Abstract
When enteric group 58 was first described as a distinct new group of Enterobacteriaceae in 1985, there were only five known human isolates: four from wounds and one from feces. In 1996, we investigated the first blood isolate of enteric group 58, a case of sepsis in a 33-year-old woman receiving total parenteral nutrition. Fifteen additional clinical isolates have since been identified at CDC, including several recognized from a collection of “unidentified” strains dating back to 1973. All strains were characterized with a standard set of 49 biochemical tests used for Enterobacteriaceae, and the results were analyzed to determine phenotypic relatedness and best taxonomic fit. Antibiograms were determined as a taxonomic tool. Original identifications provided by submitting laboratories encompassed a wide variety of Enterobacteriaceae, including 14 species in eight genera, the most common being Enterobacter spp., Salmonella spp., Serratia spp., Kluyvera spp., or Escherichia spp. Enteric group 58 strains have been most frequently isolated from traumatic injuries, fractures, and wounds and rarely from feces. Defining its clinical significance and distinguishing infection from colonization requires further study, but our case report indicates that serious systemic infection can occur. The vernacular name enteric group 58 was used from 1985 to 2004. In this paper, we formally name it Averyella dalhousiensis gen. nov., sp. nov., on the basis of its unique phenotype and its unique 16S rRNA gene sequence. These data indicate that enteric group 58 is not closely related to any of the existing genera or species of Enterobacteriaceae. The type strain is designated CDC9501-97, and a phenotypic definition is given based on all 21 strains.
Enteric group 58 is an infrequently encountered member of the family Enterobacteriaceae. The first strain was recognized in 1981 on the basis of a biochemical reaction pattern unlike that of any other organism in the family. By 1985, five isolates from clinical specimens had been identified by the Enteric Bacteriology Laboratories at the Centers for Disease Control and Prevention (CDC) (9). Four isolates had been recovered from lower extremity wounds, and the fifth was from stool (9). Little clinical information was submitted with these strains, but there was no indication that they had caused clinically significant infections. Farmer et al. described the biochemical characteristics of these five strains and designated them enteric group 58, which is the only proposed classification (9). Sixteen additional clinical isolates have since been identified at CDC, including a number that were recognized from a collection of unidentified organisms dating back to 1973. DNA-DNA hybridization was not done, because of its unavailability. There has been one case report describing the recovery of enteric group 58 from an orthopedic compression plate, from the Queen Elizabeth II Health Sciences Centre (QEII HSC), in an otherwise asymptomatic patient who did not require treatment with antibiotics (6). An attempt to recover this isolate from storage at the QEII HSC was unsuccessful.
Very little is known about the epidemiology, pathogenesis, or clinical significance of this group of organisms, but existing information favors an environmental or exogenous source. We report the first blood culture isolate of enteric group 58 associated with infection of a central venous catheter and describe the phenotypic characteristics of 21 enteric group 58 strains isolated from clinical specimens.
After the original study was completed, we were able to study three strains of enteric group 58 by 16S rRNA gene sequencing. These results confirmed by a second method that enteric group 58 is distinct from all of the characterized genera and species in the family Enterobacteriaceae. These later results compelled us to give enteric group 58 a scientific name, a critical step in learning more about a newly recognized bacterium.
CASE REPORT
A 33-year-old female presented to the QEII HSC with a 3-day history of rigors and fever temporally related to the intravenous (i.v.) administration of total parenteral nutrition (TPN) through a subcutaneous port, which she administered herself daily at her home. She required i.v. hyperalimentation after surgical small-bowel resection for recurrent superior mesenteric artery thrombosis, a complication of a documented hypercoagulable state. On initial examination, she appeared unwell and her temperature was 39.3°C. The port site was not erythematous or tender, and the remainder of the physical exam was unremarkable. The leukocyte count was 8.0 × 103/mm3, with 38% band forms. An initial clinical chemistry profile and a chest radiograph were normal. Medications included iron, calcium, and warfarin for chronic anticoagulation.
Treatment was initiated with i.v. ampicillin and gentamicin. Two sets of blood cultures drawn from peripheral veins yielded a gram-negative rod after one day of incubation. The bacterium was identified as Enterobacter amnigenus biotype II (Table 1, case 1 [also designated strain 1 for this paper]) by use of the hospital's automated identification system. Susceptibility results indicated resistance to ampicillin and cefazolin and susceptibility to cefotaxime, ciprofloxacin, gentamicin, and trimethoprim-sulfamethoxazole. Clinical response to i.v. antibiotics was rapid (36 to 48 h), and the subcutaneous port was not removed. On day 3, the patient was discharged from the hospital to complete a 14-day course of oral ciprofloxacin.
TABLE 1.
