Abstract
Strains of the multidrug-resistant (MDR) Salmonella enterica serovar Typhimurium isolated in Japan were examined for high-level fluoroquinolone resistance. Since the first isolation in 2000 (described in reference 13), we have identified 12 human and 5 nonhuman isolates with high-level fluoroquinolone-resistance (ciprofloxacin MIC of 24 μg/ml or more). Most of these isolates shared some features including definitive phage type (DT12/193), resistance type (ACSSuTNCp; resistant to ampicillin, chloramphenicol, streptomycin, sulfonamides, tetracycline, nalidixic acid, and ciprofloxacin), and genotype on pulsed-field gel electrophoresis that were different from those of the MDR S. enterica Typhimurium DT104. Mutations in quinolone resistance-determining regions of gyrA and parC were also conserved in almost all of the isolates despite the absence of any apparent epidemiological relationships among cases. This suggests that a specific clonal group of the serovar Typhimurium with high levels of fluoroquinolone resistance is disseminating among animals and humans in Japan.
Fluoroquinolones are commonly used in the treatment of gastrointestinal infections. Strains of the multidrug-resistant (MDR) Salmonella enterica serovar Typhimurium such as DT104 (definitive phage type [DT] 104) have dispersed to various countries (6, 15, 16). Furthermore, reports of quinolone-resistant strains of serovar Typhimurium, with decreased susceptibility to fluoroquinolones, are increasing, and such organisms may cause difficulty in the treatment of infections (12, 17). In 2000, we identified a high-level fluoroquinolone-resistant serovar Typhimurium isolate in a patient (13). High-level resistance to fluoroquinolones has been reported in other countries as well (3, 7, 11). Here, we further surveyed strains of the MDR S. enterica serovar Typhimurium isolates collected in Japan that were different from those reported previously (9). We identified several additional high-level fluoroquinolone-resistant isolates and compared them with each other and also with the first isolate in terms of resistance type, phage type, genotype, and other analyses.
MATERIALS AND METHODS
Strains.
A total of 110 MDR (resistant to more than four drugs) isolates of S. enterica serovar Typhimurium, listed in Table 1, were used. Thirty-nine were from human sources isolated between 1988 and 2003, and 71 were from nonhuman sources including cattle, poultry, pig, rat, dog, cat, and the environment isolated between 1989 and 2003. Two isolates were from companion animals, a cat and a dog, of two patients infected with high-level fluoroquinolone-resistant strains. The first isolate of high-level fluoroquinolone-resistant S. enterica serovar Typhimurium (called “the first isolate” herein) (Table 2, isolate 0) and one isolate showing decreased susceptibility to fluoroquinolones in a previous study (Table 2, isolate 100) were also used in this study (9, 13).
TABLE 1.
Distribution of R-type and phage types of S. enterica serovar Typhimurium isolates in this study
| R-type | No. of isolates by phage type (DT)
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 | 29 | 36 | 104 | 104B | 104L | 120 | 126 | 193 | 194 | 203 | U302 | RDNCa | UTb | Total | |
| Human isolates | |||||||||||||||
| ACSSuT | 10 | 2 | 5 | 1 | 18 | ||||||||||
| ACSSuTN | 2 | 2 | 4 | ||||||||||||
| ACSSuTNCp | 4 | 4 | |||||||||||||
| ACSSuTNCp+SxTpG | 8 | 8 | |||||||||||||
| ACSSuTK | 1 | 2 | 3 | ||||||||||||
| ACSSuTK+SxTpG | 1 | 1 | |||||||||||||
| ACSSuTKN+SxTp | 1 | 1 | |||||||||||||
| Total | 4 | 11 | 4 | 1 | 11 | 7 | 1 | 39 | |||||||
| Nonhuman isolates | |||||||||||||||
| ACSSuT | 36 | 10 | 2 | 1 | 1 | 2 | 1 | 53 | |||||||
| ACSSuTNCp | 2 | 2 | |||||||||||||
| ACSSuTNCp+SxTpG | 2 | 1 | 3 | ||||||||||||
| ACSSuT+SxTp | 1 | 1 | |||||||||||||
| ACSSuTK | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||||||||
| ACSSuTKN | 2 | 2 | |||||||||||||
| ACSSuTK+SxTpG | 1 | 1 | 2 | ||||||||||||
| ASSuTK | 1 | 1 | |||||||||||||
| ASSuT + SxTp | 1 | 1 | |||||||||||||
| Total | 4 | 1 | 1 | 36 | 11 | 2 | 1 | 1 | 2 | 1 | 1 | 3 | 3 | 4 | 71 |
Reacted but did not conform.
