Abstract
Daptomycin, a novel cyclic lipopeptide antibiotic, exhibits rapid bactericidal activity in vitro against most clinically relevant gram-positive organisms, including drug-resistant pathogens. Herein we describe a patient in whom methicillin-resistant Staphylococcus aureus with reduced susceptibility to daptomycin was responsible for bacteremia and progressive vertebral osteomyelitis during daptomycin therapy.
CASE REPORT
A 52-year-old Asian male with a history of Parkinson's-like illness, micronodular cirrhosis, pulmonary fibrosis requiring intermittent corticosteroid therapy, and diabetes mellitus presented with right groin pain in June 2004. Blood cultures revealed methicillin-resistant Staphylococcus aureus (MRSA). Magnetic resonance imaging (MRI) of his right hip revealed septic iliopsoas bursitis, and a transthoracic echocardiogram showed normal cardiac valves without vegetations. He underwent irrigation and drainage of the right hip and iliac bursa; cultures grew MRSA. He received a 6-week course of vancomycin with prompt resolution of bacteremia and right hip symptoms.
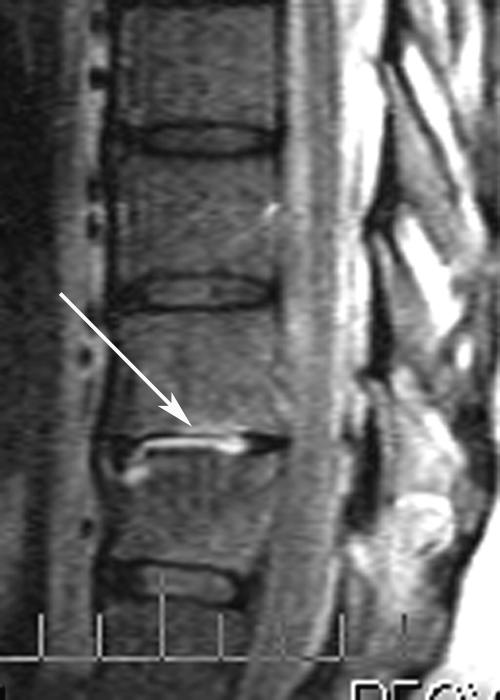
In July 2004, he presented with new-onset back pain a few days after completing vancomycin therapy. MRI of the thoracic spine revealed T10-T11 diskitis and osteomyelitis (Fig. 1). He was not bacteremic. Biopsy of the T10-T11 disk space revealed MRSA. Daptomycin was initiated at a daily dose of 6 mg/kg of body weight intravenously.
FIG. 1.
Thoracic spine MRI (July 2004) shows abnormal enhancement of T10-T11 disk (arrow) and the adjacent vertebral endplates.
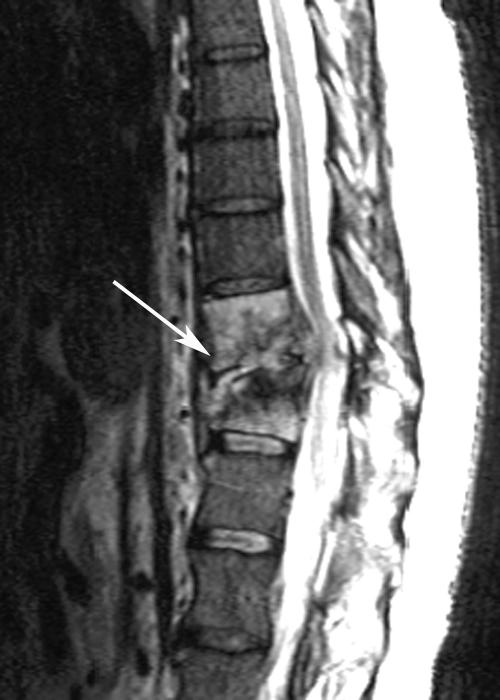
In August, during the 4th week of daptomycin therapy, he complained of increasing back pain. Repeat spine MRI showed significant progression of diskitis and osteomyelitis at T10-T11, collapse of T11 with gibbus deformity, an epidural phlegmon, and spinal cord compression (Fig. 2). His neurological exam was normal. A biopsy of the involved disk was performed; blood and T10-T11 disk cultures grew MRSA. He was started on linezolid with resolution of bacteremia. Linezolid was discontinued 10 days later due to thrombocytopenia. Quinupristin-dalfopristin (Q-D) and rifampin were initiated. Repeat MRI 2 weeks later showed progressive compression of T11 and unchanged cord compression. Conservative therapy was recommended by neurosurgery. He developed disabling myalgias and arthralgias secondary to Q-D; given the lack of alternate antimicrobials, Q-D was continued along with symptomatic therapy. In September, a computed tomography scan of the abdomen performed to evaluate persistent constipation revealed diffuse pneumatosis coli. Blood cultures and stool enzyme immunoassay for Clostridium difficile were negative. Conservative management was chosen, given the lack of abdominal pain or tenderness, hemodynamic stability, and operative risk.