Summary of 21 enteric group 58 cases
| Case | Sexb | Age | Locationa | Date isolated (mo/day/yr) | Source | Sender's identificationd | Comment(s) |
|---|---|---|---|---|---|---|---|
| 1c | F | 33 | NS | 10/12/96 | Blood | Enterobacter amnigenus biotype II | Infected “port-a-cath” |
| 2 | M | NY | 12/9/73 | Foot | Ankle isolate | ||
| 3 | F | MI | 7/21/77 | Foot | Atypical Enterobacteriaceae | Cellulitis, puncture | |
| 4 | F | 7 | TN | 8/9/79 | Head wound | Gram-negative rod (API20E: 5 104 513) | Car accident, thrown into gutter; Nocardia sp. also isolated |
| 5 | F | ONT | Human | Enterobacteriaceae | |||
| 6 | F | 64 | NJ | 9/29/80 | Atypical Enterobacter sp. | ||
| 7 | M | 32 | MI | 6/20/81 | Hip wound | Gram-negative rod | Thigh |
| 8 | M | 56 | IN | 6/23/81 | Great toe | Unidentified | Open fracture |
| 9 | M | 27 | MI | Serratia fonticola | |||
| 10 | M | 78 | MI | ||||
| 11 | M | 66 | MN | Finger | Kluyvera sp. | Saw laceration, Staphylococcus sp. also isolated | |
| 12 | M | 50 | VA | 3/14/85 | Elbow | Gram-negative bacillus | Car accident, infection of olecranon bursa |
| 13 | M | 20 | CZEC | 1983 | Stool | Serratia sp. | |
| 14 | M | NJ | 11/01/86 | Hand wound | |||
| 15 | M | 7 | OH | 5/30/87 | Foot | Salmonella sp. or Hafnia alvei (API20E: 5 304 513) | |
| 16 | F | 63 | NJ | 5/26/87 | Intestinal fluid | Escherichia coli (API20E: 5 104 513) | |
| 17 | M | 57 | OR | 9/21/87 | Stump infection | Salmonella sp. | Amputee |
| 18 | M | 35 | TN | 7/13/88 | Foot wound | Salmonella sp. O group 53 | Negative in CDC Salmonella O and H sera |
| 19 | M | 20 | IN | 6/29/88 | Femur wound | Microscan: 5 300 407 (API20E: 5 304 513) | Multiple trauma |
| 20 | M | CA | 6/11/88 | Stump | Enteric group 58 | Trauma patient | |
| 21 | M | 26 | OH | 3/26/90 | Hand | Gram-negative rod (API20E: 5 314 513) | Traumatic wrist wound |
NS, Nova Scotia; NY, New York; MI, Mississippi; TN, Tennessee; ONT, Ontario; NJ, New Jersey; IN, Indiana; MN, Minnesota; VA, Virginia; CZEC, Czechoslovakia; OH, Ohio; OR, Oregon; CA, California.
F, female; M, male.
CDC9501-97.
Microscan and API biocodes provided where available.
Two subsequent admissions to the hospital ensued (on days 45 and 52) due to fever and rigors during i.v. infusion of TPN through the subcutaneous port. On both occasions, an organism identified as E. amnigenus biotype II was isolated from blood cultures, with a susceptibility pattern similar to that obtained previously. Each time, clinical response to i.v. antibiotics was rapid. On the third hospital admission, an indium scan demonstrated uptake only in the area of the subcutaneous port. An organism identified as E. amnigenus biotype II was recovered from the hub needle after surgical removal. Neither TPN containers nor solutions were available for bacteriologic study. The patient received 2 weeks of i.v. ciprofloxacin prior to hospital discharge on day 66. She remained symptom-free after 1 year of follow-up.
The first identification of strain 1 as being enteric group 58, rather than E. amnigenus biotype II, was done at the National Laboratory for Enteric Pathogens at the Laboratory Centre for Disease Control (LCDC) in Ottawa, Ontario, Canada.
MATERIALS AND METHODS
Clinical laboratory bacteriologic methods.
Blood cultures were done using the Bactec 9240 continuous-monitoring blood culture system and Bactec Plus Aerobic/F and Standard Anaerobic/F blood culture media (Becton Dickinson, Cockeysville, Md.). Initial identification was done using the automated Vitek Senior system with NCII computer, software version AMS RO9.1 (bioMerieux Vitek, Inc., Hazelwood, Missouri) and the Vitek gram-negative identification (GNI) card. To check the accuracy of the identification, key reactions were first done with standard tube media at the QEII HSC. In addition, the reference strain (Table 1, strain 1) was also sent to the Isaac Walton Killam (IWK) Health Centre in Halifax, Nova Scotia, Canada, where it was tested on the Microscan Walkaway-40, with software version 20.55 (Dade Microscan, Inc., Sacramento, Calif.) for comparison with the Vitek system.
CDC laboratory methods and media.
The additional enteric group 58 strains used in this study were collected at the CDC beginning in 1973 and are listed in Table 1. Although each strain has been assigned a CDC strain number, for simplicity, we will use the case number and strain number interchangeably, e.g., strain 1 is the designation for the strain identified in case 1. A detailed description of media and methods used to characterize Enterobacteriaceae has been previously published (7-9, 11).