Untypeable.
TABLE 2.
Characteristics of high-level fluoroquinolone-resistant isolates of S. enterica serovar Typhimurium
| Strains | Year isolated | Sources (nonhuman) | MIC (μg/ml)a
|
Mutations in QRDRs
|
Genotype
|
R-type | PCR for resistance genes or contigs
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NA | CI | NX | GyrA | GyrB | ParC | ParE | BlnI | XbaI | DT | blaoxa30aadA1 | dhfr12-aadA2 | aac3 | catA | tetRA | ||||
| Human isolate | ||||||||||||||||||
| 1 | 2002 | >256 | 24 | 32 | S83F + D87N | S80R | Aa | Aa | 193 | ACSSuTNCp + SxTpG | + | + | + | + | + | |||
| 2 | 2001 | >256 | 24 | 32 | S83F + D87N | S80R | Ab | Aa | 12 | ACSSuTNCp | + | − | − | + | + | |||
| 3 | 2001 | >256 | 24 | 32 | S83F + D87N | S80R | Ab | Aa | 12 | ACSSuTNCp | + | − | − | + | + | |||
| 4 | 2001 | >256 | 32 | 32 | S83F + D87N | S80R | Ab | Aa | 12 | ACSSuTNCp | + | − | − | + | + | |||
| 5 | 2002 | >256 | 32 | 48 | S83F + D87N | S80R | Ac | Ab | 12 | ACSSuTNCp | + | − | − | + | + | |||
| 6 | 2002 | >256 | 24 | 32 | S83F + D87N | S80R | Aa | Aa | 193 | ACSSuTNCp + SxTpG | + | + | + | + | + | |||
| 7 | 2003 | >256 | 32 | 32 | S83F + D87N | S80R | Aa | Aa | 193 | ACSSuTNCp + SxTpG | + | + | + | + | + | |||
| 8 | 2003 | >256 | >32 | >256 | S83F + D87G | S80R | S458P | B | Ac | 193 | ACSSuTNCp + SxTpG | + | + | + | + | + | ||
| 9 | 2001 | >256 | 24 | 32 | S83F + D87N | S80R | Aa | Aa | 193 | ACSSuTNCp + SxTpG | + | + | + | + | + | |||
| 10 | 2002 | >256 | 32 | 48 | S83F + D87N | S80R | Aa | Aa | 193 | ACSSuTNCp + SxTpG | + | + | + | + | + | |||
| 11 | 2002 | >256 | 32 | 48 | S83F + D87N | S80R | Aa | Aa | 193 | ACSSuTNCp + SxTpG | + | + | + | + | + | |||
| 12 | 2003 | >256 | 24 | 32 | S83F + D87N | S80R | Aa | Aa | 193 | ACSSuTNCp + SxTpG | + | + | + | + | + | |||
| 0b | 2000 | >256 | 24 | 32 | S83F + D87N | S80R | Aa | Aa | 12 | ACSSuTNCp + SxTpG | + | + | + | + | + | |||
| Nonhuman isolate | ||||||||||||||||||
| 101 | 2001 | Cattle | >256 | 24 | 32 | S83F + D87N | S80R | Aa | Aa | 12 | ACSSuTNCp + SxTpG | + | + | + | + | + | ||
| 102c | 2001 | Dog | >256 | 24 | 24 | S83F + D87N | S80R | Ab | Aa | 12 | ACSSuTNCp | + | − | − | + | + | ||
| 103c | 2001 | Cat | >256 | 24 | 32 | S83F + D87N | S80R | Ab | Aa | 12 | ACSSuTNCp | + | − | − | + | + | ||
| 104 | 2001 | Dog | >256 | 24 | 32 | S83F + D87N | S80R | Aa | Aa | 12 | ACSSuTNCp + SxTpG | + | + | + | + | + | ||
| 105 | 2003 | NAd | >256 | 24 | 24 | S83F + D87N | S80R | Aa | Aa | 193 | ACSSuTNCp + SxTpG | + | + | + | + | + | ||
| 100 | 1997 | Cattle | >256 | 0.75 | 3 | S83F | S80R | Ab | B | 193 | ACSSuTN | + | − | − | + | + | ||
Determined by E-test. NA, nalidixic acid; CI, ciprofloxacin; NX, norfloxacin.