FIG. 2.
Thoracic spine MRI (August 2004) reveals progression of diskitis/osteomyelitis at T10-T11 (arrow) with vertebral collapse, gibbus deformity, epidural phlegmon, and spinal cord compression.
Four weeks into Q-D and rifampin therapy, he developed vomiting, renal insufficiency, worsening hepatic encephalopathy, and lactic acidosis. Abdominal computed tomography scan showed worsening pneumatosis coli with possible perforation. Repeat blood cultures were negative. His family opted for comfort care, and the patient died. An autopsy revealed compression fracture of T10-T11 vertebrae with no evidence of active osteomyelitis, micronodular cirrhosis, bronchopneumonia, and submucosal colonic edema. Postmortem cultures of the T10-T11 disks and vertebrae were negative.
Clinical isolates were identified as Staphylococcus aureus based on colony morphology and Staphaurex (Remel) slide coagulase test. Antimicrobial susceptibility testing was performed by automated microdilution broth testing (Microscan; Dade Behring). Methicillin resistance was confirmed by growth on commercially prepared oxacillin screen agar (BBL). Vancomycin screen agar (BBL) was used to screen for reduced susceptibility to vancomycin. The MIC of vancomycin was determined using Etest strips (AB Biodisk). Inducible clindamycin resistance was detected by the D-test as described elsewhere (3).
Patient's clinical isolates of MRSA were sent to the daptomycin reference laboratory (Laboratory Specialists, Inc., Westlake, OH). Daptomycin susceptibility was determined by Kirby-Bauer disk diffusion and microdilution panels manufactured by TREK Diagnostics (Cleveland, OH). The daptomycin-containing wells were adjusted to contain physiological levels of calcium (50 mg/liter) after the addition of cation-adjusted Mueller-Hinton broth (5). The daptomycin susceptibility breakpoint for S. aureus was ≤1 μg/ml or a zone diameter of ≥16 mm. Pulsed-field gel electrophoresis (PFGE) was performed on available MRSA isolates.
Five MRSA isolates from the patient were available, including one dating back 6 months prior to his current illness (Table 1). Vancomycin MICs increased from 2.0 μg/ml (isolates obtained before and immediately after vancomycin therapy) to 4.0 μg/ml (after 4 weeks of daptomycin therapy). Daptomycin MICs increased from 0.5 μg/ml (isolate prior to daptomycin therapy) to 4.0 μg/ml (blood and T10-T11 disk isolate obtained during the 4th week of daptomycin therapy) (Table 1). All MRSA isolates (i) demonstrated inducible clindamycin resistance by the D-test; (ii) were susceptible to gentamicin, chloramphenicol, linezolid, Q-D, trimethoprim-sulfamethoxazole, and rifampin; and (iii) were indistinguishable by PFGE.
TABLE 1.
Vancomycin and daptomycin susceptibilities for MRSA isolates obtained prior to and during the course of daptomycin therapy
| Date (mo yr) | Specimen | Vancomycin MIC (Sa = ≤4 μg/ml) | Daptomycin zone diameter (S = ≥16 mm) | Daptomycin MIC (S = ≤1 μg/ml) |
|---|---|---|---|---|
| January 2004 | Sputum | 2.0 | 20 | NTb |
| June 2004 | Blood | 2.0 | 19 | 0.5 |
| July 2004 | T10-T11 disk | 2.0 | NT | NT |
| August 2004 | Blood | 4.0 | 14 | 4.0 |
| August 2004 | T10-T11 disk | 4.0 | 15 | 4.0 |
S, susceptibility breakpoint.
NT, not tested.
