Abstract
The detection of fungal pathogens in clinical samples by PCR requires the use of extraction methods that efficiently lyse fungal cells and recover DNA suitable for amplification. We used quantitative PCR assays to measure the recovery of DNA from two important fungal pathogens subjected to six DNA extraction methods. Aspergillus fumigatus conidia or Candida albicans yeast cells were added to bronchoalveolar lavage fluid and subjected to DNA extraction in order to assess the recovery of DNA from a defined number of fungal propagules. In order to simulate hyphal growth in tissue, Aspergillus fumigatus conidia were allowed to form mycelia in tissue culture media and then harvested for DNA extraction. Differences among the DNA yields from the six extraction methods were highly significant (P < 0.0001) in each of the three experimental systems. An extraction method based on enzymatic lysis of fungal cell walls (yeast cell lysis plus the use of GNOME kits) produced high levels of fungal DNA with Candida albicans but low levels of fungal DNA with Aspergillus fumigatus conidia or hyphae. Extraction methods employing mechanical agitation with beads produced the highest yields with Aspergillus hyphae. The MasterPure yeast method produced high levels of DNA from C. albicans but only moderate yields from A. fumigatus. A reagent from one extraction method was contaminated with fungal DNA, including DNA from Aspergillus and Candida species. In conclusion, the six extraction methods produce markedly differing yields of fungal DNA and thus can significantly affect the results of fungal PCR assays. No single extraction method was optimal for all organisms.
Noncultivation methods are increasingly being used to overcome the poor diagnostic sensitivities and long turnaround times associated with the detection and identification of fungal pathogens in clinical samples by cultivation. One of these cultivation-independent methods is real-time PCR, which can rapidly detect and quantify fungal nucleic acid sequences in human tissue samples. Real-time quantitative PCR (qPCR) assays have detection thresholds that approach single-molecule sensitivity, and thus, little additional assay sensitivity can be achieved in the PCR itself by techniques such as targeting genes with multiple copies per fungal genome or increasing the total amount of DNA tested. The ultimate sensitivity of any PCR assay for the detection of fungal pathogens depends on the efficient lysis of fungal cells in the tissue sample and the purification of DNA that is free of PCR inhibitors. Fungi have cell walls that impede lysis and the recovery of nucleic acids. Few studies have focused on the critical DNA extraction stage of sample processing, in contrast to the multitude of studies on fungal PCR assay methods. Furthermore, highly sensitive and specific nucleic acid-based methods for the detection of fungi necessitate the use of DNA extraction reagents that are free of contaminating fungal nucleic acids.
We sought to compare DNA extraction methods by using qPCR to measure the amount of fungal DNA liberated from two important fungal pathogens that infect humans, Aspergillus fumigatus and Candida albicans. Candida albicans is a model yeast pathogen, and Aspergillus fumigatus is a model filamentous fungal pathogen. We elected to test DNA extraction in spiked bronchoalveolar lavage (BAL) fluid because BAL fluid is the specimen most commonly subjected to fungal PCR at our institution and has been shown to contain PCR inhibitors. Although Aspergillus fumigatus is a common cause of pneumonia in immunocompromised patients, pneumonia due to Candida albicans is much less common (4, 11, 12). The inoculation of BAL fluid with known numbers of Aspergillus fumigatus conidia or Candida albicans yeast cells allows one to test DNA extraction methods using well-defined numbers of fungal propagules. However, Aspergillus fumigatus normally exists as hyphal structures in tissue. Thus, while useful for the accurate quantitation of fungal propagules subjected to DNA extraction, conidia do not represent the clinically relevant structure. One can mimic hyphal growth in tissue by germinating Aspergillus fumigatus conidia in tissue culture media and allowing them to form mycelial mats. Hyphae can then be harvested for extraction. We also sought to assess the contamination of commercial DNA extraction kits with Aspergillus and Candida DNA.
MATERIALS AND METHODS
Preparation of BAL fluid.
BAL fluid samples from patients undergoing evaluation for pneumonia at the Fred Hutchinson Cancer Research Center were pooled. Aliquots from this pool were subjected to extraction (MasterPure yeast method [MPY]) and fungal DNA quantitation by qPCR to assure that the pool was free of Aspergillus and Candida DNA. Samples of this pooled BAL fluid were then spiked with fungal propagules and subjected to the various DNA extraction methods noted below.
Cultivation of fungi and quantitation of fungal propagules.
