Abstract
Sequence analysis of an 850-bp fragment internal to the aspecific drug efflux gene adeB revealed 11 sequence types (STs) among a collection of 50 multidrug-resistant Acinetobacter baumannii (MDRAB) strains, including members of pan-European clones I, II, and III. The delineation of STs conformed with the intraspecific grouping of these strains previously determined by different DNA fingerprinting methods. Larger strain collections need to be screened to further explore the potential of sequence-based adeB typing as a universal tool for the monitoring of MDRAB clones.
Acinetobacter baumannii is one of the most frequently isolated nonfermentative gram-negative species from critically ill and immunocompromised patients among intensive care units patients worldwide. Strains of this opportunistic pathogen can be involved in a range of nosocomial infections, such as ventilator-associated pneumonia, bloodstream infections, and meningitis (1), and are acquiring resistance to multiple antibiotics at an increasing rate (4, 9). A. baumannii infections can occur as sporadic cases associated with single genotypes but can also give rise to epidemic outbreaks caused by genotypically highly related isolates, such as those belonging to the pan-European (pE) multidrug-resistant A. baumannii (MDRAB) clones I, II, and III (3, 18). Thus far, the intraspecific differentiation of MDRAB has mainly relied on the single or combined use of DNA fingerprinting methods, including ribotyping, amplified fragment length polymorphism (AFLP) analysis, pulsed-field gel electrophoresis (PFGE) of macrorestriction fragments, and repetitive DNA element PCR (rep-PCR) (2, 3, 7, 8, 13, 18). Apart from differences in genotypic resolution, labor intensity, and reproducibility, all these techniques share the major drawback that interlaboratory comparison remains problematic because fingerprinting data are generally not portable. DNA sequence-based methods, on the other hand, facilitate unambiguous and global comparisons of the findings between different laboratories and are expected to outcompete banding pattern-based methods in long-term epidemiological studies (17). As opposed to other human pathogens, however, the search for genes that can be used in single or multilocus sequence typing (MLST; http://www.mlst.net) at the intraspecific or strain level is ongoing for A. baumannii. In previous papers (7, 8, 15), we reported that members of pan-European MDRAB clones I, II, and III as well as a number of genotypically unrelated MDRAB strains all shared the aspecific drug efflux gene adeB, together with various combinations of genes that specifically confer resistance to aminoglycosides (AGs) and tetracyclines. The adeB gene makes up part of the adeABC gene cluster, which encodes a resistance-nodulation-cell division-type three-component efflux system that was recently described for A. baumannii strain BM4454 (10). In this strain, adeABC conferred low-level resistance to several aminoglycosides and reduced susceptibilities to various other antimicrobial agents. Triggered by the observation that the adeB gene was present in a set of genotypically related and unrelated MDRAB strains from different geographical origins and time periods, the current study set out to investigate whether adeB is a suitable locus for sequence-based identification of intraspecific groups among MDRAB strains.