Daptomycin (Cubicin; Cubist Pharmaceuticals), a cyclic lipopeptide antibiotic, exhibits rapid concentration-dependent bactericidal activity against most gram-positive pathogens, including isolates resistant to methicillin, vancomycin, and linezolid (2). It is currently approved by the U.S. Food and Drug Administration for the treatment of complicated skin and skin structure infections caused by S. aureus (including methicillin-resistant strains), Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus dysgalactiae subsp. equisimilis, and Enterococcus faecalis (vancomycin-susceptible strains only) at a dose of 4 mg/kg/day administered intravenously (2). Daptomycin is currently being studied for a variety of other infections, including gram-positive bacteremia and endocarditis (1). Its unique mechanism of action involves binding to bacterial membranes and rapid depolarization of membrane potential; the resultant inhibition of protein, DNA, and RNA synthesis results in bacterial cell death (2). Hence, cross-resistance with other antimicrobial classes has not been reported. The activity of daptomycin is dependent on the presence of calcium ions; it is two- to fourfold more active when tested in 50 mg/liter of calcium (similar to the normal human serum concentration of ionized calcium) (5).
The convenience of once-daily dosing, minimal adverse effects, paucity of significant drug interactions, bactericidal activity against drug-resistant pathogens, and low potential for the development of drug resistance have prompted clinicians to use daptomycin for the treatment of deep-seated infections, such as endocarditis and osteomyelitis, despite the absence of published human studies. In a rat model of experimental MRSA endocarditis, daptomycin (with or without rifampin) at a dose equivalent to 6 mg/kg once daily in humans was superior to vancomycin monotherapy (12). In intravenous drug abusers being treated for gram-positive endocarditis and bacteremia, the bactericidal killing rate of daptomycin was significantly decreased in patient serum samples than the rate in broth (11). This may have been related to the high degree of protein binding (≥93%) of daptomycin. In the same study, the bactericidal killing rate of daptomycin was greater when killing curves were performed with test strains in logarithmic- versus stationary-phase growth (11).
Clinical and laboratory studies on the effectiveness of daptomycin in treatment of bone and joint infections have been limited to date. In an experimental rabbit model of MRSA tibial osteomyelitis, 41% of daptomycin- and 39% of vancomycin-treated rabbits had negative tibial cultures for MRSA after 28 days of therapy (8). Compared to daptomycin, vancomycin was present in significantly higher levels in both infected and uninfected bone; daptomycin was not detectable in uninfected bone. The concentrations of vancomycin and daptomycin in bone were 6.0% and 1.3% of the corresponding peak serum concentrations (8). High protein binding of daptomycin was again considered to play a role. A patient with MRSA vertebral osteomyelitis was successfully treated with a 12-week course of daptomycin (6 mg/kg once daily) after he developed significant allergic reactions to vancomycin and linezolid (7). A bone biopsy specimen obtained during Q-D therapy continued to grow MRSA with reduced susceptibility to Q-D. Intraoperative cultures during daptomycin therapy were negative. Another report describes successful daptomycin therapy in 9 of 10 patients with drug-resistant gram-positive bone and joint infections (eight with MRSA and one each with Enterococcus and Streptococcus); relapse on daptomycin therapy in one patient was blamed on infrequent dosing (4). Six patients had osteomyelitis, and five patients had septic arthritis (4). Details of daptomycin dosage, location of infection, and possible surgical debridement were not provided.
The emergence of daptomycin resistance during therapy has been extremely uncommon (6, 13). During phase 2 and 3 trials, one clinical isolate of daptomycin-resistant S. aureus and E. faecalis were identified among more than 1,000 infected subjects (2). Three isolates of S. aureus with daptomycin MICs of 8 μg/ml were found among 88 staphylococcal isolates obtained from diverse geographic regions between 1996 and 2001 (6). The emergence of daptomycin resistance and treatment failure was reported in two cases of MRSA vertebral osteomyelitis (daptomycin dose of 6 mg/kg daily) (10). Recurrent MRSA bacteremia was noted in both patients. Increasing MIC to daptomycin was seen in isolates of MRSA obtained before and after daptomycin therapy, and all isolates from a given patient were indistinguishable by PFGE (10). Another report described a patient with prolonged MRSA bacteremia due to portal vein thrombophlebitis treated with daptomycin who subsequently developed daptomycin resistance (9).
Our patient relapsed with vertebral osteomyelitis after 6 weeks of vancomycin therapy for MRSA bacteremia and right ileopsoas bursitis. Recurrence of MRSA bacteremia and progression of vertebral osteomyelitis were noted during the 4th week of daptomycin therapy. Daptomycin MICs increased to levels considered to be nonsusceptible. The exact mechanism of resistance to daptomycin has not been identified. Increased protein binding and low daptomycin concentration in bone may have led to clinical failures even though daptomycin's activity is enhanced in the presence of high calcium concentrations (8). The patients described by us and Rezai et al. (10) failed a higher dose of daptomycin than is currently recommended for complicated skin and skin structure infections (2). It is unclear whether unique strain characteristics and/or certain clinical presentations of MRSA infections are associated with the emergence of daptomycin resistance. Caution and close monitoring are necessary when daptomycin is utilized for the treatment of osteomyelitis until further clinical and experimental investigations can document its efficacy in this setting.
