Abstract
The sporadic emergence of Klebsiella pneumoniae strains resistant to cefepime and cefpirome was observed in Greek hospitals during 1996. Examination of six epidemiologically distinct strains and clones selected in vitro provided indications that resistance is due to the cooperation of decreased outer membrane permeability and hydrolysis of the cephalosporins by SHV-5 β-lactamase, which was produced in large amounts.
Cephalosporin-resistant Klebsiella pneumoniae strains producing extended-spectrum β-lactamases (ESBLs) of the TEM and SHV types have been implicated in nosocomial infections (1, 3, 5, 8, 17). ESBLs are active against most expanded-spectrum cephalosporins, such as ceftazidime (CAZ) and cefotaxime (CTX), but they are unable to hydrolyze cefoxitin (FOX) (7). The SHV-5 β-lactamase is one of the most prevalent ESBLs worldwide (11). In Greek hospitals an enzyme of this type predominates among cephalosporin-resistant K. pneumoniae isolates (8, 13, 17), conferring high-level resistance to CAZ and reduced susceptibility to CTX. The “fourth-generation” cephalosporins (4GCs) cefepime (CPM) and cefpirome (CPO) have been found to be more active than CTX (16; unpublished data). During 1996, resistance to CPM and CPO was occasionally noticed among SHV-5-producing K. pneumoniae strains. The former antibiotic was introduced in Greece in 1995. In the present work we have attempted to analyze the mechanisms that provide resistance to CPM and CPO in these strains. The possible clonal relation of the isolates was investigated by a PCR-based typing technique.
Seven CPM- and CPO-resistant K. pneumoniae strains (MICs, ≥32 μg/ml) which were isolated from patients treated in the intensive care units (ICUs) of three Athens hospitals during 1996 were examined.
The ERIC2-PCR method was used to type the isolates (6). Extracted genomic DNA (100 ng from each strain) was amplified in a final volume of 50 μl containing 20 mM Tris-Cl (pH 8.3), 50 mM KCl, 0.2 mM (each) deoxynucleoside triphosphate, 2 mM MgCl2, 50 pmol of the ERIC2 primer (5′-AAGTAAGTGACTGGGGTGAGCG-3′), and 0.5 U of Taq DNA polymerase (GIBCO-BRL). The PCR products were separated in 1.2% agarose.
A cephalosporin-susceptible strain (strain S7) and a clinical isolate (isolate NS1) with the typical resistance phenotype conferred by SHV-5 were used as controls. Plasmid pSHA2, which encodes a 36-kDa porin protein of K. pneumoniae (10), and plasmid pEL1, which codes for SHV-5 β-lactamase (13), were used to transform the K. pneumoniae strains by electroporation. For the in vitro selection of mutants, strain S7 was plated on nutrient agar containing CPM (0.5 or 1 μg/ml). Susceptibility to β-lactams was evaluated by the Etest (AB Biodisk). Clarified ultrasonic extracts of bacterial cell suspensions were used as β-lactamase preparations. β-Lactamase specific activity was estimated by using nitrocefin (1 U was the amount of enzyme that hydrolyzed 1 nmol of nitrocefin/min/mg of protein at 37°C and pH 7). Isoelectric focusing (IEF) of the β-lactamases was performed in polyacrylamide gels containing ampholytes (pH range, 3.5 to 9.5). The maximum rates of hydrolysis of CPM, CPO, and CTX by the SHV-5 β-lactamase were estimated by UV spectrophotometry (4). The Vmax values were expressed as the values relative to that for cephaloridine, which was set at 100. Outer membrane protein (OMP) preparations were obtained after selective solubilization and removal of cytoplasmic material from sonicated cell suspensions with sodium lauryl sarcosinate (10). The preparations were run on discontinuous polyacrylamide gels and stained with Coomassie brilliant blue.