For the purpose of this study, 50 well-documented MDRAB strains mostly from European hospitals were selected from previous studies in which they were typed by one or multiple DNA fingerprinting methods and in which it was shown that they contained the adeB gene (Table 1). This selection included members of pE clones I, II, and III but also clinical strains from the Czech Republic previously allocated to three ribotypes, five genotypically unrelated clinical strains, and veterinary isolate LMG 22458. Most of the MDRAB selected strains were resistant to one or multiple aminoglycosides (Table 1), to several fluoroquinolones (18), and to tetracycline (7, 8). Susceptibility to aminoglycosides was determined by the disk diffusion method with BBL Mueller-Hinton II agar (Becton Dickinson and Company), according to the recommendations of CLSI (formerly NCCLS) (12), by using the following disks (Oxoid Ltd., Basingstoke, United Kingdom): kanamycin (30 μg), gentamicin (10 μg), tobramycin (10 μg), amikacin (30 μg), and netilmicin (30 μg). Total genomic DNA was prepared by using a protocol based on the method of Pitcher and coworkers (16). For adeB detection, a common PCR core mix (total volume, 50 μl) was used that consisted of 1× PCR buffer (Applied Biosystems, Warrington, United Kingdom), deoxynucleoside triphosphates (dNTPs; Applied Biosystems) at a concentration of 200 μM of each dNTP, 1 U of AmpliTaq DNA polymerase (Applied Biosystems), and 20 pmol of each primer (Sigma-Genosys Ltd., Cambridgeshire, United Kingdom). A 50-ng portion of intact total DNA was used as the PCR template. Detection and partial sequence analysis of adeB were performed with the previously published PCR primer pair O3 (5′-GTATGAATTGATGCTGC-3′) and O4 (5′-CACTCGTAGCCAATACC-3′) that targets a 979-bp fragment internal to this gene in A. baumannii strain BM4454, which was used as a positive control in PCR (10). PCR amplifications were performed in a GeneAmp 9600 PCR system (Perkin-Elmer) by using the following temperature program: initial denaturation at 94°C for 5 min; 25 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 1 min, and extension at 72°C for 1 min; and a final extension at 72°C for 7 min. Partial sequencing of adeB positions 1635 to 2484 was performed by using the BigDye Terminator (version 3.1) ready reaction cycle sequencing kit on an ABI Prism 3100 genetic analyzer (Applied Biosystems). As a control, the sequence of the internal adeB fragment of A. baumannii strain BM4454 (EMBL accession no. AF370885) (10) was redetermined. Sequence alignments and comparisons were performed by using BioNumerics software (version 3.5; Applied Maths, St.-Martens Latem, Belgium) and the BioEditor program (5).
TABLE 1.
Typing and AG resistotype data for 50 multidrug-resistant A. baumannii strainsd
| Original strain no. | LMG deposit no. | Geographical origin | Yr of isolation | Formerly typed as: | Typing method(s) used [reference(s)] | adeB ST | AG resistotype |
|---|---|---|---|---|---|---|---|
| UZG S91 01483 | Gent, B | 1991 | pE clone I | (GTG)5-PCR (8) | I | K-Ga | |
| RUH 3247 | LMG 22453 | Leuven, B | 1990 | pE clone I | AFLP (3,13), ribotyping (3, 13), (GTG)5-PCR (8) | I | K-G-N-Tb |
| NIPH 470 | C̆. Budĕjovice, CR | 1997 | pE clone I | AFLP (13), ribotyping (13), (GTG)5-PCR (8) | I | K-N-Ab | |
| NIPH 7 | LMG 22454 | Prague, CR | 1991 | pE clone I | AFLP (13), ribotyping (13), (GTG)5-PCR (8) | I | K-G-Ab |
| NIPH 290 | Pr̆íbram, CR | 1994 | pE clone I | AFLP (13), ribotyping (13), (GTG)5-PCR (8) | I | K-G-N-T-Ab | |