The first isolate in reference 13.
Associated with cases human isolates no. 2 and 4, respectively.
NA, not available
Antimicrobial susceptibility testing.
Antimicrobial susceptibility testing was performed using a disk diffusion method as previously described according to the standards outlined by the Clinical and Laboratory Standards Institute (formerly, the National Committee for Clinical Laboratory Standards) (9, 14). Escherichia coli ATCC 25922 was used as a quality control strain. Disks with the following drugs (with abbreviations used in naming the typical resistance type [R-type]) were used: ampicillin (A), 10 μg; chloramphenicol (C), 30 μg; streptomycin (S), 10 μg; tetracycline (T), 30 μg; ciprofloxacin (Cp), 5 μg; kanamycin (K), 30 μg; cefotaxime (CE), 30 μg; trimethoprim-sulfamethoxazole (Sx), 1.25 μg and 23.75 μg, respectively; trimethoprim (Tp), 5 μg; gentamicin (G),10 μg; nalidixic acid (N), 30 μg; and sulfisoxazole (Su), 250 μg (Becton Dickinson Microbiology Systems, Cockeysville, MD). MICs were determined with an E-test (AB Biodisk, Solona, Sweden), which was performed according to the manufacturer's recommendations.
Bacteriophage typing.
Bacteriophage typing was performed in accordance with the method of the Health Protection Agency, London, United Kingdom (1).
PFGE.
Pulsed-field gel electrophoresis (PFGE) was performed by using S. enterica serovar Braenderup H9812 as a standard strain (8). Resulting profiles were interpreted based on visual analysis. A photograph of representative PFGE profiles was scanned and saved in TIFF format to be analyzed using Fingerprinting II software (Bio-Rad Laboratories Inc., Hercules, CA). Similarity was determined using the Dice coefficient, and clustering was based on the unweighted pair group method with arithmetic averages with a band position tolerance of 1.2%. Profiles with minor variations (three or fewer band differences) were designated as different subtypes (indicated by different lowercase letters) within a type (indicated by the same uppercase letters), which corresponded to similarities of approximately 90% or more in this study. Otherwise, profiles were designated as different genotypes (indicated by different uppercase letters) when similarities were less than 90%.
Analyses of QRDR.
DNA sequences of the quinolone resistance-determining regions (QRDRs) of the gyrA, gyrB, parC, and parE genes were determined as described previously (5, 13).
Cloning of genes involved in resistance to antimicrobials other than quinolone.
PCR for the detection of genetic elements such as the class 1 integrons was conducted as described previously (9). The amplified 2-kb fragments were cloned into a HincII-digested pSTV28 vector (Takara Bio Inc., Shiga, Japan), and transformants resistant to ampicillin or trimethoprim were obtained. Purified plasmids were sequenced by using M13-47 and M13-RV (Takara Bio Inc.) as primers. Searches for homologues of the resulting sequences in the EMBL database were conducted with the WU-BLAST program.