Aspergillus fumigatus (ATCC strain B5233) was grown on 5 ml of Sabouraud dextrose agar in a 50-ml tissue culture flask at 37°C for 2 days and then left at 25°C to sporulate until mature. The agar was overlaid with 5 ml of sterile 0.1% Tween 20 filtered to 0.2 μm, and the flask was placed on a rotary shaker for 10 min. The solution of A. fumigatus conidia and hyphal fragments was harvested with a syringe and passed through a 5.0-μm polycarbonate filter (Millipore Corporation) to remove hyphae. Conidia were washed two times by centrifuging the filtrate at 3,000 × g for 20 min, removing all of the supernatant, and resuspending the pellet in fresh 0.1% Tween 20. After the second centrifugation, the pellet was resuspended in 15 ml of 0.1% Tween 20 and the solution was placed on ice. Microscopic examination of the preparation was done to confirm the absence of hyphal elements. The concentration of conidia was determined by manual cell counting with a hemocytometer. Aliquots (0.1 ml) of BAL fluid were each inoculated with 28,000 conidia and subjected to the DNA extraction protocols.
A clinical isolate of Candida albicans recovered from blood culture was grown in Sabouraud dextrose broth at 37°C in a shaking incubator for 1 day. The yeast cells were washed twice in 10 mM Tris-1 mM EDTA buffer by centrifugation at 14,000 × g for 5 min, then resuspended in buffer and placed on ice. The cells were manually counted with a hemocytometer. Yeast cells (42,000) were inoculated into each 0.1-ml aliquot of BAL fluid for DNA extraction.
Cultivation of Aspergillus in tissue culture media to form mycelia.
To create mycelia for DNA extraction, 2,800 washed A. fumigatus conidia were inoculated into wells of a 96-well tissue culture plate (Costar, Corning, NY). Each well contained 0.1 ml of Dulbecco's modified Eagle medium (Invitrogen, Carlsbad, CA). The plates were incubated at 35°C and 5% CO2 in a humidified tissue culture incubator for 24 h. Visual inspection of the plates using an inverted phase-contrast microscope was used to confirm the presence of extensive mycelial mats. Although the number of A. fumigatus genomes could not be independently quantified by a technique such as that used for counting conidia, we sought to produce roughly equivalent hyphal masses by inoculating tissue culture media with a known number of Aspergillus conidia and then allowing the conidia to germinate into hyphae for 24 h in culture. The hyphae were harvested from wells by mixing with a pipette and transferring 0.1 ml of resuspended hyphae to a microcentrifuge tube for DNA extraction.
Preparation of fungal genomic DNA for use in qPCR standards.
Aspergillus fumigatus (ATCC strain B5233) was grown on 5 ml of Sabouraud dextrose agar in a 50-ml tissue culture flask at 37°C as described above. The agar was overlaid with 10 ml of sterile 0.1% Tween 20 filtered to 0.2 μm, and a stir bar was used to break hyphae by spinning on a stir plate. Hyphal fragments were pelleted by centrifugation at 3,200 × g for 15 min and resuspended in sterile water. A clinical isolate of Candida albicans was grown in Sabouraud dextrose broth at 37°C overnight. The yeasts were pelleted by centrifugation at 3,200 × g for 15 min and resuspended in sterile water. Resuspended fungi were processed through the MasterPure yeast DNA extraction method as described below. The purified nucleic acid was treated with 5 units of RiboShredder RNase mixture (Epicenter, Madison, WI) at 37°C for 50 min to degrade RNA. The DNA was precipitated with isopropanol and sodium acetate, washed with 70% ethanol, and resuspended in Tris-EDTA buffer. The optical density of the genomic DNA was measured with a spectrophotometer at 260 nm in order to quantify the stock concentration for subsequent dilution in qPCR standards.
DNA extraction methods.
Manufacturers' instructions were followed for all methods except where noted.
Method MPY (MasterPure yeast DNA purification kit [Epicenter, Madison, WI]) employs a nonenzymatic method for the lysis of fungi followed by a salting-out procedure to precipitate proteins and an alcohol precipitation step to purify DNA.
Method UCS (UltraClean soil DNA isolation kit [MoBio, Inc., Solana Beach, CA]) uses a bead matrix and lysis buffer to pulverize cells by horizontal shaking on a vortex mixer, followed by adsorption of DNA to a spin filter, a wash step, and the elution of DNA in buffer. The protocol was followed per the manufacturer's instructions, using the alternative protocol for maximum yields. Microcentrifuge tubes with sample and bead matrices were attached to a horizontal platform on a vortex mixer and agitated vigorously for 10 min. Each sample was split into 2 volumes, and 650 μl of solution S3 was added to each tube of supernatant prior to addition to the two spin columns. The final DNA eluates were combined.