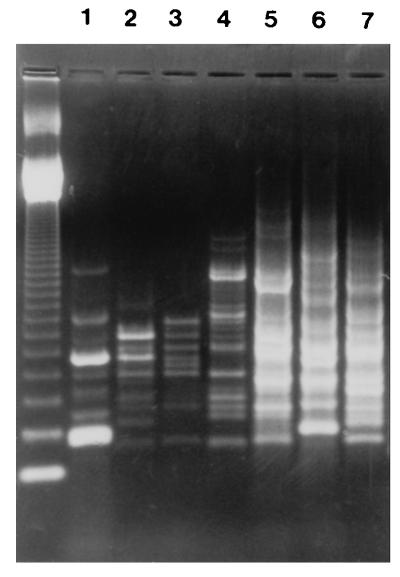
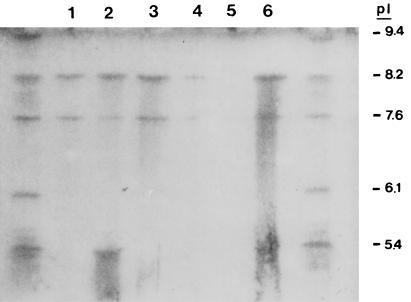
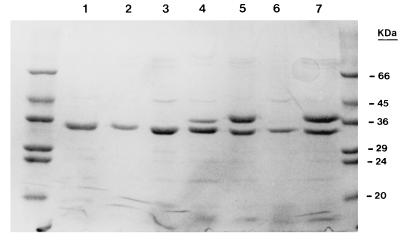
Six different ERIC2-PCR patterns were obtained for the 4GC-resistant isolates of K. pneumoniae. Two isolates from an ICU (isolates RL5 and RL7) displaying similar ERIC2-PCR patterns were considered outbreak isolates, and only one isolate from this pair (isolate RL5) was examined further (Fig. 1). The isolates were resistant to CAZ, CTX, CPM, and CPO. They were also resistant to FOX and the piperacillin-tazobactam combination (Table 1). The strains possessed a β-lactamase with a pI of 8.2. Substrate and inhibition profiles confirmed that the enzyme was SHV-5. Two strains also expressed a second β-lactamase, presumably TEM-1, that focused at 5.4 (Table 1 and Fig. 2). Quantitation of SHV-5 expression showed that the strains produced at least 2.5-fold-higher amounts than strain NS1 (Table 1). Hydrolysis studies showed that SHV-5 hydrolyzed CPM and CPO faster than it hydrolyzed CTX at Vmax. The hydrolysis rates for CPM, CPO, and CTX relative to that for cephaloridine were 42, 60, and 11, respectively. Analysis of the outer membrane profiles revealed that the resistant strains lacked a major OMP of 36 kDa (Fig. 3).
FIG. 1.
ERIC2-PCR patterns of seven 4GC-resistant clinical K. pneumoniae isolates (lanes 1 to 7). Isolates LR5 (lane 5) and LR7 (lane 7) displayed similar patterns. Molecular mass markers are in the leftmost lane.
TABLE 1.
