Abstract
Lyme disease is usually diagnosed and treated based on clinical manifestations. However, laboratory testing is useful for patients with confusing presentations and for validation of disease in clinical studies. Although cultivation of Borrelia burgdorferi is definitive, prior investigations have shown that no single test is optimal for Lyme disease diagnosis. We applied high-volume blood culture, skin biopsy culture, PCR, and serodiagnosis to a cohort of patients with suspected Lyme disease acquired in Maryland and southern Pennsylvania. The study was performed to confirm the relative utility of culture and to identify laboratory testing algorithms that will supplement clinical diagnosis. Overall, 30 of 86 patients (35%) were culture positive, whereas an additional 15 of 84 (18%) were seropositive only (51% total sero- and culture positive), and PCR on skin biopsy identified 4 additional patients who were neither culture nor seropositive. Among 49 laboratory test-positive patients, the highest sensitivity (100%) for diagnosis was obtained when culture, skin PCR, and serologic tests were used, although serologic testing with skin PCR was almost as sensitive (92%). Plasma PCR was infrequently positive and provided no additional diagnostic value. Although culture is definitive and has a relatively high sensitivity, the results required a mean of 3.5 weeks to recovery. The combination of acute-phase serology and skin PCR was 75% sensitive, offering a practical and relatively rapid alternative for confirming clinical impression. The full battery of tests could be useful for patients with confusing clinical signs or for providing strong laboratory support for clinical studies of Lyme disease.
Lyme disease, caused by the deer tick-borne spirochetal pathogen Borrelia burgdorferi, is the most common vector-borne disease within the United States (4, 13). Human infection can result in neurologic, cardiovascular, or musculoskeletal disorders (1, 12). In the early stages, patients can be asymptomatic or have erythema migrans (EM) with headache, muscle aches, lymphadenopathy, or fever. Early dissemination can follow within days or weeks and is highlighted by clinical evidence of skin, nervous system, heart, or joint involvement. Months after infection, untreated patients can develop chronic major manifestations. Clinical diagnosis of Lyme disease is usually straightforward with presence of a typical EM rash but in some cases can be difficult because of nonspecific signs and symptoms (10). Diagnosis can be supported by serology, which has limitations in sensitivity and specificity (7, 10, 14). Previous evaluations of Lyme disease laboratory diagnostics were mainly based upon clinical diagnosis as the gold standard; thus, to determine their relative diagnostic utilities, we evaluated existing diagnostic tests among a cohort of patients that included many with culture-proven disease. We compared high-volume plasma and skin biopsy culture, serological testing by enzyme-linked immunosorbent assay (ELISA) with supplemental immunoglobulin M (IgM) and IgG Western blotting, and skin/plasma PCR for Lyme disease diagnosis among patients presenting with clinical suspicion of Lyme disease.
(This work was presented in part at the 114th General Meeting of the American Society for Microbiology in New Orleans, La., 23 to 27 May 2004.)
MATERIALS AND METHODS
Patient specimens.
Plasma and skin biopsy samples were collected from adult patients from Maryland and southern Pennsylvania who were evaluated at The Johns Hopkins Medical Institutions and were determined to have findings suspicious for Lyme disease by one of the study physicians. Specimens were collected for the two consecutive summers of 2001 and 2002. Patients were not enrolled if they were currently or had recently taken antibiotics. In total, 86 patients were included in this study. From this patient population, 81 plasma, 47 skin biopsy, 3 cerebrospinal fluid (CSF), and 84 acute/convalescent-phase serum specimens were obtained. Samples were promptly delivered to the laboratory for rapid processing and inoculation of cultures usually within 1 day.
Plasma culture.
A method modified from that of Wormser et al. (15) was used as follows: 20 to 25 ml of EDTA-anticoagulated blood was drawn from each patient by using sterile blood culture venipuncture techniques. After centrifugation at 350 × g for 15 min, plasma was removed from the cells using sterile disposable pipettes. Three milliliters of plasma was inoculated into each of three separate culture flasks containing 60 ml of complete Barbour-Stoenner-Kelly II (BSKII) medium without antibiotics. For 14 patients, sufficient plasma was available for inoculation into only two culture flasks. Any remaining plasma was frozen at −70°C for PCR tests.
Skin biopsies.