Method FDNA (FastDNA kit [Qbiogene, Irvine, CA]) uses a bead matrix and lysis buffer to pulverize cells by agitation in a FastPrep agitator for high-speed cell disruption, followed by adsorption of DNA to glass milk, a wash step, and elution of DNA in buffer. We used lysing matrix A, cell lysis solution-yeast lysis buffer, and the spin column protocol. Samples were agitated for two 30-second runs at a speed of 5 m per second.
Method MPPL (MasterPure plant leaf DNA purification kit [Epicenter, Madison, WI]) uses a nonenzymatic lysis procedure, alcohol precipitation of DNA, and a cleanup procedure to bind PCR inhibitors, followed by a reprecipitation of DNA. The 0.1 ml of input sample was combined with 0.3 ml of the plant DNA extraction solution and ground with a disposable plastic micropestle prior to DNA precipitation.
Method YL-GNOME (yeast cell lysis preparation kit plus GNOME kit [Qbiogene, Irvine, CA]) uses two kits to lyse fungal cells and purify DNA. The yeast cell lysis kit uses enzymes for the digestion of fungal cell walls. The resulting spheroplasts are then subjected to further lysis in the GNOME kit by using a lysis buffer and protease mix. Protein is precipitated with a salting-out procedure, and then DNA is precipitated with alcohol. The first step of the yeast lysis procedure was centrifugation of the BAL or tissue culture sample with the addition of yeast enzyme enhancer to the pellet. The manufacturer's instructions were followed, except that the steps were appropriately scaled down in size, with the addition of 0.1 ml of yeast enzyme enhancer, 2 μl of yeast enzyme salts, and 20 μl of spheroplasting enzyme mix per sample. The resulting digest was then added to 0.4 ml of cell suspension solution in the GNOME kit, 0.1 ml of cell lysis solution was added, and the lysis and purification proceeded with volumes scaled to the input sample volume.
Method SM (SoilMaster DNA extraction kit [Epicenter, Madison, WI]) uses a hot detergent lysis procedure to break open cells, a salting-out procedure to precipitate protein, a column chromatography step to remove PCR inhibitors, and an alcohol precipitation step to purify DNA. The optional step of vortex mixing at 37°C for 10 min was not performed.
Method QIAMP-S (QIAamp DNA stool mini kit [QIAGEN, Valencia, CA]) uses lysis buffer, proteinase K, and heat at 70°C to break open cells. Inhibitors in the lysate are bound to an insoluble matrix (InhibitEX tablet) and pelleted by centrifugation. DNA in the supernatant is bound to a spin column, washed, and eluted in buffer. Note that this method was not used to compare DNA extraction yields due to the detection of contaminating fungal DNA. See “Identification of contaminating fungal DNA in extraction reagents” below.
All DNA extractions were performed in quadruplicate except for the analysis of Aspergillus conidia by methods MPPL, YL-GNOME, and SM, which were performed in duplicate. Input and output volumes for each method are listed in Table 1.
TABLE 1.
Comparison of DNA extraction methods based on costs, times, sample volumes, and additional reagents required
| Extraction method | Cost/test | Processing time (h:min)a
|
Sample vol | Vol recovered | Additional reagents/equipment (not supplied) | Equipment | |
|---|---|---|---|---|---|---|---|
| Minimum | Maximum | ||||||
| MPY | $1.81 | 0:40 | 1:20 | 100 μl | 50 μl | Isopropanol, ethanol, microcentrifuge tubes | Heat block, microcentrifuge |
| MPPL | $3.23 | 0:55 | 1:40 | 100 μl | 50 μl | Isopropanol, ethanol, microcentrifuge tubes | Heat block, microcentrifuge |
| SM | $4.54 | 0:50 | 1:55 | 100 μl | 300 μl | Microcentrifuge tubes | Heat block, microcentrifuge |
| UCS | $3.00 | 0:35 | 2:40 | 100 μl | 50 μl | None | Vortex adapter, microcentrifuge |
| FDNA | $2.56 | 0:20 | 1:15 | 100 μl | 100 μl | None | Microcentrifuge, FastPrep machine |
| YL-GNOME | $5.93 | 3:30 | 4:00 | 100 μl | 100 μl | Isopropanol, microcentrifuge tubes | Heat block, microcentrifuge |
The minimum processing times reflect extractions of single samples, whereas the maximum times reflect extractions of 12 samples each.