β-Lactam susceptibility, β-lactamase content, and OMP characteristics of clinical and control K. pneumoniae isolates and laboratory clones
| Strain | Source | Etest MIC (μg/ml)
|
β-Lactamase content
|
Presence of a 36-kDa OMPa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CAZ | CTX | FOX | PTZb | CPM | CPO | pI | U | |||
| RL1 | ICU 1, urine | >128 | >128 | >128 | >64 | 48 | 64 | 8.2 and 5.4 | 1,125 | − |
| RL2 | ICU 1, sputum | >128 | >128 | >128 | >64 | 64 | 96 | 8.2 | 1,832 | − |
| RL3 | ICU 1, blood | >128 | 96 | >128 | >64 | 32 | 48 | 8.2 and 5.4 | 1,617 | − |
| RL4 | ICU 2, urine | >128 | >128 | >128 | >64 | 32 | 64 | 8.2 | 980 | − |
| RL5 | ICU 3, sputum | >128 | 128 | >128 | >64 | 32 | 64 | 8.2 | 1,320 | − |
| RL6 | ICU 3, sputum | >128 | >128 | >128 | >64 | 32 | 96 | 8.2 | 1,012 | − |
| RL7 | ICU 3, sputum | >128 | 128 | >128 | >64 | 32 | 64 | 8.2 | NTc | NT |
| NS1 | 64 | 8 | 3 | 16 | 2 | 4 | 8.2 | 356 | + | |
| S7 | 0.4 | 0.05 | 4 | 2 | 0.03 | 0.1 | <20 | + | ||
| S7-M | 2 | 2 | 128 | 4 | 1.5 | 2 | <20 | − | ||
| S7(pEL1) | >128 | 64 | 4 | >64 | 12 | 16 | 8.2 | 1,806 | + | |
| S7-M(pEL1) | >128 | 96 | 128 | >64 | 64 | 96 | 8.2 | 1,693 | − | |
| S7-M(pEL1-pSHA2) | >128 | 32 | 16 | >64 | 8 | 8 | 8.2 | 1,470 | + | |
+ and −, presence and absence of a 36-kDa OMP, respectively.
PTZ, piperacillin-tazobactam (tazobactam was used at a fixed concentration of 4 μg/ml).
NT, not tested.
FIG. 2.
IEF of β-lactamase preparations of K. pneumoniae strains. Lanes: 1, RL1; 2, RL4; 3, RL5; 4, NS1; 5, S7; and 6, S7(pEL1). Control β-lactamases are on both sides of the gel. Their pIs are indicated on the right.
FIG. 3.
SDS-PAGE of OMP preparations of K. pneumoniae strains. Lanes: 1, RL1; 2, RL4; 3, RL5; 4, NS1; 5, S7; 6, S7-M; and 7, S7-M(pEL1-pSHA2). The molecular masses of the control proteins are indicated on the right.
In vitro mutants were obtained at a frequency of 10−8 after plating strain S7 on medium containing CPM (1 μg/ml). The MICs of the β-lactams for the representative mutant clone S7-M were found to be elevated. The MICs of CPM, CTX, and FOX were affected to a greater extent compared with those of the other β-lactams (Table 1). The β-lactam MICs, however, were below the breakpoints except for that of FOX. The MIC of FOX was 128 μg/ml. Electrophoresis of the OMPs of S7-M showed that this clone did not express detectable quantities of the 36-kDa protein (Fig. 3). Plasmid pEL1 was used to transform S7 and S7-M. An increase in the MICs of all β-lactams examined except FOX was observed for the transformants S7(pEL1) and S7-M(pEL1). The quantities of β-lactamase produced by the latter clones were similar to those produced by the 4GC-resistant clinical strains (Table 1). The β-lactam resistance phenotype of S7-M(pEL1) resembled those of the 4GC-resistant strains. Clone S7-M(pEL1) was transformed with plasmid pSHA2 encoding a previously characterized K. pneumoniae porin protein (10). Plasmid pSHA2 is a pACYC84 derivative with an origin of replication from plasmid p15A which allows for coexistence with pEL1, a ColE1 plasmid. Expression of the cloned porin in S7-M(pEL1-pSHA2) was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of the OMP preparations (Fig. 3). The MICs of all β-lactams except CAZ were reduced; the MIC of CAZ remained above 128 μg/ml. The most pronounced reductions were observed for CPO and FOX. Introduction of the second plasmid did not influence β-lactamase production significantly (Table 1).
Typing by ERIC2-PCR showed that different K. pneumoniae clones resistant to 4GCs have emerged independently in Athens hospitals. The appearance of such strains is indicative of changes in the selective pressure exerted by β-lactams and coincided with the introduction of novel antibiotics, such as CPM, in our clinical setting. Notably, all strains were collected from patients in ICUs, where CPM is most commonly used.