| NIPH 1605 | Sedlc̆any, CR | 2001 | pE clone I | AFLP (13), ribotyping (13), (GTG)5-PCR (8) | I | K-G-Tb | |
| RUH 436 | LMG 10539 | Utrecht, NL | 1984 | pE clone I | AFLP (3, 13), ribotyping (3, 13), (GTG)5-PCR (8) | I | K-Gb |
| RUH 2037 | Venlo, NL | 1986 | pE clone I | AFLP (3, 13), ribotyping (3, 13), (GTG)5-PCR (8) | I | K-Gb | |
| RUH 875 | LMG 10543 | Dordrecht, NL | 1984 | pE clone Ic | AFLP (3, 13), ribotyping (3, 13), (GTG)5-PCR (8) | I | K-G-Tb |
| RUH 510 | LMG 10523 | Nijmegen, NL | 1984 | pE clone I | AFLP (3, 13), ribotyping (3, 13), (GTG)5-PCR (8) | I | K-Gb |
| RUH 3242 | Basildon, UK | 1989 | pE clone I | AFLP (3, 13), ribotyping (3, 13), (GTG)5-PCR (8) | I | K-Gb | |
| RUH 3239 | London, UK | 1980s | pE clone I | AFLP (3, 13), ribotyping (3, 13), (GTG)5-PCR (8) | I | K-Gb | |
| RUH 3282 | Salford, UK | 1990 | pE clone I | AFLP (3, 13), ribotyping (3, 13), (GTG)5-PCR (8) | I | K-G-N-Ab | |
| RUH 3238 | Sheffield, UK | 1987 | pE clone I | AFLP (3, 13), ribotyping (3, 13), (GTG)5-PCR (8) | I | K-Gb | |
| HPA A2166 | Hospital A, UK | 2000 | pE clone I | (GTG)5-PCR (8) | I | Ka | |
| HPA A0661 | LMG 22456 | Hospital J, UK | 2000 | pE clone I | (GTG)5-PCR (8) | I | K-G-Aa |
| DGK 4982 | LMG 22458 | Gent, B | 1998 | pE clone II | (GTG)5-PCR (8) | II | K-Ga |
| UZG POR 03034 | Gent, B | Unknown | pE clone II | (GTG)5-PCR (8) | II | Ka | |
| NIPH 330 | Tábor, CR | 1994 | pE clone II | AFLP (13), ribotyping (13), (GTG)5-PCR (8) | II | K-Gb | |
| NIPH 455 | Jihlava, CR | 1996 | pE clone II | AFLP (13), ribotyping (13), (GTG)5-PCR (8) | II | K-G-Ab | |
| NIPH 1469 | Prague, CR | 2001 | pE clone II | AFLP (13), ribotyping (13), (GTG)5-PCR (8) | II | K-Ab | |
| NIPH 24 | LMG 22457 | Prague, CR | 1991 | pE clone II | AFLP (13), ribotyping (13), (GTG)5-PCR (8) | II | K-Gb |
| RUH 3422 | Odense, DK | 1984 | pE clone II | AFLP (3, 13), ribotyping (3, 13), (GTG)5-PCR (8) | II | Kb | |
| BM4454 | Paris, F | 1999 | pE clone II | (GTG)5-PCR (8) | II | —a | |
| RUH 134 | LMG 10541 | Rotterdam, NL | 1982 | pE clone IIc | AFLP (3, 13), ribotyping (3, 13), (GTG)5-PCR (8) | II | K-Gb |
| RUH 3240 | Newcastle, UK | 1989 | pE clone II | AFLP (3, 13), ribotyping (3, 13), (GTG)5-PCR (8) | II | G-Nb | |
| RUH 3245 | Salisbury, UK | 1989 | pE clone II | AFLP (3, 13), ribotyping (3, 13), (GTG)5-PCR (8) | II | G-Nb | |
| HPA A2161 | Hospital A, UK | 2000 | pE clone II | (GTG)5-PCR (8) | II | K-G-N-Aa | |
| HPA A0372 | LMG 22460 | Hospital H, UK | 2000 | pE clone II-like | (GTG)5-PCR (8) | II | K-G-N-Aa |
| UZG S91 00973 | Gent, B | 1991 | pE clone III | (GTG)5-PCR (7) | III | K-G-Aa | |
| UZG S91 02796 | LMG 22452 | Gent, B | 1991 | pE clone III | (GTG)5-PCR (7) | III | K-G-Aa |
| UZG F93 03448 | LMG 22863 | Gent, B | 1993 | pE clone III | (GTG)5-PCR (7) | III | K-G-Aa |
| 06A201 | Lille, F | 1997 | pE clone III | AFLP (14,18), ribotyping (14), (GTG)5-PCR (7) | III | K-G-T-Ab | |
| 04C048 | Paris, F | 1997 | pE clone III | AFLP (14,18), ribotyping (14), (GTG)5-PCR (7) | III | K-G-T-Ab | |
| 10C070 | Genova, I | 1998 | pE clone III | AFLP (18), (GTG)5-PCR (7) | III | K-G-T-Aa | |
| 12A133 | Utrecht, NL | 1997 | pE clone III | AFLP (14,18), ribotyping (14), (GTG)5-PCR (7) | III | K-G-T-Ab | |
| 18D047 | Barcelona, S | 1997 | pE clone III | AFLP (18), (GTG)5-PCR (7) | III | —a | |
| 17C085 | Madrid, S | 1998 | pE clone III | AFLP (14,18), ribotyping (14), (GTG)5-PCR (7) | III | K-G-T-Ab | |
| 16D083 | Sevilla, S | 1997 | pE clone III | AFLP (18), (GTG)5-PCR (7) | III | K-G-T-Aa | |