Quantitative PCR methods.
Two TaqMan-based PCR assays were used to measure fungal DNA using a GeneAmp 7900 sequence detection system (Applied Biosystems, Foster City, CA) with primers that target highly conserved regions of the fungal 18S rRNA gene and 5′ nuclease probes complementary to Aspergillus species or Candida species 18S rRNA genes. For the Aspergillus fumigatus assay, we used primers Fun-18S-995F (5′-CGATYAGATACCGTYGTAGTC-3′), Fun-18S-1217R (5′TGTCTGGACCTGGTGAGTTT-3′), and a 6-carboxyfluorescein (FAM)-labeled probe with a Black Hole quencher (BHQ1) (5′-FAM-TTTCTATGATGACCCGCTCGGCA-BHQ1-3′). For the Candida albicans qPCR assay, we used primers Fun-18S-1313F (5′-SCGATAACGAACGAGACCT-3′) and Fun-18S-1467R (5′-TAGCGCGCTGCGGCCCAGA-3′) with a VIC (Applied Biosystems)-labeled probe and a 6-carboxytetramethylrhodamine (TAMRA) quencher (5′-VIC-CTAAATAGTGSTGCTAGCWTTTGC-TAMRA-3′). The concentration of each primer was 200 nM, and the concentration of each probe was 100 nM. We used Universal master mix (Applied Biosystems) for all qPCR reactions and ran each sample in a 50-μl volume consisting of 5 μl of target DNA and 45 μl of master mix with primers and probe. PCR conditions included a 2-minute incubation at 50°C to inactivate previous amplicons with uracil-DNA glycosylase, followed by a 10-minute incubation at 95°C to activate the Taq Gold polymerase. Forty-five cycles of PCR, consisting of 15 seconds at 95°C, 30 seconds at 55°C, and 30 seconds at 65°C, were performed. All qPCR assays contained 4 no-template control samples (negative controls) and 12 samples consisting of Aspergillus fumigatus or Candida albicans genomic DNA (as appropriate) added to reactions in duplicate to produce standards of 1,000 pg, 100 pg, 10 pg, 1 pg, 100 fg, and 20 fg of fungal genomic DNA. The threshold cycle values from the genomic DNA standards were used to create a standard curve to assess the amount of fungal DNA in samples subjected to the various DNA extraction methods. All samples from extraction replicates were run in duplicate. Amplification controls were performed on DNA extracted from each method; 5 μl of extracted DNA was combined with 1 μl of 1,000-pg fungal genomic DNA standard, and qPCR was performed on this mixture. If PCR inhibitors are present in the extracted DNA, the threshold cycle for that sample shifts to a higher cycle number compared to the 1,000-pg standard without exogenous sample DNA. Digest controls consisted of sterile UV-irradiated water processed through each of the DNA extraction methods and then analyzed by qPCR.
Identification of contaminating fungal DNA in extraction reagents.
Because Aspergillus DNA was detected in digest controls from the QIAMP-S extraction method (QIAamp DNA stool mini kit; QIAGEN) and was linked to a tablet used to bind PCR inhibitors (InhibitEX tablet; QIAGEN), we used broad-range 18S rRNA gene PCR with analysis of cloned products to identify the contaminating species (2).
Statistical analysis.
Each sample of extracted DNA was subjected to qPCR in duplicate, and extractions were performed in duplicate or quadruplicate. Mean quantities of fungal DNA detected with each DNA extraction method for each experimental system were plotted along with the standard deviations for the replicates. Analysis of variance with additional Bonferroni-protected contrasts was performed to compare extraction methods within each organism and experimental system. Such comparisons were considered statistically significant when P values were less than 0.05/(number of contrasts performed). A Bonferroni correction was preferable to Scheffe's method, as the number of contrasts was relatively small in each case. Some contrasts were performed to confirm that no significant difference in performances had been observed. In other cases, a significant difference was expected. Levene's test was initially performed to ascertain whether the assumption of homogeneous variances was valid. Contrasts of interest were selected on the basis of graphical analysis. SAS 9.2 and Excel 2000 for Windows were used for statistical and graphical analyses, respectively.
RESULTS
Levels of fungal DNA recovered with the six extraction methods are displayed in Fig. 1 to 3. Overall analysis of variance tests for differences by extraction method were significant at P < 0.0001 for all three organisms/experimental systems. Table 2 displays the log differences and P values for various comparisons of mean DNA levels between extraction methods in each of the three experimental systems. Log differences translate to ratios on the original scale. For example, a log difference of 0.580 (row 2) means that the average count for the first method or group of methods is 100.580, or 3.8, times larger than the other, and a log difference of 3.574 (row 6) indicates that the first method or group of methods is 3,750 times larger than the second.