Skin biopsies were conducted on EM lesions that met Centers for Disease Control and Prevention (CDC) surveillance criteria (3) and only in patients who did not receive antecedent antibiotic therapy. For qualifying EM lesions, a 2-mm punch biopsy was obtained from the leading edge, and if multiple EM lesions were identified, the biopsy was taken from the primary or most erythematous lesion. Skin biopsy specimens were transported to the laboratory in incomplete BSKII, without rabbit serum or bovine serum albumin but supplemented with 40 μg of rifampin. One half of each skin biopsy sample was ground in a sterile disposable tissue grinder using ∼0.4 ml of the incomplete BSK. The ground sample was then inoculated into a sterile plastic conical tube containing 6 ml of complete BSKII without antibiotics. The remaining half of the skin biopsy was frozen at −70°C for future PCR testing.
CSF.
CSF specimens were sent in sterile containers. Three milliliters was inoculated into separate culture flasks containing 9 ml of complete BSKII medium.
Incubation and examination.
All cultures were incubated at 34 to 35°C in 5 to 10% CO2 for 8 weeks. Each culture flask was examined weekly for the presence of spirochetes by acridine orange staining of methanol-fixed preparations using a fluorescence microscope. Presumptive positives were confirmed by acridine orange staining in a wet mount, examining for corkscrew motility. All positive cultures had aliquots frozen at −70°C for further PCR tests.
PCR.
In addition to positive culture confirmation, 31 plasma and 23 skin biopsy samples were tested by real-time PCR using an ABI Taqman 7700 targeting flaB (8). This assay was shown to detect as few as 4.5 B. burgdorferi genome equivalents per reaction mixture in pilot validation studies. Some samples were confirmed with conventional PCR for B. burgdorferi ospA as previously described (6). DNA was prepared using a QIAamp DNA mini kit following the manufacturer's tissue protocol instructions (QIAgen). We used 500-μl volumes for plasma and culture specimens, and the remaining half of the tissue biopsy specimens were in a 200-μl elution volume. For conventional ospA PCR, amplicons were detected after agarose gel electrophoresis and ethidium bromide staining.
To determine analytical sensitivity, we counted cultured B. burgdorferi cells by dark-field microscopy using a hemacytometer and prepared DNA for use as a quantified standard. Matrices of distilled water, CSF, plasma, and transport medium were tested with the same B. burgdorferi standard DNA to determine their effects on PCR results. Specificity of the PCR assays for Borrelia burgdorferi was tested against DNA prepared from cultured Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Staphylococcus epidermidis, Treponema pallidum, Ehrlichia chaffeensis, and Anaplasma phagocytophilum.
Serology.
Serodiagnosis was established using CDC/Association of State and Territorial Public Health Laboratory Directors criteria on acute-phase serum, convalescent-phase serum, or both (2). In brief, serum samples obtained from prospective patients at their first visit and in convalescence (several weeks to several months later) were tested initially by ELISA (either IgG/IgM Borrelia burgdorferi ELISA [Trinity Biotech, Wicklow, Ireland] or VIDAS Lyme IgG and IgM [bioMérieux, Durham, NC]). Reactive or equivocal samples were then tested by Western blotting, as recommended (Focus Diagnostics, Cypress, California, or MarDx Diagnostics, Inc., Carlsbad, California) (2, 14).
Clinical assessments.
An initial clinical impression was determined at the time of presentation and before results of Lyme disease diagnostic testing were available. The assessment was based upon CDC case surveillance criteria excluding laboratory confirmatory methods (3). Each patient was assessed as probable, possible, or unlikely and compared for agreement with the qualitative results of individual or combinations of laboratory test results. A probable case was defined as a patient with EM or history of EM and a characteristic clinical syndrome with headache, VIIth cranial nerve palsy, or arthritis. Possible cases were defined in those with tick exposure, a rash not typical for EM, or summertime fever without identifiable source. Unlikely cases were defined as patients concerned about Lyme disease who lacked characteristic CDC case surveillance clinical manifestation criteria. Patients were also classified into early localized (EM with or without fever or lymphadenopathy only for less than 120 days), early disseminated (multiple EM, cranial neuropathy, radiculopathy, large joint arthritis, carditis, peripheral neuropathy, or other potential central nervous system manifestations for less than 120 days), or late (suspected manifestations for ≥120 days) to determine if certain tests were more likely to be revealing.
Statistical analysis.
