogs. Office Int. Epizoot. Bull. 2004(2):50.
- 2.de Jong, M. D., V. C. Bach, T. Q. Phan, M. H. Vo, T. T. Tran, B. H. Nguyen, M. Beld, T. P. Le, H. K. Truong, V. V. Nguyen, T. H. Tran, Q. H. Do, and J. Farrar. 2005. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N. Engl. J. Med. 352:686-691. [DOI] [PubMed] [Google Scholar]
- 3.Dowdle, W. R., and M. A. Hattwick. 1977. Swine influenza virus infections in humans. J. Infect. Dis. 136(Suppl.):S386-389. [DOI] [PubMed] [Google Scholar]
- 4.Fouchier, R. A., T. M. Bestebroer, S. Herfst, L. Van Der Kemp, G. F. Rimmelzwaan, and A. D. Osterhaus. 2000. Detection of influenza A viruses from different species by PCR amplification of conserved sequences in the matrix gene. J. Clin. Microbiol. 38:4096-4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koopmans, M., R. Fouchier, B. Wilbrink, A. Meijer, G. Natrop, A. D. M. E. Osterhaus, J. E. van Steenbergen, M. du Ry van Beest Holle, M. A. E. Conyn van Spaendonck, and A. Bosman. 2003. Update on human infections with highly pathogenic avian influenza virus A/H7N7 during an outbreak in poultry in the Netherlands. Eurosurveill. Wkly. 7:1-4. [Google Scholar]
- 6.Morley, P. S., J. R. Bogdan, H. G. Townsend, and D. M. Haines. 1995. Evaluation of Directigen Flu A assay for detection of influenza antigen in nasal secretions of horses. Equine Vet. J. 27:131-134. [DOI] [PubMed] [Google Scholar]
- 7.Mumford, E. L., J. L. Traub-Dargatz, M. D. Salman, J. K. Collins, D. M. Getzy, and J. Carman. 1998. Monitoring and detection of acute viral respiratory tract disease in horses. J. Am. Vet. Med. Assoc. 213:385-390. [PubMed] [Google Scholar]
- 8.Office International des Epizooties. 1996. Conclusions and recommendations from the consultation meeting of OIE and W.H.O. experts on equine influenza, Newmarket, United Kingdom, 18-19 September 1995. Office Int. Epizoot. Bull. 108:482-484. [Google Scholar]
- 9.Office International des Epizooties. 2000. Equine influenza, p. 546-557. Manual of standards for diagnostic tests and vaccines. Office International des Epizooties, Paris, France.
- 10.Quinlivan, M., A. Cullinane, M. Nelly, K. Van Maanen, J. Heldens, and S. Arkins. 2004. Comparison of sensitivities of virus isolation, antigen detection, and nucleic acid amplification for detection of equine influenza virus. J. Clin. Microbiol. 42:759-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sovinová, O., B. Tümová, F. Pouska, and J. Nemec. 1958. Isolation of a virus causing respiratory disease in horses. Acta Virol. 2:52-61. [PubMed] [Google Scholar]
- 12.van Maanen, C., G. J. van Essen, J. Minke, J. M. Daly, and P. J. Yates. 2003. Diagnostic methods applied to analysis of an outbreak of equine influenza in a riding school in which vaccine failure occurred. Vet. Microbiol. 93:291-306. [DOI] [PubMed] [Google Scholar]
- 13.Wadell, G. H., M. B. Teigland, and M. M. Siegel. 1963. A new influenza virus associated with equine respiratory disease. J. Am. Vet. Med. Assoc. 143:587-590. [PubMed] [Google Scholar]
- 14.Webster, R. G. 1993. Are equine 1 influenza viruses still present in horses? Equine Vet. J. 25:537-538. [DOI] [PubMed] [Google Scholar]
- 15.Webster, R. G., and Y. J. Guo. 1991. New influenza virus in horses. Nature 351:527. [DOI] [PubMed] [Google Scholar]