| NIPH 1717 | Prague, CR | 2001 | Ribotype R2-6 | AFLP (13), ribotyping (13), (GTG)5-PCR (8) | IV | Kb | |
| NIPH 301 | LMG 22459 | Slaný, CR | 1994 | Ribotype R2-7 | AFLP (13), ribotyping (13), (GTG)5-PCR (8) | IV | K-G-Nb |
| NIPH 335 | Tábor, CR | 1994 | Ribotype R21-16 | AFLP (13), ribotyping (13), (GTG)5-PCR (8) | V | K-G-Tb | |
| NIPH 1445 | Plzen̆, CR | 2000 | Ribotype R21-16 | AFLP (13), ribotyping (13) | V | K-G-Tb | |
| NIPH 1497 | Prague, CR | 2001 | Ribotype R23-19 | AFLP (13), ribotyping (13) | VI | K-G-N-Tb | |
| NIPH 1683 | Prague, CR | 2001 | Ribotype R23-19 | AFLP (13), ribotyping (13) | VI | K-Gb | |
| HPA A1100 | Hospital G, UK | 2000 | Ungrouped | (GTG)5-PCR (8) | VII | K-Aa | |
| HPA A1392 | LMG 22461 | Hospital I, UK | 2000 | Ungrouped | (GTG)5-PCR (8) | VIII | —a |
| AC 658 | São Paulo, BR | 1997 | Ungrouped | (GTG)5-PCR (8) | IX | K-G-A-Na | |
| NIPH 1734 | Mladá Boleslav, CR | 2001 | Ribotype R24-20 | AFLP (13), ribotyping (13) | X | K-G-N-T-Ab | |
| NIPH 47 | Prague, CR | 1991 | Ribotype R8-1 | AFLP (13), ribotyping (13), (GTG)5-PCR (8) | XI | K-G-Tb |
Data from this study.
Data from reference 14.
pE clone reference strain proposed by Dijkshoom et al. (3).
Abbreviations: B; Belgium; BR, Brazil; CR, Czech Republic; DK; Denmark; F, France; I, Italy; NL, The Netherlands; S, Spain; DGK, Faculteit Diergeneeskunde, Ghent University, Ghent, Belgium; HPA, Health Protection Agency, London, United Kingdom; LMG, BCCM/LMG Bacteria Collection, Ghent University, Ghent, Belgium; NIPH, Collection of A. Nemec, National Institute of Public Health, Prague, Czech Republic RUH, Collection of L. Dijkshoom, Leiden University Medical Center, Leiden, The Netherlands; UZG, Universitair Ziekenhuis Gent, Ghent, Belgium; —, no resistance to the five AGs tested.
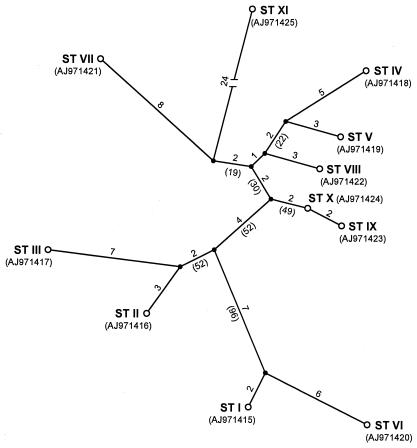
In the present study, the previously designed primer pair O3-O4 (10) was used for sequence analysis of an internal region of the adeB gene in order to investigate whether this gene contains polymorphic sites that are potentially useful for sequence-based identification of intraspecific groups in MDRAB. As a result of this partial sequencing approach, 11 different adeB sequence types (STs; STs I to XI) could be defined (Fig. 1) among a collection of 50 MDRAB strains representing six previously delineated intraspecific groups and five genotypically unrelated strains. By definition, an adeB gene was considered to represent a distinct ST when its 850-bp partial sequence differed in at least one position from all other sequences. STs that were shared by two or more strains (i.e., STs I to VI) all displayed a complete internal sequence identity, whereas the number of base conversions between individual STs ranged from 2 to 45 (Fig. 1). Although the majority of these conversions were found to represent silent mutations, a number of substitutions resulted in amino acid sequence polymorphisms (Table 2). The polymorphic sites at positions 551, 584, 606, and 730 may be of diagnostic value for discrimination of pE clones I, II, and III; but clearly, more strains of these intraspecific MDRAB groups need to be investigated to verify this finding. The partially redetermined nucleotide sequence of the adeB gene of strain BM4454 (10) differed from the original sequence (EMBL accession no. AF370885) at two positions, i.e., positions 1974 and 2295, where in both cases the sequence representing ST II (EMBL accession no. AJ971416) contained an A instead of a G.