Where appropriate, means of groups were compared using Student's t test, or proportions of groups were compared using the χ2 test or by comparing 95% confidence intervals. A P value of <0.05 was considered significant.
RESULTS
Serology.
Serum samples were obtained from 84 of 86 enrolled patients, including acute-phase samples from all, and convalescent-phase samples from 47 were obtained at a median of day 29 after the acute-phase sample. At least one serologic result was available for 80 of the 82 patients who had plasma cultures performed, for 47 who had skin cultures, for 57 patients who had plasma PCR, and for 23 who had skin PCR. Among 23 patients who had skin PCR, plasma/skin culture and serologic results were available for 22, and culture results only were available for one other patient. Combining acute and convalescent serologic results, 36 of 84 (43%) were seropositive. Among acute-phase samples, 21 (25%) were seropositive, including 8 with IgG and 17 with IgM by immunoblotting. Among the 47 convalescent-phase sera, 25 (53%) were seropositive (6 IgG and 20 IgM). Only 47 of 84 (56%) patients returned for serologic testing, suggesting a potential bias for patients with clear features of Lyme disease, such as EM. However, the proportions of patients with and without EM who returned for convalescent serology were similar (P = 0.61; χ2 test). Serologic results among culture- and PCR-positive individual patients follow.
Plasma, skin, and CSF cultures.
Plasma cultures from 82 patients were prepared, including triplicate cultures for 70 and duplicate cultures for 12. Plasma was not received for four additional enrolled patients. B. burgdorferi was cultivated from 22 patient plasma specimens (27%), including 8 for whom all three flasks were positive, 2 for whom both of the two inoculated flasks were positive, 4 for whom two of three cultures were positive, 1 for whom one of two cultures was positive, and 7 for whom one of three cultures was positive. Of the remaining 60 patients, 182 flasks had no growth throughout the 8 weeks, and 8 flasks were discarded for heavy bacterial contamination, including all 3 flasks for one patient only. The time to recovery from plasma ranged from 7 to 49 days, with a mean time to culture detection of 24 days. The mean interval before processing and cultivation was similar between culture-positive and culture-negative patients (1.06 days ± 1.11 standard deviation [SD] versus 1.02 days ± 0.99 SD; P = 0.90) and between culture-positive samples and culture-negative, seropositive patients (1.06 days ± 1.11 SD versus 1.32 days ± 1.07 SD; P = 0.44). Among the 22 patients with positive plasma cultures, all had either localized or disseminated EM skin lesions, and a description was available for 20. Of these, 12 were described as typical (central erythema, central clearing, or homogeneous), 7 were described as atypical (blue, vesicle, punctum, or size of <5 cm), and for one patient there were multiple lesions.
The average symptom duration among plasma culture-positive patients was 6 days (median, 4 days), whereas for those who were plasma culture negative, this interval was 64 days (median, 10 days). Among the 79 patients who had both serologic testing and plasma culture results available, 17 (22%) were both seropositive and culture positive, 18 (23%) were seropositive but culture negative, 4 (5%) were seronegative and culture positive, and 40 (51%) were negative for both.
Skin biopsies were obtained for culture from 47 patients; 15 (32%) cultures grew B. burgdorferi. Mean time to recovery of B. burgdorferi from skin biopsies was 25 days, with a range of 14 to 56 days. Among these, 9 (19%) were both seropositive and culture positive, 6 (13%) were seronegative and culture positive, 16 (34%) were seropositive and culture negative, and 16 (34%) were negative for both. Among 49 patients for whom the character of a skin lesion was reported, B. burgdorferi was cultured from skin biopsy of 7 of 28 (25%) with typical EM, 5 of 17 (29%) with atypical EM, and all 3 (100%) with multiple EM. Of these, other laboratory evidence of Lyme disease was obtained for 23 (30% skin culture positive) with typical EM, 11 (45% skin culture positive) with atypical EM, and all 3 with multiple EM. Of the three CSF specimens, one grew B. burgdorferi, one culture had no growth, and one culture was contaminated and unable to be analyzed.
Overall, when plasma and skin culture results were combined for the 86 patients, 30 (35%) were either plasma or skin culture positive, including 8 from both plasma and skin, 14 from plasma only, 7 from skin only, and 1 from both skin and CSF. Of the 46 patients with both plasma and skin cultures attempted, 25 (54%) were positive in either, while 8 were positive in both, 10 were positive only in plasma, and 7 were positive only in skin. The remaining 21 (46%) were negative in both skin and plasma. However, among the 84 patients with both serologic and culture results available, 21 (25%) were both seropositive and culture positive, 7 (8%) were seronegative but culture positive, 15 (18%) were seropositive and culture negative, and 41 (49%) were both seronegative and culture negative.