The efficacies of 4GCs against infections caused by enterobacteria producing ESBLs have not been fully assessed (9, 15). CPM and CPO appear to be labile to the activities of various TEM and SHV derivatives (15). As shown here, the rates of hydrolysis of CPM and CPO by SHV-5 are higher than the rate observed for CTX. The higher levels of activity of the former antibiotics can be attributed to their high rates of penetration through the cell walls of gram-negative organisms (12). Increased MICs of CPM and CPO are expected in variants overexpressing SHV-5. Examination of the clinical strains and the laboratory clones of K. pneumoniae showed that up-regulation of SHV-5 expression is the main mutational event leading to decreased susceptibility to 4GCs. However, overexpression of the enzyme at the levels observed here is not adequate to provide resistance above the breakpoints, and a concomitant decrease in outer membrane permeability is required. The loss of the 36-kDa OMP alone caused a marginal increase in the MICs of CPM and CPO. This information indicates that the resistance phenotype of the clinical isolates is due to the synergistic activity of enhanced hydrolysis and decreased penetration rates of CPM and CPO. This double requirement may account for the low frequency of occurrence of K. pneumoniae isolates resistant to 4GCs. A similar combination has been found to cause decreased susceptibility to carbapenems in K. pneumoniae (2).
It is not known whether the resistant isolates described here have been selected by CPM. Overproduction of SHV-5 affects most expanded-spectrum cephalosporins and penicillin-inhibitor combinations (3). Moreover, decreased permeability affects virtually all β-lactams (10), suggesting that the isolates may have been selected by antibiotics other than 4GCs. Whatever the selective agents were, the emergence of such strains indicates that 4GCs must be used cautiously in hospitals where the prevalence of ESBL-producing enterobacteria is high. Changes in the MICs of 4GCs, resulting from either elevated production of an ESBL or decreased permeability, may pass unnoticed, particularly when automatic systems that use a limited number of antibiotic dilutions around the breakpoint are used. Apart from the possibility of the selection of resistant variants, there is an additional concern regarding the use of 4GCs against SHV-5 β-lactamase-producing K. pneumoniae. As has been shown for other hydrolyzable cephalosporins, the presence of an ESBL may lead to treatment failures even when the microorganism appears to be susceptible by the standard in vitro tests. This has been attributed to the large amounts of β-lactamase at sites of infection, where the number of bacteria is high, and is analogous to the inoculum effect observed in vitro (14). We propose that CPM and CPO not be used against ceftazidime-resistant K. pneumoniae isolates.
Acknowledgments
We are grateful to C. A. Owen for helpful suggestions and L. Martinez-Martinez for providing plasmid pSHA2.
REFERENCES
- 1.Bingen E H, Desjardins P, Arlet G, Bourgeois F, Mariani-Kurkdjian P, Lambert-Zechovsky N Y, Denamur E, Philippon A, Elion J. Molecular epidemiology of plasmid spread among extended broad-spectrum β-lactamase-producing Klebsiella pneumoniae isolates in a pediatric hospital. J Clin Microbiol. 1993;31:179–184. doi: 10.1128/jcm.31.2.179-184.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford P A, Urban C, Mariano N, Projan S J, Rahal J J, Bush K. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the loss of an outer membrane protein. Antimicrob Agents Chemother. 1997;41:563–569. doi: 10.1128/aac.41.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.French G L, Shannon K P, Simmons N. Hospital outbreak of Klebsiella pneumoniae resistant to broad-spectrum cephalosporins and β-lactam–β-lactamase inhibitor combinations by hyperproduction of SHV-5 β-lactamase. J Clin Microbiol. 