FIG. 1.
Unrooted maximum-parsimony tree of multiple aligned partial adeB sequences representing 11 STs, indicated by capital Roman letters I to XI followed by the EMBL accession number in parentheses. The number of base conversions over the tree is indicated along the phylogenetic distance lines, and bootstrap percentages are indicated in parentheses for analysis of 100 replicates.
TABLE 2.
Polymorphic amino acid positions for 11 adeB STs in A. baumannii
| adeB ST | Amino acid at the following polymorphic amino acid positiona:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 551 | 584 | 606 | 642 | 645 | 660 | 730 | 768 | |
| I | A | T | A | D | S | P | L | A |
| II | T | T | T | D | S | P | F | A |
| III | A | I | T | D | S | P | L | A |
| IV | A | T | T | A | T | P | L | A |
| V | A | T | T | A | T | Q | L | A |
| VI | A | T | T | D | S | P | L | A |
| VII | A | T | T | D | T | P | L | T |
| VIII | A | T | T | A | T | P | L | A |
| IX | A | T | T | A | T | P | L | A |
| X | A | T | T | A | T | P | L | A |
| XI | A | T | T | D | T | P | L | A |
Aligned nucleotide sequences of partial adeB fragments (positions 1635 to 2484 according to the numbering of the adeB sequence with EMBL accession no. AF370885) were translated into amino acid sequences by using a polymorphism statistics tool in Bionumerics. Polymorphic amino acid positions were identified with the BioEdit sequence alignment editor.
The delineation of the 11 adeB STs completely agreed with the intraspecific diversity among the 50 MDRAB strains previously assessed by DNA fingerprinting methods such as ribotyping, AFLP analysis, PFGE, and/or rep-PCR. The most remarkable finding was that all members of pE clones I (n = 16), II (n = 13), and III (n = 10) belonged to the same adeB ST, i.e., STs I, II, and III, respectively (Table 1). The strains of pE clones I, II, and III included for this study were selected in a way that they represented hospital units from four to six different European countries and that they were isolated at different time points during the past 20 years. The fact that members of a given pE clone with different geographical and/or temporal histories all shared the same adeB ST thus indicates that sequence-based typing of this gene may be a potential tool for the quick identification of new members of these widespread clones. As evidenced by the data obtained with the Czech MDRAB strains previously allocated to HindIII-HincII ribotyping groups R21-16 and R23-19 and, accordingly, also grouped in two AFLP clusters (13) (Table 1), partial adeB sequencing may also have the potential to identify less widespread intraspecific MDRAB groups. The two representative strains of both groups displayed an identical adeB ST; and the two corresponding STs, STs V and VI, appeared to be unique and clearly distinct from STs I to III (Fig. 1). Interestingly, strains NIPH 1717 and NIPH 301, which were previously assigned to the highly related ribotypes R2-6 and R2-7 and that grouped in the same AFLP cluster (13), were also joined together by adeB typing as members of ST IV (Table 1). The finding that the five genotypically unrelated MDRAB strains from the Czech Republic, the United Kingdom, and Brazil (Table 1) each exhibited a unique adeB ST may indicate that these strains represent distinct intraspecific lineages in A. baumannii (Fig. 1); but further evidence, e.g., evidence based on MLST, is awaited to substantiate this. The fact that strains AC 658 and NIPH 1734, which represent the closely related adeB STs IX and X (Fig. 1), respectively, also displayed highly similar but not identical (GTG)5-PCR band patterns (G. Huys, unpublished data) again illustrates the good overall agreement found between the adeB sequence-based grouping and the intraspecific grouping based on DNA fingerprinting. On the other hand, our sequencing data also indicate that the 850-bp region of the adeB gene analyzed in this study is probably not variable enough to discriminate MDRAB strains at the subclonal level. For example, clone II strains NIPH 1469 and RUH 3240 shared a ribotype (ribotype R4-2) slightly different from the other clone II ribotypes and grouped in a distinct AFLP subcluster of clone II (13), and yet, both strains belonged to ST II (Table 1). Possibly, sequencing of longer or multiple fragments of adeB or other components of the adeABC gene cluster may increase the resolution of the method.