Genomic DNA of the first isolate prepared using a Wizard Genome Purification kit (Promega Corp., Madison, WI) was completely digested with EcoRI or HindIII and ligated with pSTV28 and pBlueScript SK(+) (Stratagene Corp., La Jolla, CA) vectors. Clones with gentamicin or chloramphenicol resistance were selected on LB plates containing the corresponding antimicrobials. An EcoRI fragment conferring gentamicin resistance was cloned into the vector pSTV28. A HindIII fragment conferring chloramphenicol resistance was cloned into pBlueScript. Purified plasmids were sequenced, and searches for homologues of the resulting sequences were conducted as described above.
Detection of resistance genes other than quinolone resistance genes.
Primer pairs for contigs of blaOXA-30-aadA1 (ampicillin and streptomycin resistance) and dhfr12-aadA2 (trimethoprim and streptomycin resistance), for the aac3 gene (gentamicin resistance) and the catA gene (chloramphenicol resistance), and for the tetRA contig (tetracycline resistance) were designed based on the corresponding sequences of GenBank accession numbers AF550415 and AF326777 (Table 3). PCR was performed essentially as described previously (9). Briefly, the reaction was run at 94 C for 20 s, 55 C for 20 s, and 72 C for 2 min for 35 cycles after denaturing at 94 C for 3 min using a boiled bacterial suspension as a template.
TABLE 3.
Primers used to detect the resistance genes and contigs
| Name | Sequence | Source (accession no.) |
|---|---|---|
| oxa30-1321F | 5′-CGCAAGAAATAACCCAAAAAATTG-3′ | AY224185 |
| aadA1-3′ | 5′-TTATTTGCCGACTACCTTGG-3′ | |
| dhfr12-352F | 5′-GCTAACTACCGCGCCAC-3′ | AF550415 |
| aadA2-3′ | 5′-TCATTTACCAACTGACTTGATG-3′ | |
| aac3-3′ | 5′-CTTAGCGAGCGTCGTCTTTC-3′ | |
| aac3-5′ | 5′-TTCGTTCCACTGAGCGTCAG-3′ | |
| catA-3′ | 5′-TTACGCCCCGCCCTGCC-3′ | AF326777 |
| catA-5′ | 5′-ATGGAGAAAAAAATCACTGGATATACC-3′ | |
| tetR-3′ | 5′-TTAAGACCCACTTTCACATTTAAG-3′ | |
| tetA-C | 5′-CTAAGCACTTGTCTCCTG-3′ |
RESULTS
A total of 110 isolates of MDR S. enterica serovar Typhimurium were tested for antimicrobial susceptibility and phage type. As shown in Table 1, definitive phage type DT104 and related strains with the typical resistance type R-ACSSuT were dominant both in human and in nonhuman isolates.
Human isolates.
Among 39 MDR strains, 12 were resistant to ciprofloxacin. All 12 were isolated after 2001. Four isolates were R-ACSSuTNCp and DT12, and eight were R-ACSSuTNCp+SxTpG and DT193 (Table 1). For all the isolates, fluoroquinolone MICs were 24 μg/ml or more, indicating a high level of resistance (Table 2). Sequence analysis of the QRDRs of the gyrA, gyrB, parC, and parE genes revealed that all but one isolate harbored the same combination of three point mutations, two in GyrA (S83F, TCC to TTC; and D87N, GAC to AAC) and one in ParC (S80R, AGC to CGC), which are the same as those found in the first isolate (Table 2, isolate 0) (13). For one isolate (no. 8) the norfloxacin MIC was much higher (>256 μg/ml) than for the other isolates. A total of four point mutations were found in this isolate: two in GyrA (S83F, TCC to TTC; and D87G, GAC to GGC), one in ParC (S80R, AGC to CGC), and one in ParE (S458P, TCG to CCG).