FIG. 1.
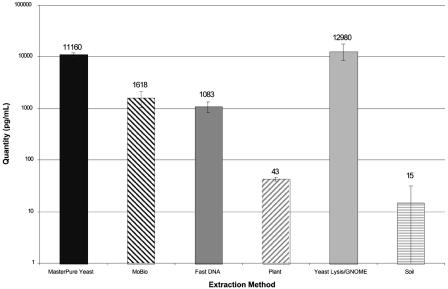
Mean levels of Candida DNA detected in BAL fluid spiked with C. albicans yeast cells and subjected to six DNA extraction methods. Fungal DNA levels were measured using quantitative PCR. Error bars indicate standard deviations for replicate extractions. The MPY and YL-GNOME extraction methods produced the highest levels of Candida DNA.
FIG. 3.
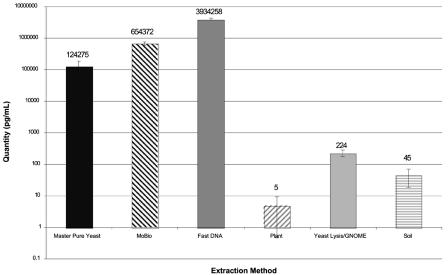
Mean levels of Aspergillus DNA in tissue culture media inoculated with A. fumigatus conidia, allowed to form mycelia, and subjected to six DNA extraction methods. Fungal DNA levels were measured using quantitative PCR. Error bars indicate standard deviations for replicate extractions. The FDNA method produced the highest levels of Aspergillus DNA from hyphae.
TABLE 2.
Comparison of mean fungal DNA levels recovered by extraction method
| Isolate | Contrast | Log difference | P valuea |
|---|---|---|---|
| A. fumigatus conidia | FDNA vs UCS | −0.027 | 0.8860 |
| FDNA and UCS vs MPY | 0.580 | 0.0191 | |
| MPY vs MPPL and SM | 1.757 | <.0001 | |
| A. fumigatus hyphae | FDNA vs UCS | 0.784 | 0.0017 |
| UCS vs MPY | 0.779 | 0.0018 | |
| MPY vs MPPL and SM | 3.574 | <.0001 | |
| C. albicans | YL-GNOME vs MPY | 0.036 | 0.8639 |
| FDNA vs UCS | −0.161 | 0.4524 | |
| MMPL vs SM | 0.890 | 0.0005 | |
| YL-GNOME and MPY vs FDNA and UCS | 0.966 | <.0001 | |
| FDNA and UCS vs MMPL and SM | 1.918 | <.0001 |
P values shown in bold are significant at 0.05 by Bonferroni correction for contrasts on that organism.
Figure 1 shows the quantity of Candida DNA detected in BAL fluid samples experimentally inoculated with C. albicans yeast forms and subjected to the six different DNA extraction methods. Five contrasts were performed to examine differences in extraction methods for C. albicans yeast cells, thus requiring a P value of <0.01 for significance with the Bonferroni correction (Table 2). The MPY and YL-GNOME methods both produced high levels of Candida DNA, with more than 11,000 pg DNA/ml detected in BAL fluid, and these methods were not significantly different from each other. The UCS method (1,618 pg/ml) and the FDNA method (1,083 pg/ml) yielded levels of DNA that were not significantly different from each other, but the recovery of fungal DNA with these methods was significantly less than the recoveries obtained with the MPY and YL-GNOME methods (P < 0.0001). The MPPL and SM methods produced dramatically lower levels of Candida DNA compared to the other four methods, and these differences were highly statistically significant (P < 0.0001).