At present, little is known about the distribution and evolutionary history of the adeB gene in A. baumannii. So far, the gene has mainly been detected in A. baumannii outbreak strains (6, 7, 8) that exhibit resistance to multiple drugs, usually including one or more AGs. Recently, it was found that adeB can be up-regulated in MDRAB (6) and that it can also occur in drug-susceptible A. baumannii strains (15), in which the gene may be cryptic due to the regulatory control by the two-component AdeRS system (11). Because of this stringent control, it is difficult to predict the presence of adeB in A. baumannii based on the AG resistance profile. In addition, many European MDRAB strains are known to carry one or several specific AG resistance genes (14) that can potentially reinforce the AG resistance spectrum encoded by adeB. This effect is highly pronounced for pE clones I and II, in which the AG resistotypes differed significantly among adeB-positive strains (Table 1). In the course of a PCR-based screening survey for adeB in 32 environmental A. baumannii isolates of aquatic and terrestrial origins, it was found that none of the strains investigated harbored the efflux gene (Huys, unpublished). Provided that the use of additional (degenerated) primer sets confirm these findings, this suggests that the gene is probably not omnipresent in A. baumannii and was acquired by a number of strains from an exogenous source at some point in time. For this reason, it is expected that adeB typing will primarily be useful for clinical isolates of this species.
Based on partial sequencing analysis, the present study has demonstrated that the delineation of adeB STs among genotypically related and unrelated MDRAB strains corroborated extremely well the genotypic clustering of these strains previously obtained with various banding pattern-based methods. However, it is clear that more extended collections of clinical and environmental isolates need to be investigated in order to obtain better insight into the distribution of the adeB gene among A. baumannii strains and to further validate the use of adeB typing for the rapid identification of known intraspecific groups or the delineation of new intraspecific lineages in this species. When it is integrated in a dynamic Internet-based platform, sequence-based typing of adeB could prove to be a useful tool for worldwide monitoring of MDRAB clones.
Nucleotide sequence accession numbers.
A selection of the sequences representing the 11 adeB sequence types reported in this study have been submitted to EMBL under accession numbers AJ971415 to AJ971425 (Fig. 1).
Acknowledgments
G.H. is a postdoctoral fellow of the Fund for Scientific Research-Flanders (Belgium) (F.W.O.-Vlaanderen). A.N. was supported by grant 8554-3 of the Internal Grant Agency of the Ministry of Health of the Czech Republic.
We thank L. Dijkshoorn, N. Woodford, M. Vaneechoutte, S. Brisse, and V. Magalhães for the kind gifts of strains. P. Dawyndt is acknowledged for excellent assistance in sequence data processing.