Acknowledgments
Holenarasipur R. Vikram has served as a member of the Regional Advisory Board for Cubist Pharmaceuticals.
Laura M. Koeth is a consultant for Cubist Pharmaceuticals, and Laboratory Specialists, Inc., is financially supported by Cubist Pharmaceuticals.
REFERENCES
- 1.Carpenter, C. F., and H. F. Chambers. 2004. Daptomycin: another novel agent for treating infections due to drug-resistant gram-positive pathogens. Clin. Infect. Dis. 38:994-1000. [DOI] [PubMed] [Google Scholar]
- 2.Cubist Pharmaceuticals. 2003. Cubicin (daptomycin for injection) (package insert). Cubist Pharmaceuticals, Lexington, Mass.
- 3.Fiebelkorn, K. R., S. A. Crawford, M. L. McElmeel, and J. H. Jorgensen. 2003. Practical disk diffusion method for detection of inducible clindamycin resistance in Staphylococcus aureus and coagulase-negative staphylococci. J. Clin. Microbiol. 41:4740-4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finney, M. S., C. W. Crank, and J. Segreti. 2004. Use of daptomycin to treat drug-resistant Gram-positive bone and joint infections, abstr. 427, p. 120. Program Abstr. 42nd Annu. Meet. Infect. Dis. Soc. Am., Boston, Mass. Infectious Diseases Society of America, Alexandria, VA.
- 5.Fuchs, P. C., A. L. Barry, and S. D. Brown. 2000. Daptomycin susceptibility tests: interpretive criteria, quality control, and effect of calcium on in vitro tests. Diagn. Microbiol. Infect. Dis. 38:51-58. [DOI] [PubMed] [Google Scholar]
- 6.Jevitt, L. A., A. J. Smith, P. P. Williams, P. M. Raney, J. E. McGowan, Jr., and F. C. Tenover. 2003. In vitro activities of daptomycin, linezolid, and quinupristin-dalfopristin against a challenge panel of staphylococci and enterococci, including vancomycin-intermediate Staphylococcus aureus and vancomycin-resistant Enterococcus faecium. Microb. Drug Resist. 9:389-393. [DOI] [PubMed] [Google Scholar]
- 7.Juthani-Mehta, M., H. R. Vikram, J. L. Gorelick, and J. M. Rappeport. 2003. Successful therapy of methicillin-resistant Staphylococcus aureus (MRSA) vertebral osteomyelitis (VOM) with daptomycin, abstr. 298, p. 83. Program Abstr. 41st Annu. Meet. Infect. Dis. Soc. Am., San Diego, Calif. Infectious Diseases Society of America, Alexandria, VA.
- 8.Mader, J. T., and K. Adams. 1989. Comparative evaluation of daptomycin (LY146032) and vancomycin in the treatment of experimental methicillin-resistant Staphylococcus aureus osteomyelitis in rabbits. Antimicrob. Agents Chemother. 33:689-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangili, A., I. Bica, D. R. Snydman, and D. H. Hamer. 2005. Daptomycin-resistant, methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 40:1058-1060. [DOI] [PubMed] [Google Scholar]
- 10.Rezai, K., J. P. Quinn, R. Hayes, K. Lolans, R. A. Weinstein, and M. K. Hayden. 2004. Emergence of daptomycin resistance and treatment failure in two cases of S. aureus osteomyelitis, abstr. K97-a. Program Abstr. 44th Annu. Intersci. Conf. Antimicrob. Agents Chemother. 2004. American Society for Microbiology, Washington, D.C.
- 11.Rybak, M. J., E. M. Bailey, K. C. Lamp, and G. W. Kaatz. 1992. Pharmacokinetics and bactericidal rates of daptomycin and vancomycin in intravenous drug abusers being treated for gram-positive endocarditis and bacteremia. Antimicrob. Agents Chemother. 36:1109-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakoulas, G., G. M. Eliopoulos, J. Alder, and C. T. Eliopoulos. 2003. Efficacy of daptomycin in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:1714-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Streit, J. M., R. N. Jones, and H. S. Sader. 2004. Daptomycin activity and spectrum: a worldwide sample of 6737 clinical gram-positive organisms. J. Antimicrob. Chemother. 53:669-674. [DOI] [PubMed] [Google Scholar]