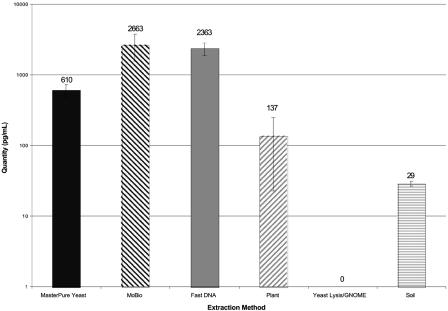
Figure 2 displays the quantity of Aspergillus DNA detected in BAL fluid samples experimentally inoculated with A. fumigatus conidia and subjected to the six different DNA extraction methods. Three contrasts were performed to examine differences in extraction methods for A. fumigatus conidia; therefore, P values of <0.017 were considered statistically significant after Bonferroni correction. The UCS and FDNA methods both employ bead beating for the physical disruption of cells; these methods produced the highest yields of Aspergillus DNA (>2,000 pg/ml) and were not significantly different from each other. The UCS and FDNA methods produced higher levels of DNA than the MPY method (610 pg/ml), but the difference was not statistically significant after Bonferroni correction (P = 0.0191). The MPY method was significantly better than the MPPL, YL-GNOME, and SM methods (P < 0.0001). The latter three methods all had DNA yields of less than 200 pg/ml. Although the YL-GNOME method performed well when extracting DNA from C. albicans, this method performed poorly when extracting DNA from Aspergillus conidia. Modest PCR inhibition was detected when Aspergillus conidia were extracted with the YL-GNOME method, resulting in a 1-log drop in assay sensitivity detected by a shift in threshold cycle when the samples were spiked with 1,000 pg of Aspergillus genomic DNA. A combination of poor DNA extraction and modest PCR inhibition likely accounts for the absence of Aspergillus DNA detected with the YL-GNOME method. PCR inhibitors were not detected with any other extraction methods.
FIG. 2.
Mean levels of Aspergillus DNA detected in BAL fluid spiked with A. fumigatus conidia and subjected to six DNA extraction methods. Fungal DNA levels were measured using quantitative PCR. Error bars indicate standard deviations for replicate extractions. The UCS and FDNA methods produced the highest levels of Aspergillus DNA from conidia.
It is possible to estimate the efficiency of extraction by comparing the amount of fungal DNA recovered with the amount of fungal DNA initially inoculated with intact organisms in BAL fluid. Unfortunately, the multicellular natures of Aspergillus hyphae and budding Candida yeast cells make estimates of initial cell counts highly inaccurate. In contrast, Aspergillus conidia are easily counted as separate cells with a hemocytometer, and each conidium contains a single genome. On the basis of an estimated Aspergillus genome mass of 31.6 fg, we would expect to recover 8,848 pg of Aspergillus DNA per ml of BAL fluid after inoculation with 280,000 conidia at 100% extraction efficiency. The conidium extraction efficiencies for the methods studied were 30.1% for UCS, 26.7% for FDNA, 6.9% for MPY, 1.6% for MPPL, 0.3% for SM, and 0% for YL-GNOME. When 10-fold and 100-fold fewer conidia were added to BAL fluid and extracted with the UCS method, extraction efficiencies were 8.7% and 9.9%, respectively. BAL fluid spiked with 280 conidia still yielded DNA levels of 8.6 pg/ml, or 860 fg per 0.1 ml of sample.
Figure 3 displays the quantities of Aspergillus DNA detected in tissue culture media experimentally inoculated with A. fumigatus conidia, cultured to form mycelial mats, and then harvested for DNA extraction by using the six methods. Three contrasts were performed to examine differences in extraction methods for A. fumigatus hyphae; therefore, P values of <0.017 were considered significant after Bonferroni correction. The FDNA method produced the highest DNA yield at 3,934,258 pg/ml of culture medium and was significantly better than the UCS method (654,372 pg/ml; P = 0.0017). The UCS method was significantly better than the MPY method (124,276 pg/ml; P = 0.0018). The MPY method was significantly better (P = 0.0001) than the remaining three methods of MPPL (5 pg/ml), YL-GNOME (225 pg/ml), and SM (46 pg/ml).
Attempts to use the QIAMP-S DNA extraction method on BAL fluid were unsuccessful because Aspergillus and other fungal DNA was detected in digest controls, indicating contamination of the reagents. We isolated the contamination to the InhibitEX tablets used to bind PCR inhibitors. No further comparisons were possible with this DNA extraction method.
Several factors besides the recovery of DNA must be considered when selecting a DNA extraction method. Table 1 displays the cost per sample, processing time, sample volume, additional reagents, and equipment for each DNA extraction method. The MPY method was the least expensive method, had few manipulations, and could be completed in about an hour with a few samples. The YL-GNOME method was the most expensive approach because it required two separate kits and required the most processing time to produce DNA. The UCS and FDNA methods had similar reagent costs, but the FDNA method requires the purchase of a separate agitator, whereas the UCS method can use a vortex mixer for agitation. More manipulations were required in the UCS method than in the FDNA method, leading to a longer processing time. In general, the DNA extraction methods generating high yields of fungal DNA (MPY, UCS, and FDNA) gave reasonably reproducible results in replicate samples, reflected in the standard deviations for the means in Fig. 1 to 3.