PCR.
All positive plasma and skin cultures were confirmed to contain B. burgdorferi DNA by PCR. By quantitative PCR, of 57 plasma samples tested, only 2 had sufficient B. burgdorferi DNA present for detection (2.1 × 105 and 2.4 × 105 borreliae/ml); both patients were seropositive but neither had B. burgdorferi isolated from plasma. Thus, of 57 plasmas tested, B. burgdorferi DNA was detected in 2 of 33 (6%) culture-positive or seropositive patients and in none of 16 from patients who were culture positive only; one plasma tested by PCR was contaminated and could not be cultured.
Of the 47 skin biopsies obtained, sufficient residual material was available to conduct quantitative PCR on 23. Among these, 9 were seronegative and culture negative, 4 were B. burgdorferi skin culture positive, 10 were B. burgdorferi plasma culture positive (3 had B. burgdorferi isolated from both skin and plasma), 13 were seropositive, and 14 (61%) were positive by either culture or serology. Of the 23 tested, 10 were positive by skin biopsy PCR, including all 4 who were skin biopsy culture positive, 5 who were seropositive, 5 who were plasma culture positive, and 6 who were either skin/plasma culture positive or seropositive. Compared to the combined culture and serology results, 6 (26%) were both skin PCR and sero/culture positive, 8 (35%) were skin PCR negative but sero/culture positive, and 5 (22%) were negative for all three tests. Of interest, 4 (17%) patients with B. burgdorferi DNA detected in skin were both seronegative and skin/plasma culture negative. All four of these skin biopsies were confirmed to contain B. burgdorferi genomic DNA based upon separate amplification of ospA.
Comparisons with qualitative clinical assessments.
Qualitative clinical assessments were made prospectively for 82 patients, of whom 25 were classified as unlikely, 32 as possible, and 25 as probable to have Lyme disease. Overall, initial serologic tests agreed with possible or probable clinical Lyme disease diagnosis in only 50% (40/80) of cases, increasing to 69% (55/80) when follow-up serologic tests were included (Table 1). Agreement of individual tests with initial possible or probable clinical Lyme disease diagnosis was otherwise poor, including skin culture (41% [19/46 cases]) and skin PCR (48% [11/23 cases]), except for plasma culture (59% [46/79 cases]). The combinations of tests that agreed best with initial possible or probable clinical Lyme disease diagnosis were serology, any culture, or skin PCR at 82% (67/82 patients), serology or skin PCR at 75% (60/80 patients), and plasma or skin culture at 66% (54/82 patients).
TABLE 1.
Agreement of laboratory diagnostic tests with initial clinical assessment for Lyme disease
| Laboratory diagnostic test (no. tested) | Initial clinical assessment for Lyme disease [no. for which test result agreed (%)]
|
|
|---|---|---|
| Probablea | Probable or possiblea | |
| Initial serology (80) | 32 (40) | 40 (50) |
| Any serology (80) | 21 (26) | 55 (69) |
| Skin biopsy culture (46) | 10 (22) | 19 (41) |
| Plasma culture (79) | 10 (13) | 46 (58) |
| Any culture (82) | 3 (4) | 54 (66) |
| Any serology or any culture (82) | 14 (17) | 63 (77) |
| Skin biopsy PCR (23) | 9 (39) | 11 (48) |
| Plasma PCR (55) | 27 (49) | 15 (27) |
| Any serology, any culture, or any PCR (82) | 18 (22) | 67 (82) |
Probable Lyme disease is EM or history of EM and a characteristic clinical syndrome with headache, VIIth cranial nerve palsy, or arthritis; possible Lyme disease is tick exposure, rash not typical for EM, or summertime fever without identifiable source.
Test combinations.