REFERENCES
- 1.Bergogne-Bérézin, E., and K. J. Towner. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bou, G., G. Cervero, M. A. Dominguez, C. Quereda, and J. Martinez-Beltran. 2000. PCR-based DNA fingerprinting (REP-PCR, AP-PCR) and pulsed-field gel electrophoresis characterization of a nosocomial outbreak caused by imipenem- and meropenem-resistant Acinetobacter baumannii. Clin. Microbiol. Infect. 6:635-643. [DOI] [PubMed] [Google Scholar]
- 3.Dijkshoorn, L., H. Aucken, P. Gerner-Smidt, P. Janssen, M. E. Kaufmann, J. Garaizar, J. Ursing, and T. L. Pitt. 1996. Comparison of outbreak and nonoutbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J. Clin. Microbiol. 34:1519-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gales, A. C., R. N. Jones, K. R. Forward, J. Linares, H. S. Sader, and J. Verhoef. 2001. Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: geographic patterns, epidemiological features, and trends in the SENTRY Antimicrobial Surveillance Program (1997-1999). Clin. Infect. Dis. 32(Suppl. 2):S104-S113. [DOI] [PubMed] [Google Scholar]
- 5.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 6.Higgins, P. G., H. Wisplighoff, D. Stefanik, and H. Seifert. 2004. Selection of topoisomerase mutations and overexpression of adeB mRNA transcripts during an outbreak of Acinetobacter baumannii. J. Antimicrob. Chemother. 54:821-823. [DOI] [PubMed] [Google Scholar]
- 7.Huys, G., M. Cnockaert, A. Nemec, L. Dijkshoorn, S. Brisse, M. Vaneechoutte, and J. Swings. 2005. Repetitive-DNA-element PCR fingerprinting and antibiotic resistance of pan-European multi-resistant Acinetobacter baumannii clone III strains. J. Med. Microbiol. 54:851-856. [DOI] [PubMed] [Google Scholar]
- 8.Huys, G., M. Cnockaert, M. Vaneechoutte, N. Woodford, A. Nemec, L. Dijkshoorn, and J. Swings. 2005. Distribution of tetracycline resistance genes in genotypically related and unrelated multi-resistant Acinetobacter baumannii strains from different European hospitals. Res. Microbiol. 156:348-355. [DOI] [PubMed] [Google Scholar]
- 9.Karlowsky, J. A., D. C. Draghi, M. E. Jones, C. Thornsberry, I. R. Friedland, and D. F. Sahm. 2003. Surveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2001. Antimicrob. Agents Chemother. 47:1681-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magnet, S., P. Courvalin, and T. Lambert. 2001. Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob. Agents Chemother. 45:3375-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchand, I., L. Damier-Piolle, P. Courvalin, and T. Lambert. 2004. Expression of the RNA-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob. Agents Chemother. 48:3298-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial disk susceptibility testing. Approved standard M2-A7. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.Nemec, A., L. Dijkshoorn, and T. J. K. van der Reijden. 2004. Long-term predominance of two pan-European clones among multi-resistant Acinetobacter baumannii strains in the Czech Republic. J. Med. Microbiol. 53:147-153. [DOI] [PubMed] [Google Scholar]
- 14.Nemec, A., L. Dolzani, S. Brisse, P. van den Broek, and L. Dijkshoorn. 2004. Diversity of aminoglycoside-resistance genes and their association with class 1 integrons among strains of pan-European Acinetobacter baumannii clones. J. Med. Microbiol. 53:1233-1240. [DOI] [PubMed] [Google Scholar]
- 15.Nemec, A., and M. Maixnerová. 2004. Aminoglycoside resistance of Acinetobacter baumannii hospital strains in the Czech Republic. Klin. Mikrobiol. Infekc. Lek. 10:223-228. [PubMed] [Google Scholar]
- 16.Pitcher, D. G., N. A. Saunders, and R. J. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8:151-156. [Google Scholar]
- 17.Urwin, R., and M. C. Maiden. 2003. Multi-locus sequence typing: a tool for global epidemiology. Trends Microbiol. 11:479-487. [DOI] [PubMed] [Google Scholar]
- 18.van Dessel, H., L. Dijkshoorn, T. Van der Reijden, N. Bakker, A. Paauw, P. Van den Broek, J. Verhoef, and S. Brisse. 2004. Identification of a new geographically widespread multiresistant Acinetobacter baumannii clone from European hospitals. Res. Microbiol. 155:105-112. [DOI] [PubMed] [Google Scholar]