Biochemical testing and computer analysis (CDC).
All strains were studied with biochemical tests normally used to characterize strains of Enterobacteriaceae (7-9, 11). Tests were read at 24 h, 48 h, and 7 days. Tests were repeated if the results disagreed with the sender's results and for all tests that had strain-to-strain variation (such as citrate utilization and urea hydrolysis [Table 2]). The biochemical test results were analyzed with two different computer programs, GEORGE (version 99B) and STRAIN MATCHER (9). GEORGE compares the test strain with over 150 named taxa (genera, species, subspecies, biogroups, and enteric groups) in the family Enterobacteriaceae. It lists 24 different mathematical scores that indicate how well the test strain fits the taxa that are the closest biochemical matches. It also lists the biochemical tests that are incompatible with the taxon chosen as the best biochemical fit. It is based on the “normalized likelihood” method of Lapage et al. (9, 10, 14). STRAIN MATCHER does a “strain by strain” analysis and compares the test strain to over 11,000 individual strains in the database. The final printout lists the 60 strains from the database that are the closest biochemical matches of the test strain (9).
TABLE 2.
Biochemical reactions of 21 enteric group 58 strainsa
| Testb | Cumulative % positive on day:
|
Reaction for type strain CDC9501-97c | ||
|---|---|---|---|---|
| 1 | 2 | 7 | ||
| Indole production | 0 | − | ||
| Methyl red | 100 | + | ||
| Voges-Proskauer (O'Meara) (7, 9, 11) | 0 | − | ||
| Citrate utilization (Simmons) | 75 | 85 | 100 | + |
| H2S on triple sugar iron agar | 0 | 0 | 0 | − |
| H2S on peptone iron agar | 0 | 0 | 0 | − |
| Urea hydrolysis (Christensen) | 35 | 65 | 70 | + (2) |
| Phenylalanine deaminase | 0 | − | ||
| Lysine decarboxylase (Moeller) | 100 | 100 | 100 | + |
| Arginine dihydrolase (Moeller) | 0 | 0 | 10 | − |
| Ornithine decarboxylase (Moeller) | 90 | 95 | 95 | + |
| Motility | 100 | 100 | 100 | + |
| Gelatin hydrolysis (22°C) | 0 | 0 | 0 | − |
| KCN test (% resistant to cyanide) | 85 | 95 | 95 | + |
| Malonate utilization | 68 | 90 | 90 | + |
| d-Glucose | ||||
| Acid production | 100 | 100 | 100 | + |
| Gas production | 45 | 80 | 80 | + |
| Acid production from: | ||||
| Adonitol | 0 | 0 | 0 | − |
| l-Arabinose | 100 | 100 | 100 | + |
| d-Arabitol | 0 | 0 | 0 | − |
| Cellobiose | 100 | 100 | 100 | + |
| Dulcitol | 65 | 75 | 75 | + |
| Erythritol | 0 | 0 | 0 | − |
| d-Galactose | 93 | 100 | 100 | + |
| Glycerol | 5 | 21 | 95 | + (3) |
| myo-Inositol | 0 | 0 | 0 | − |
| Lactose | 0 | 10 | 95 | + (4) |
| Maltose | 100 | 100 | 100 | + |
| d-Mannitol | 100 | 100 | 100 | + |
| d-Mannose | 100 | 100 | 100 | + |
| Melibiose | 0 | 0 | 0 | − |
| α-Methyl-d-glucoside | 5 | 42 | 9 | + (7) |
| Raffinose | 0 | 0 | 0 | − |
| l-Rhamnose | 100 | 100 | 100 | + |
| Salicin | 65 | 95 | 100 | + (2) |
| d-Sorbitol | 100 | 100 | 100 | + |
| Sucrose | 0 | 0 | 35 | ? |
| Trehalose | 100 | 100 | 100 | + |
| d-Xylose | 100 | 100 | 100 | + |
| Esculin hydrolysis | 0 | 16 | 100 | + (4) |
| Mucate fermentation | 0 | 5 | 5 | − |
| Tartrate fermentation (Jordan) | 58 | 68 | 68 | − |
| Acetate utilization | 5 | 26 | 79 | + (3) |
| Lipase (corn oil) | 0 | 0 | 5 | − |
| DNase (25°C and 36°C) | 0 | 0 | 0 | − |
| Nitrate reduction to nitrite | 100 | + | ||
| Oxidase | 0 | − | ||
| ONPG test | 89 | 100 | 100 | + |
| Yellow pigment production | 0 | 0 | 0 | − |
| Citrate utilization (Christensen) | 79 | 95 | 95 | − |
| Tyrosine clearing | 0 | 0 | 0 | − |
A total of 21 strains were studied, but some strains were not tested for every reaction.
Sources, references, and test characteristics are given in parentheses. ONPG, o-nitrophenyl-β-d-galactopyranoside.