Figure 1 shows a comparison of results using various methodologies. Since no single method was able to identify all patients with objective laboratory evidence of Lyme disease, we sought to identify a profile of diagnostic tests that would provide the highest possible sensitivity. Thus, we defined a reference Lyme disease patient population based upon one or more of the accepted diagnostic tests (culture, serology per CDC/Association of State and Territorial Public Health Laboratory Directors criteria [2], and PCR of skin or plasma confirmed by targeting two B. burgdorferi genes). Not all tests were obtained on all patients; thus, denominators varied for purposes of calculating proportions and were based upon the number of patients who had the test and who were either seropositive, plasma or skin culture positive, or skin biopsy PCR positive.
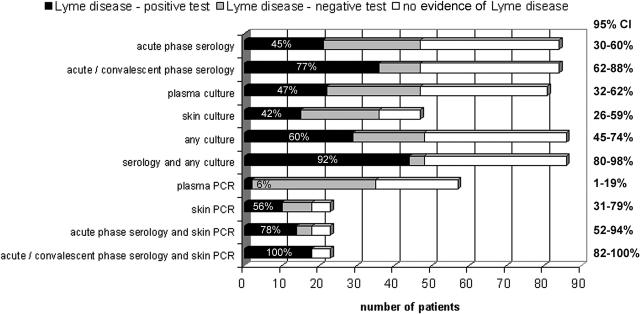
FIG. 1.
Number of patients tested by each method who had laboratory evidence of Lyme disease and who were positive (black bars) or negative (gray bars) by each test or test combination compared with the total number of enrolled subjects for whom each test was conducted. The percentages of patients who tested positive out of the total with laboratory evidence of Lyme disease who had that test or test combination are shown on the bars. The numbers of patients with no laboratory evidence of Lyme disease are shown with the white bars. The 95% CI for each sensitivity determination is indicated on the right side of the panel.
Of the applied methods, plasma PCR tests were performed on 57 patients, of whom 33 were positive by either culture or serology and 2 were positive only by skin biopsy PCR. Among these, the plasma PCR proved to be the least sensitive diagnostic procedure, identifying only 6% (2 of 35) of patients with laboratory evidence of Lyme disease, and was never the sole positive test. The most sensitive diagnostic test remained application of acute and convalescent serology (47 patients), the frequent reference for Lyme disease laboratory diagnosis, which was 77% (36/47; 95% confidence interval [CI], 62 to 88%) sensitive, followed by cumulative plasma and skin culture at 60% (29/48; 95% CI, 45 to 74%), skin biopsy PCR at 56% (10/18; 95% CI, 31 to 79%), plasma culture alone at 47% (22/47; 95% CI, 32 to 62%), and skin culture alone at 42% (15/36; 95% CI, 26 to 60%). With the exception of plasma PCR, any of the described methods when combined with one or more methods increased the positive percentage rate. The most sensitive approach proved to be the combination of acute and convalescent serology with skin biopsy PCR (100% of 18 patients; 95% CI, 82 to 100%), whereas acute and convalescent serology coupled with skin and plasma culture was nearly as sensitive, detecting 92% (44/48; 95% CI, 80 to 98%) of all patients. The sensitivity of acute-phase serology coupled with skin PCR, both which can be analyzed within days of presentation, yields a sensitivity rate of 78% (14/18; 95% CI, 52 to 94%), a marked but not significant improvement over the 45% sensitivity of acute-phase serology alone and unhelpful in the absence of rash.
When patients with any laboratory evidence of Lyme disease were divided into disease phase groups (33 early localized, 12 early disseminated, 3 late), no differences in the proportion of positive tests or combinations described above were found (χ2 test), although too few patients with late Lyme disease were available for critical analysis.
DISCUSSION
The diagnosis of Lyme disease is primarily based on clinical findings that include EM, often accompanied by muscle aches, fever, headache, and lymphadenopathy, after tick exposure in areas where B. burgdorferi is endemic (10, 13). When the typical or classical clinical presentation and exposure are evident, it is usually appropriate to treat patients, since additional laboratory testing does not improve the posttest probability of infection. However, when accepted signs and/or symptoms are unsatisfactory or inconclusive, laboratory testing may be needed for improving diagnostic certainty. Serologic testing is the mainstay for laboratory-based diagnosis, although modern technologies have provided other tools that variably contribute, including PCR and culture (5, 9, 10, 15). The utility of specific laboratory diagnostics, culture in particular, has also been emphasized given the need for gold standards for evaluation of new diagnostic tests and for use in laboratory authentication of Lyme disease in investigations of new treatments and vaccines (10-12). The utility of high-volume blood cultures for diagnosis of Lyme disease has been primarily advanced by a single investigational group (15). The studies reported here using similar methodologies strongly support this approach for in vitro recovery of B. burgdorferi as a definitive diagnostic method. However, when compared to a composite diagnostic definition comprising clinical and laboratory findings, no single test, including culture from plasma or skin, achieves a high rate of diagnostic sensitivity (10).