1996;34:358–363. doi: 10.1128/jcm.34.2.358-363.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gazouli M, Tzouvelekis L S, Prinarakis E, Miriagou V, Tzelepi E. Transferable cefoxitin resistance in enterobacteria from Greek hospitals and characterization of a plasmid-mediated group 1 β-lactamase (LAT-2) Antimicrob Agents Chemother. 1996;40:1736–1740. doi: 10.1128/aac.40.7.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gouby A, Neuwirth C, Bourg G, Bouziges N, Carles-Nurit M J, Despaux E, Ramuz M. Epidemiological study by pulsed-field gel electrophoresis of an outbreak of extended-spectrum β-lactamase-producing Klebsiella pneumoniae in a geriatric hospital. J Clin Microbiol. 1994;32:301–305. doi: 10.1128/jcm.32.2.301-305.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hulton C S, Higgins C F, Sharp P M. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enterobacteria. Mol Microbiol. 1991;5:825–834. doi: 10.1111/j.1365-2958.1991.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 7.Jacoby G A, Medeiros A A. More extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1991;35:1697–1704. doi: 10.1128/aac.35.9.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Legakis N J, Tzouvelekis L S, Hatzoudis G, Tzelepi E, Gourkou A, Pitt T L, Vatopoulos A C. Klebsiella pneumoniae in Greek hospitals. Dissemination of plasmids coding an SHV-5 type β-lactamase. J Hosp Infect. 1995;31:177–187. doi: 10.1016/0195-6701(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 9.Livermore D M. β-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995;8:557–584. doi: 10.1128/cmr.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Martinez L, Hernandez-Alles S, Alberti S, Tomas J M, Benedi V J, Jacoby G A. In vivo selection of porin-deficient mutants of Klebsiella pneumoniae with increased resistance to cefoxitin and expanded-spectrum cephalosporins. Antimicrob Agents Chemother. 1996;40:342–348. doi: 10.1128/aac.40.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medeiros, A. A. 1997. Evolution and dissemination of β-lactamases accelerated by generations of β-lactam antibiotics. Clin. Infect. Dis. 24(Suppl. 1):19–45. [DOI] [PubMed]
- 12.Nikaido H, Liu W, Rosenberg E Y. Outer membrane permeability and β-lactamase stability of dipolar ionic cephalosporins containing methoxyimino substituents. Antimicrob Agents Chemother. 1990;18:337–342. doi: 10.1128/aac.34.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prinarakis E E, Tzelepi E, Gazouli M, Mentis A F, Tzouvelekis L S. Characterization of a novel SHV β-lactamase variant that resembles the SHV-5 enzyme. FEMS Microbiol Lett. 1996;139:229–234. doi: 10.1111/j.1574-6968.1996.tb08207.x. [DOI] [PubMed] [Google Scholar]
- 14.Rice L B, Yao J D C, Klimm K, Eliopoulos G M, Moellering R C., Jr Efficacy of different β-lactams against an extended-spectrum β-lactamase-producing Klebsiella pneumoniae strain in the rat intra-abdominal abscess model. Antimicrob Agents Chemother. 1991;35:1243–1244. doi: 10.1128/aac.35.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanders, C. C. 1996. In vitro activity of fourth generation cephalosporins against Enterobacteriaceae producing extended-spectrum β-lactamases. J. Chemother. 8(Suppl. 2):57–62. [PubMed]
- 16.Tzouvelekis L S, Tzelepi E, Mentis A F, Legakis N J. In vitro activity of cefpirome against selected clinical enterobacterial isolates with β-lactamase-mediated resistance. Infection. 1995;23:384–388. doi: 10.1007/BF01713572. [DOI] [PubMed] [Google Scholar]
- 17.Vatopoulos A C, Philippon A, Tzouvelekis L S, Komninou Z, Legakis N J. Prevalence of a transferable SHV-5 type β-lactamase in clinical isolates of Klebsiella pneumonia and Escherichia coli in Greece. J Antimicrob Chemother. 1990;26:635–648. doi: 10.1093/jac/26.5.635. [DOI] [PubMed] [Google Scholar]