Symbols: +, positive at 24 h (or at 48 h for tests not done at 24 h); −, negative at end of appropriate incubation time. A number in parentheses indicates the day the reaction became positive if it was delayed.
Growth at 4°C (CDC).
Strains of enteric group 58 (including strain 1) were streaked onto sheep blood agar, MacConkey agar, and Trypticase soy agar and incubated at 4°C for up to 21 days. “No growth at 4°C” was indicated by the absence of visible colonies. Kluyvera cryocrescens, a psychrophilic species of Enterobacteriaceae which grows and produces visible colonies after 5 to 7 days under these conditions, was used as a growth control.
Antibiograms as a taxonomic tool (CDC).
Antibiograms were determined by the disk diffusion method of Bauer et al. (2). We used the “standard taxonomic set” of 12 antibiotics (Table 3) that has been used in the CDC's Enteric Reference Laboratory since 1972 for testing cultures of Enterobacteriaceae and Vibrionaceae (8).
TABLE 3.
“Taxonomic antibiograms” (disk diffusion method) of 10 enteric group 58 strains
| Case | Zone size (mm) fora:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CL | NA | SD | GM | S | K | Te | C | P | AM | CB | CF | |
| 21 | 13 | 21 | 18 | 19 | 16 | 20 | 18 | 23 | 6 | 6 | 18 | 6 |
| 6 | 13 | 22 | 25 | 24 | 17 | 22 | 17 | 26 | 6 | 6 | 11 | 6 |
| 7 | 13 | 23 | 23 | 25 | 16 | 24 | 19 | 23 | 6 | 10 | 21 | 6 |
| 8 | 13 | 22 | 27 | 23 | 16 | 19 | 16 | 25 | 6 | 6 | 20 | 6 |
| 9 | 13 | 23 | 19 | 23 | 15 | 22 | 19 | 25 | 6 | 6 | 8 | 6 |
| 13 | 12 | 21 | 6 | 20 | 15 | 19 | 17 | 20 | 6 | 6 | 18 | 6 |
| 16 | 14 | 22 | 23 | 20 | 15 | 19 | 16 | 22 | 6 | 6 | 19 | 6 |
| 17 | 13 | 20 | 26 | 18 | 13 | 18 | 17 | 21 | 6 | 12 | 20 | 6 |
| 19 | 13 | 23 | 25 | 20 | 16 | 21 | 16 | 23 | 6 | 6 | 19 | 6 |
| 20 | 12 | 20 | 27 | 20 | 14 | 18 | 15 | 20 | 6 | 6 | 20 | 6 |
CL, colistin (10 μg per disk); NA, nalidixic acid (30 μg); SD, sulfadiazine (250 μg); GM, gentamicin (10 μg); S, streptomycin (10 μg); K, kanamycin (30 μg); Te, tetracycline (30 μg); C, chloramphenicol (30 μg); P, penicillin (10 U); AM, ampicillin (10 μg); CB, carbenicillin (100 μg); CF, cephalothin (30 μg).
Molecular phylogenetic analysis of enteric group 58.
We complemented our biochemical studies with 16S rRNA gene sequence analysis, which allowed us to compare strains of enteric group 58 to named genera and species in the family Enterobacteriaceae and to propose a phylogenetic classification. Whole-cell suspensions for PCR amplification were prepared for three isolates, strain 1 (CDC9501-97), strain 4 (CDC2362-79), and strain 14 (CDC571-86), by harvesting bacterial cells from Trypticase soy agar plates containing 5% rabbit blood and resuspending the cells in 10 mM Tris-EDTA buffer, pH 8.0 (Gibco-BRL, Carlsbad, Calif.). The suspension was heated to 95°C for 10 min, and cellular debris was pelleted by centrifugation.
The 16S rRNA gene was amplified with primers 8F and 1492R (17). A volume of 2 μl of cell suspension was used as the template in the PCR, which was carried out in a total volume of 50 μl with 2.5 U of AmpliTaq Gold in 1× AmpliTaq Gold buffer (Applied Biosystems, Foster City, Calif.). Final concentrations of other reagents were as follows: 0.5 μM for each primer, 200 μM for deoxynucleoside triphosphates, and 1.5 mM for MgCl2. The thermal cycle, which was preceded by a 15-min soak at 93°C, was repeated 40 times and consisted of the following steps: 92°C for 30 s, 50°C for 60 s, and 72°C for 2 min. A subsequent extension step at 72°C was run for 5 min.
QIAquick PCR columns (QIAGEN, Valencia, Calif.) were used to purify the amplicon, which was approximately 1,480 bp. The purified template was quantified by visual comparison to the low-mass ladder (Invitrogen, Carlsbad, Calif.), following electrophoresis in a 1.5% agarose gel that was stained with ethidium bromide and illuminated with UV light.