DISCUSSION
Fungi have cell walls that impede cell lysis and the recovery of DNA using conventional extraction methods (10). Simple lysis procedures, such as the use of sequential freeze-thaw cycles or incubation with hot detergent and proteases, have not produced high yields of DNA from many fungal species. Alternative approaches for the lysis of fungal cells include the agitation of tissue samples with microspheres or particulates within a sealed tube for physical disruption (13) and the enzymatic digestion of cell wall polysaccharides to form spheroplasts followed by conventional membrane lysis procedures (3). Some DNA extraction methods for fungi, such as grinding cells frozen with liquid nitrogen using a mortar and pestle and disrupting cell walls with a probe sonicator, work well for the large-scale preparation of fungal DNA from cultures (6, 14). However, these methods are not practical for use in a clinical microbiology laboratory, where many samples must be processed and where cross contamination of samples must be scrupulously avoided. We compared the yields of fungal DNA produced from several commercial DNA extraction methods employing different lysis strategies that are suitable for use on multiple samples in a clinical microbiology laboratory setting. The use of commercial DNA extraction methods has been advocated for nucleic acid-based fungal diagnostics in order to provide standardized methods and reagents so that results can be compared between laboratories (1).
The large differences in the amounts of fungal DNA recovered with the different DNA extraction methods and detected by qPCR in this study highlight the importance of the extraction step in nucleic acid-based fungal diagnostics. For instance, there was almost a millionfold difference in DNA recovery levels between the MPPL and FDNA methods applied to Aspergillus hyphae. The SM and MPPL methods performed poorly in all tests; these methods were designed to extract DNA from bacterial, fungal, or plant sources in soil (SM) or from plant leaf material with complex cell walls (MPPL). Clearly, any fungal PCR assay that used these two extraction methods to detect Candida or Aspergillus species in tissue samples would likely suffer from unacceptably low sensitivity.
The YL-GNOME kit is designed for DNA extraction from yeasts and performed very well with Candida albicans in BAL fluid but performed poorly with Aspergillus fumigatus conidia and hyphae. Ideally, DNA extraction methods should be capable of detecting both yeast and hyphal forms of several different fungal pathogens in tissue samples submitted for fungal PCR testing. The failure of the YL-GNOME method to extract DNA from the filamentous fungal pathogen A. fumigatus makes this a poor general-purpose method for use in the clinical microbiology laboratory, where the identity of the pathogen is initially unknown.
The UCS and FDNA methods both employ agitation of the clinical sample with particulates within a microcentrifuge tube for the disruption of fungal cells, and these methods worked well with A. fumigatus hyphae and conidia. The UCS and FDNA methods performed less well than the MPY and YL-GNOME methods for the extraction of DNA from C. albicans yeast cells, demonstrating that mechanical disruption of the fungal cell wall is not always the optimal extraction approach. We found the FDNA method faster, less prone to cross contamination, and more amenable to high-throughput sample processing than the UCS method. The FDNA method has been studied previously using large inocula of propagules (107 to 108 CFU) from the organisms Candida albicans, Cryptococcus neoformans, Trichosporon beigelii, Aspergillus fumigatus, and Fusarium solani (13). Semiquantitative PCR was employed in the previous study to measure DNA recovery, and the investigators found that the high-speed cell disruption extraction method (FDNA) produced significantly greater yields from the filamentous fungi than from the yeasts, a conclusion that is supported by our study.
The MPY method is designed to extract DNA from yeasts with nonenzymatic lysis and produced good DNA recovery from C. albicans but, in our study, yielded significantly less DNA from Aspergillus conidia and hyphae than methods employing mechanical disruption, such as FDNA and UCS. The MPY method was fast and relatively cheap. Cross contamination is possible when opening sample tubes with lysis solution but can be minimized by changing gloves between samples. The MPY method can practically be performed on 20 samples or fewer in a morning for subsequent PCR the same day. The MPY method has been used by other investigators to extract DNA from filamentous fungi, though some modifications of the protocol were used (7). The utilities of other DNA extraction methods for the detection of fungi have been studied in environmental samples (5) and blood (9).