Culture is a valuable addition to the repertoire of tools for definitive identification of Lyme disease, especially when both plasma and skin cultures are simultaneously obtained, since overall 60% of all patients with objective laboratory evidence of B. burgdorferi infection and 52% of patients with single or multiple EM were culture positive. These results are remarkably similar to those of Nowakowski et al. and confirm that high-volume blood culture is a valid and appropriate approach (10). The major pitfall of culture is the long interval required (mean, 3 weeks), a factor that will diminish its application in many clinical laboratories. Surprisingly, the duration of symptoms among those with blood invasion was relatively brief, averaging 6 days (1 to 29 days); thus, B. burgdorferi strains in Maryland retain a capacity for rapid bloodstream invasion.
In accordance with the results of other studies, simple application of acute-phase serologic tests was very insensitive (45%) and, as with other studies, the use of paired acute- and convalescent-phase sera increased sensitivity among those patients with any laboratory evidence of B. burgdorferi infection to 77% (7, 10, 14). When coupled with culture results, levels of diagnostic sensitivity of >90% were obtained. However, the approach of paired acute and convalescent serological testing suffers from the same pitfall as culture in that a diagnostic serologic test may require several weeks.
Rapid detection of B. burgdorferi nucleic acids by PCR has been suggested as a valuable adjunct for diagnosis, but published reports only variably support this contention (5, 10). Recently, Liveris et al. reported a diagnostic sensitivity of 80% when using a real-time quantitative PCR for detection of B. burgdorferi DNA in skin biopsies (9). While the real-time quantitative PCR on skin biopsies did not achieve that high level of sensitivity in our hands, it was still a sensitive early marker in almost 60% of persons with EM who were tested by PCR. In contrast, amplification of B. burgdorferi DNA from plasma samples was highly insensitive (6%) and never detected B. burgdorferi DNA in the plasma of patients from whom the bacterium was eventually recovered by culture. Surprisingly, plasma PCR did detect B. burgdorferi DNA in two patients who were plasma culture negative, but it did not contribute to overall diagnostic utility, since one of the patients developed a seroconversion and the other was seropositive in both acute and convalescent samples.
Given the relatively high sensitivity of real-time quantitative PCR on skin biopsy, its combination with acute-phase serology resulted in the highest sensitivity for Lyme disease diagnosis during the early period after presentation with EM, improving from 44% (acute-phase serology alone) or 56% (skin PCR alone) to 78%. When coupled with convalescent serological testing, the sensitivity increased overall to 100%, although this figure is based on a more limited sampling of the population for whom skin PCR was conducted.
Although laboratory testing for diagnosis of Lyme disease is improving, the degree of sensitivity needed for a high level of assurance at the time of early Lyme disease is still not obtainable, even through combinations of various laboratory tests. Thus, clinical suspicion based upon well-recognized cardinal features of Lyme disease is still the most appropriate approach (16). However, where clinical uncertainty exists, a battery of additional diagnostic tests focusing initially on acute-phase serology and skin biopsy PCR, but also including culture of plasma and skin, will improve clinical confidence (10). These diagnostic tests will be most useful for the comprehensive evaluation of patients with confusing clinical presentations or when applied for definitive identification of Lyme disease for clinical studies of new therapeutics or vaccines. The overall value of culture cannot be overestimated, since the sensitive recovery of B. burgdorferi from high-volume blood cultures and skin biopsies provides an unequivocal confirmation of infection and the ability to evaluate differences in strains with relationship to virulence and pathogenicity.
Acknowledgments
We thank the Clinical Microbiology laboratory for support of the project and Gary P. Wormser, Susan Bittker, and the remaining laboratory members for training in the high-volume blood culture method.