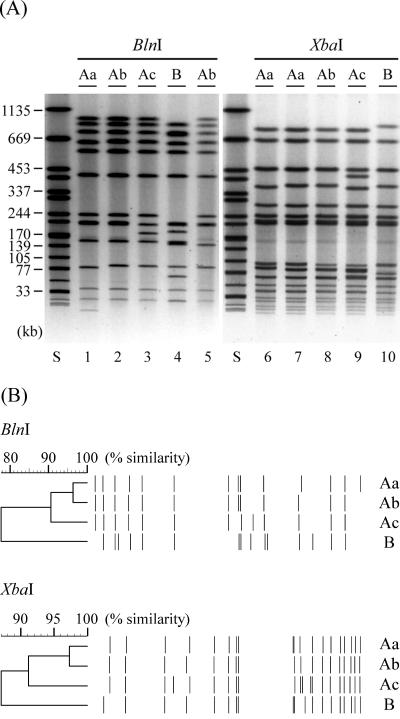
The 13 high-level fluoroquinolone-resistant isolates of S. enterica serovar Typhimurium, including the first isolate (isolate 0), were subjected to PFGE. They were classified into two types (A and B) by BlnI digestion and one type (A) by XbaI digestion (Table 2 and Fig. 1). Type A identified by BlnI or XbaI contained three subtypes. Almost all of the isolates were typed as A. Notably, isolates typed as Aa based on both endonuclease-digested profiles were predominant (n = 8). Similarity calculated by computer software was more than 75% even between the profiles classified as different types by BlnI-digestion (Fig. 1B).
FIG. 1.
(A) Representative PFGE profiles of high-level fluoroquinolone-resistant strains of S. enterica serovar Typhimurium. Total DNA of isolates no. 0 (lanes 1 and 6), no. 2 (lanes 2 and 7), no. 5 (lanes 3 and 8), no. 8 (lanes 4 and 9), and no. 100 (lanes 5 and 10) was digested with BlnI (lanes 1 to 5) or XbaI (lanes 6 to 10). Lane S, S. enterica serovar Braenderup H9812 digested with XbaI. Sizes of the bands of H9812 are indicated on the left. Genotypes from Table 2 are indicated at the top of the panel. (B) Dendrograms calculated using the representative PFGE profiles. The top part of the panel is based on the BlnI-digested profiles. The lower part is based on the XbaI-digested profiles. Genotypes are indicated on the right.
Nonhuman isolates.
Among 71 MDR strains, 5 were resistant to ciprofloxacin (Table 1). All five were isolated after 2001, similar to the human isolates. Two (Table 2, isolates 102 and 103) were R-ACSSuTNCp and DT12 and isolated from a dog and a cat which were companions of two of the human subjects (isolates 2 and 4, respectively) infected with the isolates with the same properties, though detailed epidemiological data were unavailable and the cause and effect relationship remains unclear. Two (isolates 101 and 104) were R-ACSSuTNCp+SxTpG and DT12 and isolated from cattle and a dog, and one (isolate 105) was R-ACSSuTNCp+SxTpG and DT193 (Table 1). All of them showed similar features to human isolates in terms of MICs, mutations in QRDRs, and PFGE profiles (Table 2).
Since PFGE profiles of the high-level fluoroquinolone-resistant strains of both human and nonhuman sources were quite similar to each other and different from those of MDR DT104, we visually screened BlnI-digested PFGE profiles of serovar Typhimurium isolates from this and previous studies (9). Among 497 isolates that did not include those with high-level fluoroquinolone-resistance, 1 was found to be quite similar to the major type Ab of the high-level fluoroquinolone-resistant isolates in its BlnI-digested profile, although its XbaI-digested profile was somewhat different and typed as B (Fig. 1). This strain (no. 100) was isolated from cattle in 1997. It was identified as R-ACSSuTN and DT193 and showed decreased susceptibility to fluoroquinolones and two point mutations, one in GyrA (S83F, TCC to TTC) and, surprisingly, one in ParC (S80R, AGC to CGC) (Table 2).