Sequence determination was accomplished with a dye terminator cycle sequencing kit (Beckman Coulter, Fullerton, Calif.) with approximately 200 ng of template per 15 μl reaction mixture. A total of 20 ng of primer was used in each reaction. Sequences were determined with primers 8F, 357F, 515F, 980F, 1290F, 357R, 515R, 734R, 980R, and1492R (17). Chromatograms for each isolate were imported into the program SEQMAN (Lasergene, Madison, Wis.) and assembled into contigs. A consensus for each isolate was determined from the chromatograms and exported. The sequences for the three isolates were then aligned, and an unambiguous consensus of 1,375 bp representing enteric group 58 was exported and used for a BLAST search of the GenBank database and the Ribosomal Database Project II (RDP II). The 20 most closely matched sequences from GenBank were downloaded and aligned using Clustal X (22) with default settings. An alignment of 1,317 bp was imported into the program MEGA (13), and a gene tree was constructed with the neighbor-joining algorithm (18) from Jukes-Cantor corrected distances that excluded positions containing gaps. A measure of support for the branching order of the tree was estimated by the bootstrap procedure implemented in MEGA. Next, a total of 42 sequences that included the diversity of taxa that closely matched enteric group 58 were aligned using Clustal X (22) with default settings. Sequences for Citrobacter spp., Salmonella spp., and Escherichia spp. were included as out-groups in the phylogenetic analysis. An alignment of 1,283 bp was imported into the program MEGA (13), and a gene tree was constructed as described above.
Nucleotide sequence accession numbers.
GenBank accession numbers for the three sequences are as follows: for strain 1 (CDC9501-97), DQ158206; for strain 4 (CDC2362-79), DQ158204; and for strain 14 (CDC571-86), DQ158205.
RESULTS
Clinical microbiology laboratory evaluation.
Strain 1 grew well in both Bactec Plus Aerobic/F and Standard Anaerobic/F blood culture bottles within 24 h. The gram-negative rod seen on the Gram stain was subsequently identified as E. amnigenus biotype II with 99% certainty by the hospital's automated Vitek identification system. The biocode was 31317203620. In additional “tube” biochemical tests (48 h), strain 1 was urea positive (Christensen's urea agar), lysine decarboxylase positive (Moeller method), and weakly esculin positive (modified esculin agar). These three results are atypical for E. amnigenus biotype II and were in disagreement with the automated Vitek system. Also, the Voges-Proskauer test was negative, making the identification of E. amnigenus biotype II even more unlikely. Strain 1 was also sent to the IWK Health Centre in Halifax, Nova Scotia, Canada, where it was identified by the Microscan Walkaway-40 automated identification system as Yokenella regensburgei with 92.3% certainty. The biocode was 131501404456-640. Because of these discrepant results, strain 1 was sent to the National Laboratory for Enteric Pathogens at the Laboratory Centre for Disease Control in Ottawa, Canada, where it was identified as enteric group 58 with the assistance of the two CDC computer programs which are used routinely for strain analysis.
Enteric group 58 was not included in the Vitek or the Microscan gram-negative database, based upon review of technical manuals furnished by the manufacturer of each system.
Summary of enteric group 58 clinical isolates.
Strain 1 was compared with 20 other strains studied at CDC since 1973 (Table 1). Patient demographics and accompanying comments are provided where the submitter furnished them. Enteric group 58 has been isolated in North America and in Europe. It is most commonly recovered from traumatic injuries, fractures, and wound sites but has been recovered from stool and now from blood. Enteric group 58 has been most commonly misidentified as Enterobacter spp., Salmonella spp., Serratia spp., Kluyvera spp., or Escherichia spp.
CDC biochemical profiles.
Strains of enteric group 58 are gram-negative, fermentative, nonpigmented rods that are oxidase negative. They possess the general characteristics of the family Enterobacteriaceae. The biochemical reactions of 21 enteric group 58 strains are summarized in Table 2. These strains were found by computer analysis to be most closely related to 14 different species in eight genera (data not shown). The strains of enteric group 58 all clustered together (results not shown), indicating their phenotypic similarity and separation from other Enterobacteriaceae. Strain 1 failed to grow on laboratory media at 4°C within 21 days.
Taxonomic antibiograms.
Taxonomic antibiograms were determined at the time of strain submission to the CDC. This was done as a taxonomic tool (8) rather than to provide information for antibiotic usage for patients with infection. Historically, these strains were received at several laboratories within the CDC, not all of which utilized antibiograms for taxonomic purposes. Data were available for 10 enteric group 58 strains, including strain 1, and are summarized in Table 3. Although the overall susceptibility pattern was uniform, there was some strain-to-strain variation for sulfadiazine and ampicillin. The results of an “unidentified clinical strain” can be compared to the results in this table to assist in confirming a likely identification as enteric group 58.
Analysis of 16S rRNA gene sequences.