REFERENCES
- 1.Auwaerter, P. G., J. Aucott, and J. S. Dumler. 2004. Lyme borreliosis (Lyme disease): molecular and cellular pathobiology and prospects for prevention, diagnosis and treatment. Expert Rev. Mol. Med. 2004:1-22. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1995. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. Morb. Mortal. Wkly. Rep. 44:590-591. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1997. Case definitions for infectious conditions under public health surveillance. Morb. Mortal. Wkly. Rep. 46(RR10):1-55. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2004. Lyme disease—United States, 2001-2002. Morb. Mortal. Wkly. Rep. 53:365-368. [PubMed] [Google Scholar]
- 5.Dumler, J. S. 2001. Molecular diagnosis of Lyme disease: review and meta-analysis. Mol. Diagn. 6:1-11. [DOI] [PubMed] [Google Scholar]
- 6.Hofmeister, E. K., R. B. Markham, J. E. Childs, and R. R. Arthur. 1992. Comparison of polymerase chain reaction and culture for detection of Borrelia burgdorferi in naturally infected Peromyscus leucopus and experimentally infected C.B-17 scid/scid mice. J. Clin. Microbiol. 30:2625-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson, B. J., K. E. Robbins, R. E. Bailey, B. L. Cao, S. L. Sviat, R. B. Craven, L. W. Mayer, and D. T. Dennis. 1996. Serodiagnosis of Lyme disease: accuracy of a two-step approach using a flagella-based ELISA and immunoblotting. J. Infect. Dis. 174:346-353. [DOI] [PubMed] [Google Scholar]
- 8.Leutenegger, C. M., N. Pusterla, C. N. Mislin, R. Weber, and H. Lutz. 1999. Molecular evidence of coinfection of ticks with Borrelia burgdorferi sensu lato and the human granulocytic ehrlichiosis agent in Switzerland. J. Clin. Microbiol. 37:3390-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liveris, D., G. Wang, G. Girao, D. W. Byrne, J. Nowakowski, D. McKenna, R. Nadelman, G. P. Wormser, and I. Schwartz. 2002. Quantitative detection of Borrelia burgdorferi in 2-millimeter skin samples of erythema migrans lesions: correlation of results with clinical and laboratory findings. J. Clin. Microbiol. 40:1249-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nowakowski, J., I. Schwartz, D. Liveris, G. Wang, M. E. Aguero-Rosenfeld, G. Girao, D. McKenna, R. B. Nadelman, L. F. Cavaliere, G. P. Wormser, and the Lyme Disease Study Group. 2001. Laboratory diagnostic techniques for patients with early Lyme disease associated with erythema migrans: a comparison of different techniques. Clin. Infect. Dis. 33:2023-2027. [DOI] [PubMed] [Google Scholar]
- 11.Smith, R. P., R. T. Schoen, D. W. Rahn, V. K. Sikand, J. Nowakowski, D. L. Parenti, M. S. Holman, D. H. Persing, and A. C. Steere. 2002. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann. Intern. Med. 136:421-428. [DOI] [PubMed] [Google Scholar]
- 12.Steere, A. C., V. K. Sikand, F. Meurice, D. L. Parenti, E. Fikrig, R. T. Schoen, J. Nowakowski, C. H. Schmid, S. Laukamp, C. Buscarino, D. S. Krause, et al. 1998. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N. Engl. J. Med. 339:209-215. [DOI] [PubMed] [Google Scholar]
- 13.Steere, A. C., J. Coburn, and L. Glickstein. 2004. The emergence of Lyme disease. J. Clin. Investig. 113:1093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trevejo, R. T., P. J. Krause, V. K. Sikand, M. E. Schriefer, R. Ryan, T. Lepore, W. Porter, and D. T. Dennis. 1999. Evaluation of two-test serodiagnostic method for early Lyme disease in clinical practice. J. Infect. Dis. 179:931-938. [DOI] [PubMed] [Google Scholar]
- 15.Wormser, G. P., S. Bittker, D. Cooper, J. Nowakowski, R. B. Nadelman, and C. Pavia. 2001. Yield of large-volume blood cultures in patients with early Lyme disease. J. Infect. Dis. 184:1070-1072. [DOI] [PubMed] [Google Scholar]
- 16.Wormser, G. P., R. B. Nadelman, R. J. Dattwyler, D. T. Dennis, E. D. Shapiro, A. C. Steere, T. J. Rush, D. W. Rahn, P. K. Coyle, D. H. Persing, D. Fish, B. J. Luft, et al. 2000. Practice guidelines for the treatment of Lyme disease. Clin. Infect. Dis. 31(Suppl. 1):1-14. [DOI] [PubMed] [Google Scholar]