Genes involved in resistance to antimicrobials other than quinolone.
All the high-level fluoroquinolone-resistant isolates were MDR. We further characterized their resistance genes by using the first isolate as representative and investigated whether the resulting characteristics were conserved among them.
Since all the high-level fluoroquinolone-resistant isolates were resistant to sulfisoxazole, PCR for the detection of class 1 integrons was conducted. Approximately 2-kb fragments were amplified (data not shown). Sequence analysis revealed that the fragments contained two different PCR products; the sequence of one was 99% identical to an integron of E. coli Ec1484R (GenBank accession no. AY224185) containing blaOXA-30 and aadA1, and the sequence of the other was 99% identical to an integron of the Citrobacter freundii plasmid pCTX-M3 (GenBank accession no. AF550415) containing dhfr12 and aadA2.
DNA fragments conferring resistance to other antimicrobial agents, gentamicin and chloramphenicol, were also cloned. Partial sequence analyses suggested that they contained sequences nearly identical with certain regions of C. freundii pCTX-M3, flanked by the dhfr12 and aac3 genes, and with a catA-flanking region of Shigella flexneri 2a Tn21 (GenBank accession no. AF326777, which also contains the tetRACD contig).
Thus, we designed primer pairs for the suspected contigs and genes. PCR with the primer sets was applied to all the high-level fluoroquinolone-resistant isolates from both human and nonhuman sources. Results are summarized in Table 2. Each gene or contig was detected in isolates with the corresponding resistance, that is, blaOXA-30-aadA1, catA, and tetRA were detected in all of the isolates, while dhfr12-aadA2 and aac3 were detected in the R-TpG isolates. The blaOXA-30-aadA1, catA, and tetRA genes were also detected in isolate 100.
DISCUSSION
A high-level fluoroquinolone-resistant isolate of the MDR S. enterica serovar Typhimurium was first identified in Japan in 2000 (13). In this study, we further identified 12 human and 5 nonhuman isolates. Although two of the nonhuman isolates might be associated with two human cases, no epidemiological relationships have been found among the others so far. Nevertheless, it is intriguing that all but one had the same point mutations in QRDRs as the first isolate. Strains with these multiple mutations in the QRDRs were recently reported in other countries, and some had the same combination of mutations as found in this study (3, 4, 7, 11). This suggests the dissemination of a clonal group of high-level fluoroquinolone-resistant strains of S. enterica serovar Typhimurium sharing common features in various countries, as suggested in a study of the nalidixic acid-resistant S. enterica serovar Enteritidis (10), or else a tendency to acquire such mutations in QRDRs in order to confer high-level resistance to fluoroquinolones.
Our study also indicates that almost all of the high-level fluoroquinolone-resistant human isolates had indistinguishable or quite similar PFGE profiles, though their profiles differed from those observed in MDR strains of S. enterica serovar Typhimurium DT104 (9). Our study also suggests that they would share not only common resistance types but also common resistance genes in addition to mutations in QRDRs, which would be different from resistance genes found in SGI1 in MDR DT104 (2). This is also true for the nonhuman isolates. These results suggest that a specific clonal group of high-level fluoroquinolone-resistant serovar Typhimurium has disseminated among animals and humans in Japan.