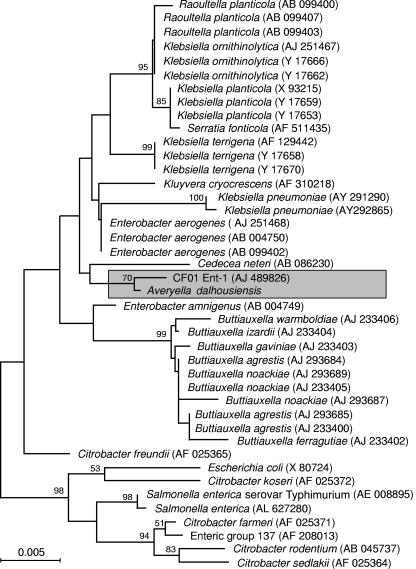
The three 16S rRNA gene sequences for the enteric group 58 isolates were identical except for three positions where the sequence of one or more strains was ambiguous. An unambiguous consensus sequence of 1,375 bp was deduced from the alignment of the three individual isolate sequences. The 16S rRNA gene sequence representing enteric group 58 shared 99.6% similarity (five bases differed from the consensus sequence) with sequence AJ489826 in GenBank, which was obtained from an unnamed member of the family Enterobacteriaceae, designated CF01Ent-1 (4). The next two closest matches were sequences from two strains of Enterobacter aerogenes, where the sequence similarities were 99.2% (11 nucleotides differed from the consensus sequence) (GenBank AJ251468) and 99.1% (12 nucleotides differed from the consensus sequence) (GenBank AB004750), and the next closest was GenBank AF310218, a sequence from Kluyvera cryocrescens that shared 98.8% similarity (16 nucleotides differed from the consensus sequence) with the enteric group 58 sequence.
In the phylogenetic analysis, the sequence for enteric group 58 clustered most closely with that from an isolate designated CF01Ent-1. Strain CF01Ent-1 was described previously (4) as perhaps a new unnamed species of the family Enterobacteriaceae, on the basis of rpoB and 16S rRNA gene sequencing studies. As was the case with many isolates of enteric group 58, the CF01Ent-1 strain was isolated from an infected wound. Our isolates differ from this strain at 5 out of the 1,253 nucleotide positions (0.39%), a value that falls within the range of divergence values between species of several enteric genera (e.g., Buttiauxella, 0.0 to 1.0%, and Salmonella, 0.31 to 1.8%) and within the range of differences between 16S rRNA operons within a single genome (0.0 to 1.3%) (5).
The sequences from enteric group 58 and CF01Ent-1 form a separate group that is distinct from other well-supported clusters of sequences in the neighbor-joining tree (Fig. 1). The results presented here are consistent with those of Bonnet et al. (4), who found that the 16S rRNA gene sequence from CF01Ent-1 did not show a close relationship to sequences from other genera in the family Enterobacteriaceae.
FIG. 1.
Relationships among 16S rRNA sequences as determined by a neighbor-joining algorithm. The accession number for each sequence drawn from GenBank is shown in parentheses. The numbers at the nodes of the tree represent the percentage of bootstrap replications in which that particular node was resolved in at least 50% of the replications. The scale bar indicates the branch length that corresponds to 0.005 nucleotide substitutions per nucleotide site.
Proposal of Averyella dalhousiensis gen. nov., sp. nov., as a new genus and species in the family Enterobacteriaceae.
Based on the phenotypic and genetic differences from all other named genera, species, and enteric groups of the family Enterobacteriaceae, we are naming enteric group 58 Averyella dalhousiensis gen. nov., sp. nov. The genus name Averyella was formed as a neo-Latin feminine noun from the name “Avery” to honor the microbiologist Oswald T. Avery, born in Halifax, Nova Scotia, Canada, also the location of Dalhousie University. Avery demonstrated that the “transforming principle” of the pneumococcus was the chemical compound DNA (1).
The species name dalhousiensis is formed as a neo-Latin feminine adjective from Dalhousie University, the source of the first published individual case (6) and the source of this first case of septicemia (case 1). We designate the type strain (holotype) of A. dalhousiensis CDC9501-97 (case 1 in this paper). Two additional strains will be deposited at the ATCC: CDC2362-79 (case 4) and CDC571-86 (case 14). ATCC numbers will be available in the GenBank sequence files. The phenotypic description is given in Tables 1 to 3 and in the text. A. dalhousiensis is defined in terms of its type strain, and future studies will be needed to determine if all 21 strains should continue to be classified in the same species. The sequencing data for strain CF01Ent-1 indicate possible genetic diversity in the genus Averyella. Future studies should include this strain, but we propose that it be reclassified as Averyella sp. CF01Ent-1 until additional genotypic and phenotypic data become available.