Aspergillus and Candida DNA were not detected in extraction controls consisting of sterile water processed through each of the six DNA extraction methods compared in this study. However, the consistent detection of contaminating fungal DNA when using a seventh DNA extraction method (QIAMP-S) highlights the importance of testing reagents for fungal contamination in order to avoid false-positive PCR results. Reagents may be sterile and still contain amplifiable microbial DNA. We selected the QIAMP-S method for testing because it employs a matrix that binds PCR inhibitors in stool (InhibitEX tablet) and we suspected that it might bind PCR inhibitors found in mucus, sputum, and BAL fluid. Our testing of multiple tablets from multiple lots showed that there was a high level of fungal DNA contamination of this matrix. Two fungal genus-specific 18S rRNA gene PCR assays were used in this study. Broad-range fungal PCR presents an additional challenge, since contamination may arise from many fungal sources (8). The extraction of fungal DNA from clinical samples is a critical step in the process of detecting and identifying fungal pathogens by PCR. Our results demonstrate that different DNA extraction methods may produce dramatically different yields of fungal DNA. We have identified several methods that are well suited for the recovery of DNA from the human pathogens Candida albicans and Aspergillus fumigatus. Although such evaluations are somewhat subjective, we found the MPY and FDNA methods easiest to use.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases grant R01 AI054703 to D.N.F.
REFERENCES
- 1.Chen, S. C., C. L. Halliday, and W. Meyer. 2002. A review of nucleic acid-based diagnostic tests for systemic mycoses with an emphasis on polymerase chain reaction-based assays. Med. Mycol. 40:333-357. [DOI] [PubMed] [Google Scholar]
- 2.Fredricks, D. N., J. A. Jolley, P. W. Lepp, J. C. Kosek, and D. A. Relman. 2000. Rhinosporidium seeberi: a human pathogen from a novel group of aquatic protistan parasites. Emerg. Infect. Dis. 6:273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glee, P. M., P. J. Russell, J. A. Welsch, J. C. Pratt, and J. E. Cutler. 1987. Methods for DNA extraction from Candida albicans. Anal. Biochem. 164:207-213. [DOI] [PubMed] [Google Scholar]
- 4.Haron, E., S. Vartivarian, E. Anaissie, R. Dekmezian, and G. P. Bodey. 1993. Primary Candida pneumonia. Experience at a large cancer center and review of the literature. Medicine (Baltimore) 72:137-142. [PubMed] [Google Scholar]
- 5.Haugland, R. A., N. Brinkman, and S. J. Vesper. 2002. Evaluation of rapid DNA extraction methods for the quantitative detection of fungi using real-time PCR analysis. J. Microbiol. Methods 50:319-323. [DOI] [PubMed] [Google Scholar]
- 6.Haugland, R. A., J. L. Heckman, and L. J. Wymer. 1999. Evaluation of different methods for the extraction of DNA from fungal conidia by quantitative competitive PCR analysis. J. Microbiol. Methods 37:165-176. [DOI] [PubMed] [Google Scholar]
- 7.Jin, J., Y. K. Lee, and B. L. Wickes. 2004. Simple chemical extraction method for DNA isolation from Aspergillus fumigatus and other Aspergillus species. J. Clin. Microbiol. 42:4293-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loeffler, J., H. Hebart, R. Bialek, L. Hagmeyer, D. Schmidt, F. P. Serey, M. Hartmann, J. Eucker, and H. Einsele. 1999. Contaminations occurring in fungal PCR assays. J. Clin. Microbiol. 37:1200-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Löffler, J., H. Hebart, U. Schumacher, H. Reitze, and H. Einsele. 1997. Comparison of different methods for extraction of DNA of fungal pathogens from cultures and blood. J. Clin. Microbiol. 35:3311-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maaroufi, Y., N. Ahariz, M. Husson, and F. Crokaert. 2004. Comparison of different methods of isolation of DNA of commonly encountered Candida species and its quantitation by using a real-time PCR-based assay. J. Clin. Microbiol. 42:3159-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marr, K. A., R. A. Carter, F. Crippa, A. Wald, and L. Corey. 2002. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34:909-917. [DOI] [PubMed] [Google Scholar]
- 12.Masur, H., P. P. Rosen, and D. Armstrong. 1977. Pulmonary disease caused by Candida species. Am. J. Med. 63:914-925. [DOI] [PubMed] [Google Scholar]
- 13.Muller, F. M., K. E. Werner, M. Kasai, A. Francesconi, S. J. Chanock, and T. J. Walsh. 1998. Rapid extraction of genomic DNA from medically important yeasts and filamentous fungi by high-speed cell disruption. J. Clin. Microbiol. 36:1625-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Burik, J. A., R. W. Schreckhise, T. C. White, R. A. Bowden, and D. Myerson. 1998. Comparison of six extraction techniques for isolation of DNA from filamentous fungi. Med. Mycol. 36:299-303. [PubMed] [Google Scholar]