Interestingly, a nonhuman isolate (isolate 100), which was obtained from cattle in 1997 before the first high-level fluoroquinolone-resistant human isolate appeared in 2000, shared some properties with the human and nonhuman high-level fluoroquinolone-resistant strains but lacked the second mutation in gyrA. This combination of mutations in QRDRs (namely, S83F in GyrA and S80R in ParC) seems rare among isolates with decreased susceptibility to fluoroquinolones, at least in Japan, because a point mutation in GyrA (S83Y, TCC to TAC [n = 1]; D87G, GAC to GGC [n = 3]; D87Y, GAC to TAC [n = 1]; or D87N, GAC to AAC [n = 1]) but not in ParC was found in each of six nalidixic-acid resistant isolates other than the high-level fluoroquinolone-resistant ones listed in Table 1. On the other hand, this combination was conserved in all the high-level fluoroquinolone-resistant isolates. This suggests that isolate 100 may be related to ancestors of the high-level fluoroquinolone-resistant strains.
Salmonella infection is one of the most important zoonoses. It is intriguing that the human and nonhuman isolates analyzed in this study shared features in common. The following seemed to comprise a set of their core characteristics: R-ACSSuT conferred by a set of resistance determinants different from that of MDR DT104, and mutations in QRDRs of GyrA (S83F) and ParC (S80R). It is likely that the acquisition of one point mutation in GyrA (D87N) by a certain strain harboring these core characteristics such as isolate 100 would have created the strains with high-level fluoroquinolone-resistance under selective conditions and that the resulting organisms would have disseminated in animals and humans while retaining their unique PFGE profiles and sometimes acquiring or losing additional resistance (R-SxTpG). The reactions of typing phages in DT12 and DT193 in this study differed from each other only in phages 12 and 13. This suggests that the two types are related since a similar difference could be observed in the relationship between DT104 and DT104B (1), which would be supportive of the above hypothesis. More extensive retrograde surveillance might reveal presumptive intermediate quinolone- resistant strains prior to the acquisition of high-level fluoroquinolone resistance. On the other hand, no apparent epidemiological relationships have been found in each case so far. Further characterization of high-level fluoroquinolone-resistant strains of S. enterica serovar Typhimurium will help epidemiological investigations.
Acknowledgments
We thank all the municipal and prefectural public health institutes, the National Institute of Animal Health, and the National Veterinary Assay Laboratory in Japan for providing us with isolates of S. enterica serovar Typhimurium. We thank the Health Protection Agency, United Kingdom, for providing the typing phages. We also thank PulseNet, the Centers for Disease Control and Prevention, for providing the S. enterica serovar Braenderup H9812.
This work was supported by a grant from the Ministry of Health, Labor and Welfare of Japan.
REFERENCES
- 1.Anderson, E. S., L. R. Ward, M. J. Saxe, and J. D. de Sa. 1977. Bacteriophage-typing designations of Salmonella typhimurium. J. Hyg. 78:297-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd, D., G. A. Peters, A. Cloeckaert, K. S. Boumedine, E. Chaslus-Dancla, H. Imberechts, and M. R. Mulvey. 2001. Complete nucleotide sequence of a 43-kilobase genomic island associated with the multidrug resistance region of Salmonella enterica serovar Typhimurium DT104 and its identification in phage type DT120 and serovar Agona. J. Bacteriol. 183:5725-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casin, I., J. Breuil, J. P. Darchis, C. Guelpa, and E. Collatz. 2003. Fluoroquinolone resistance linked to GyrA, GyrB, and ParC mutations in Salmonella enterica Typhimurium isolates in humans. Emerg. Infect. Dis. 9:1455-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eaves, D. J., L. Randall, D. T. Gray, A. Buckley, M. J. Woodward, A. P. White, and L. J. Piddock. 