DISCUSSION
In this report, we describe the first blood isolate of enteric group 58 associated with infection of a central venous catheter. Infection is one of the leading complications of central venous catheter use, and catheter-related septicemia can be life-threatening (reviewed in references 3, 16, and 19). The source of the infection in our patient could not be determined; however, a breach in sterile technique is the most probable explanation. Colonization of the catheter hub is known to be one of the most common sources of catheter-related septicemia, especially in patients with long-term venous access devices (15, 16, 19, 20). Our patient's catheter had been in place for over 3 years, and the hub needle was changed weekly by well-trained personnel, yet the hub was colonized with enteric group 58 at the time the port was removed. This suggests that the catheter hub was colonized at an earlier date, as might occur with colonization of the skin over the insertion site by touching or showering. Hematogenous seeding of the central venous catheter from a distant site of infection is considered unlikely in the absence of another focus of infection. Contamination of the TPN solution, which often occurs in an epidemic setting and is usually caused by gram-negative bacilli, is also unlikely because quality control records did not implicate solutions prepared in the hospital pharmacy and no other patients in the home TPN program had become ill. In addition, the organism did not grow in the laboratory at 4°C after 21 days of incubation, and TPN solutions were refrigerated at all times. The TPN solution could have become contaminated when vitamin supplements were added, or the supplements may have been contaminated, but there is no evidence for this. There have been a number of case reports in the medical literature describing sepsis due to unusual organisms of low pathogenicity in patients with central venous catheters (12, 21, 23). This case highlights the fact that in the presence of an implanted medical device, enteric group 58 can cause multiple episodes of sepsis.
Since enteric group 58 was first characterized as a distinct biogroup in 1985 (9), only a small number of strains have been reported. There is little known about the epidemiology, pathogenesis, or clinical significance of this organism. Most enteric group 58 strains have been isolated from traumatic injuries, fractures, wound sites, and rarely stool (Table 1). Information submitted to CDC with clinical isolates thus far has not suggested that enteric group 58 is a significant cause of human infection. One case report of enteric group 58 recovered from an orthopedic compression plate has been published in the literature (6). This device had loosened 1 year after implantation and required removal. Infection was considered possible; however, the patient remained clinically well and never required antibiotic therapy. This isolate also came from the QEII HSC in 1987, a fact only discovered after a thorough search of the literature. Identification of this isolate was also performed at the National Laboratory for Enteric Pathogens at LCDC in Ottawa, Ontario, Canada. Correspondence with LCDC yielded two additional enteric group 58 isolates from Canada: one submitted from the province of Alberta (burn site of right foot) and the other from the province of Ontario (site unknown). Although we were unable to obtain these strains, it appears that the Ontario strain is case 5 (Table 1). These examples lead us to suspect that enteric group 58 is usually an environmental or exogenous organism of low pathogenicity. Future studies should examine clinical features associated with enteric group 58 infection, especially as it relates to symptomatic infection versus colonization of traumatic injuries and wounds. In addition, little is known about the antibiotic resistance profile of enteric group 58 or whether it might serve as a reservoir for antibiotic resistance.
After our original study was completed, we obtained phylogenetic data that compelled us to assign a scientific name to enteric group 58 in order to facilitate communication within the research community. The results of 16S rRNA gene sequencing demonstrate that enteric group 58 is not closely related to other known genera or species in the family Enterobacteriaceae. Although we consider DNA-DNA hybridization to be the “gold standard” for DNA relatedness studies, it has become increasingly difficult to obtain this type of analysis. We feel that the distinct phenotypic characteristics of enteric group 58, in combination with the divergence observed by 16S rRNA gene sequencing, warrant the proposal of a formal scientific name. Thus, we propose Averyella dalhousiensis gen. nov., sp. nov. Future studies measuring evolutionary distance should include the type strain, all other strains (including our 20 strains), and the French strain CF01Ent1 (4).
This new member of the family Enterobacteriaceae is being misidentified in clinical and public health microbiology laboratories (Table 1). Our case report illustrates that one reason for this is the fact that the organism is not included in the databases of commercial identification systems. Manufacturers of these systems should obtain these strains and include them in their databases in order to assist exploration of the clinical spectrum of disease of Averyella dalhousiensis. Until this problem is corrected, it will be difficult to determine its importance in human infections. Future studies and case reports should focus on differentiating infection from colonization and should include patients' antibody responses. We hope that this report, and the assignment of a scientific name, will increase knowledge about this new genus and species.
Acknowledgments
We thank B. L. Johnston and D. Haase of the Division of Infectious Diseases, QEII HSC, for initially bringing case 1 to our attention; K. R. Forward and D. Haldane of the Division of Medical Microbiology, QEII HSC, for encouragement and use of laboratory resources; W. A. Kennedy of the Division of Medical Microbiology, IWK Health Centre, for use of laboratory resources; D. Woodward and W. Johnson of the National Laboratory for Enteric Pathogens, LCDC, for providing the first confirmation of the identification of strain 1 as enteric group 58 and for information about the other strains they had studied; and J. P. Euzéby for helping us coin the neo-Latin names of the genus and species. Special thanks to Jamyee Pleasant-Taylor (internship coordinator) and J. J. Farmer III (faculty mentor).
Bruce H. Brown, Jr., was supported by a grant from the Minority Health Professions Foundation through a program developed by the Morehouse School of Medicine in conjunction with the Centers for Disease Control and Prevention.