2004. Prevalence of mutations within the quinolone resistance-determining region of gyrA, gyrB, parC, and parE and association with antibiotic resistance in quinolone-resistant Salmonella enterica. Antimicrob. Agents Chemother. 48:4012-4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giraud, E., A. Brisabois, J. L. Martel, and E. Chaslus-Dancla. 1999. Comparative studies of mutations in animal isolates and experimental in vitro- and in vivo-selected mutants of Salmonella spp. suggest a counterselection of highly fluoroquinolone-resistant strains in the field. Antimicrob. Agents Chemother. 43:2131-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glynn, M. K., C. Bopp, W. Dewitt, P. Dabney, M. Mokhtar, and F. J. Angulo. 1998. Emergence of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 infections in the United States. N. Engl. J. Med. 338:1333-1338. [DOI] [PubMed] [Google Scholar]
- 7.Hsueh, P. R., L. J. Teng, S. P. Tseng, C. F. Chang, J. H. Wan, J. J. Yan, C. M. Lee, Y. C. Chuang, W. K. Huang, D. Yang, J. M. Shyr, K. W. Yu, L. S. Wang, J. J. Lu, W. C. Ko, J. J. Wu, F. Y. Chang, Y. C. Yang, Y. J. Lau, Y. C. Liu, C. Y. Liu, S. W. Ho, and K. T. Luh. 2004. Ciprofloxacin-resistant Salmonella enterica Typhimurium and Choleraesuis from pigs to humans, Taiwan. Emerg. Infect. Dis. 10:60-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter, S. B., P. Vauterin, M. A. Lambert-Fair, M. S. Van Duyne, K. Kubota, L. Graves, D. Wrigley, T. Barrett, and E. Ribot. 2005. Establishment of a universal size standard strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: converting the national databases to the new size standard. J. Clin. Microbiol. 43:1045-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izumiya, H., J. Terajima, S. Matsushita, K. Tamura, and H. Watanabe. 2001. Characterization of multidrug-resistant Salmonella enterica serovar Typhimurium isolated in Japan. J. Clin. Microbiol. 39:2700-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilmartin, D., D. Morris, C. O'Hare, G. Corbett-Feeney, and M. Cormican. 2005. Clonal expansion may account for high levels of quinolone resistance in Salmonella enterica serovar Enteritidis. Appl. Environ. Microbiol. 71:2587-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ling, J. M., E. W. Chan, A. W. Lam, and A. F. Cheng. 2003. Mutations in topoisomerase genes of fluoroquinolone-resistant salmonellae in Hong Kong. Antimicrob. Agents Chemother. 47:3567-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molbak, K., D. L. Baggesen, F. M. Aarestrup, J. M. Ebbesen, J. Engberg, K. Frydendahl, P. Gerner-Smidt, A. M. Petersen, and H. C. Wegener. 1999. An outbreak of multidrug-resistant, quinolone-resistant Salmonella enterica serotype Typhimurium DT104. N. Engl. J. Med. 341:1420-1425. [DOI] [PubMed] [Google Scholar]
- 13.Nakaya, H., A. Yasuhara, K. Yoshimura, Y. Oshihoi, H. Izumiya, and H. Watanabe. 2003. Life-threatening infantile diarrhea from fluoroquinolone-resistant Salmonella enterica typhimurium with mutations in both gyrA and parC. Emerg. Infect. Dis. 9:255-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A7, 7th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 15.Rabsch, W., H. Tschape, and A. J. Baumler. 2001. Non-typhoidal salmonellosis: emerging problems. Microbes Infect. 3:237-247. [DOI] [PubMed] [Google Scholar]
- 16.Threlfall, E. J., J. A. Frost, L. R. Ward, and B. Rowe. 1994. Epidemic in cattle and humans of Salmonella typhimurium DT 104 with chromosomally integrated multiple drug resistance. Vet. Rec. 134:577. [DOI] [PubMed] [Google Scholar]
- 17.Walker, R. A., A. J. Lawson, E. A. Lindsay, L. R. Ward, P. A. Wright, F. J. Bolton, D. R. Wareing, J. D. Corkish, R. H. Davies, and E. J. Threlfall. 2000. Decreased susceptibility to ciprofloxacin in outbreak-associated multiresistant Salmonella Typhimurium DT104. Vet. Rec. 147:395-396. [DOI] [PubMed] [Google Scholar]