REFERENCES
- 1.Avery, O. T., C. M. MacLeod, and M. McCarty. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a deoxyribonucleic acid faction isolated from pneumococcus type III. J. Exp. Med. 79:137-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer, A. W., W. M. Kirby, J. C. Sherris, and M. Turck. 1966. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45:493-496. [PubMed] [Google Scholar]
- 3.Benoit, J. L., G. Carandang, M. Sitrin, and P. M. Arnow. 1995. Intraluminal antibiotic treatment of central venous catheter infections in patients receiving parenteral nutrition at home. Clin. Infect. Dis. 21:1286-1288. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet, R., C. Chanal, E. Ageron, D. Sirot, C. De Champs, P. Grimont, and J. Sirot. 2002. Inducible AmpC β-lactamase of a new member Enterobacteriaceae. Antimicrob. Agents Chemother. 46:3316-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coenye, T., and P. Vandamme. 2003. Intragenomic heterogeneity between multiple 16S ribosomal RNA operons in sequenced bacterial genomes. FEMS Microbiol. Lett. 228:45-49. [DOI] [PubMed] [Google Scholar]
- 6.Dalton, M. T., and G. S. Bezanson. 1989. The isolation, from a prosthetic device site, of a rare bacterium belonging to CDC enteric group 58. Can. J. Microbiol. 35:819-820. [Google Scholar]
- 7.Ewing, W. H. 1986. Edwards and Ewing's identification of enterobacteriaceae, 4th ed. Elsevier Science Publishing Co., Inc., New York. N.Y.
- 8.Farmer, J. J., III. 1999. Enterobacteriaceae: introduction and identification, p. 442-458. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 9.Farmer, J. J., III, B. R. Davis, F. W. Hickman-Brenner, A. McWhorter, G. P. Huntley-Carter, M. A. Asbury, C. Riddle, H. G. Wathen-Grady, C. Elias, G. R. Fanning, et al. 1985. Biochemical identification of new species and biogroups of Enterobacteriaceae isolated from clinical specimens. J. Clin. Microbiol. 21:46-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman, R. B., D. Bruce, J. MacLowry, and V. Brenner. 1973. Computer-assisted identification of bacteria. Am. J. Clin. Pathol. 60:395-403. [DOI] [PubMed] [Google Scholar]
- 11.Hickman, F. W., and J. J. Farmer III. 1978. Salmonella typhi: identification, antibiograms, serology, and bacteriophage typing. Am. J. Med. Technol. 44:1149-1159. [PubMed] [Google Scholar]
- 12.Horowitz, H. W., R. B. Nadelman, K. G. Van Horn, S. E. Weekes, L. Goyburu, and G. P. Wormser. 1987. Serratia plymuthica sepsis associated with infection of central venous catheter. J. Clin. Microbiol. 25:1562-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 14.Lapage, S. P., S. Bascomb, W. R. Willcox, and M. A. Curtis. 1973. Identification of bacteria by computer: general aspects and perspectives. J. Gen. Microbiol. 77:273-290. [DOI] [PubMed] [Google Scholar]
- 15.Linares, J., A. Sitges-Serra, J. Garau, J. L. Perez, and R. Martin. 1985. Pathogenesis of catheter sepsis: a prospective study with quantitative and semiquantitative cultures of catheter hub and segments. J. Clin. Microbiol. 21:357-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raad, I. I., and G. P. Bodey. 1992. Infectious complications of indwelling vascular catheters. Clin. Infect. Dis. 15:197-208. [DOI] [PubMed] [Google Scholar]
- 17.Sacchi, C. T., A. M. Whitney, L. W. Mayer, R. Morey, A. Steigerwalt, A. Boras, R. S. Weyant, and T. Popovic. 2002. Sequencing of 16S rRNA gene: a rapid tool for identification of Bacillus anthracis. Emerg. Infect. Dis. 8:1117-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 19.Sitges-Serra, A., J. Linares, J. L. Perez, E. Jaurrieta, and L. Lorente. 1985. A randomized trial on the effect of tubing changes on hub contamination and catheter sepsis during parenteral nutrition. J. Parenter. Enteral Nutr. 9:322-325. [DOI] [PubMed] [Google Scholar]
- 20.Sitges-Serra, A., P. Puig, J. Linares, J. L. Perez, N. Farrero, E. Jaurrieta, and J. Garau. 1984. Hub colonization as the initial step in an outbreak of catheter-related sepsis due to coagulase negative staphylococci during parenteral nutrition. J. Parenter. Enteral Nutr. 8:668-672. [DOI] [PubMed] [Google Scholar]
- 21.Spaulding, A. C., and A. L. Rothman. 1996. Escherichia vulneris as a cause of intravenous catheter-related bacteremia. Clin. Infect. Dis. 22:728-729. [DOI] [PubMed] [Google Scholar]
- 22.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong, V. K. 1987. Broviac catheter infection with Kluyvera cryocrescens: a case report. J. Clin. Microbiol. 25:1115-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]