Abstract
Human fatalities from human–wildlife conflict (HWC) represent a critical dimension of conservation, often triggering retaliatory actions and post-traumatic stress in affected communities. However, most studies focus on the economic implications of HWC, neglecting human fatalities which may have far-reaching long-term implications. This study investigates the spatial and temporal patterns of human fatalities caused by megafaunal species in Zimbabwe, using data collected from 2016 to 2022. Through spatial and statistical analyses based on the Getis-Ord Gi* hotspot analysis and Mann–Kendall trend test, we assess fatalities caused by six megafaunal species: Nile crocodile (Crocodylus niloticus), African elephant (Loxodonta africana), hippopotamus (Hippopotamus amphibius), African buffalo (Syncerus caffer), African lion (Panthera leo) and spotted hyena (Crocuta crocuta). The results of the study showed that crocodiles and elephants account for over 80% of human fatalities in Zimbabwe. These fatalities also significantly increased over the study period (p < 0.03). In contrast, fatalities involving lions, hyenas, hippos, and buffaloes showed no significant increase, indicating more stable but still concerning risks. Fatality hotspots were concentrated in Kariba, Binga and Hwange districts in northern and western Zimbabwe, highlighting areas needing urgent interventions. These insights have broader implications for HWC management across Africa, where megafaunal species frequently interact with human populations. By adopting data-driven, species-specific strategies, other countries facing similar conflicts can foster human–wildlife coexistence and improve conservation outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-04934-0.
Keywords: Retaliatory killing, Psychosocial, Mental health, Deaths, Gettis Ord G*, Coexistence
Subject terms: Biodiversity, Environmental impact, Psychology and behaviour, Sustainability, Conservation biology
Introduction
Human–wildlife interactions occur along a continuum that includes both conflict and coexistence. While human–wildlife conflict (HWC) has historically received more attention due to its immediate socio-economic and conservation implications, there is a growing recognition of the need to frame these interactions within a broader coexistence paradigm1,2. Human–wildlife coexistence refers to the dynamic state where humans and wildlife share landscapes in ways that allow for the persistence of both, despite potential risks and trade-offs1. This approach acknowledges that not all interactions are negative and that fostering tolerance, promoting shared benefits, and integrating local values and knowledge systems can support long-term conservation goals3.
However, HWC poses significant challenges globally, leading to substantial biodiversity loss and severe impacts on human lives and livelihoods4. HWC arises when wildlife needs clash with human activities, resulting in crop destruction, livestock predation, property damage, and, most critically, human injuries and fatalities5. The problem is exacerbated by the increasing human population, habitat fragmentation, and the expansion of agricultural lands into wildlife habitats, making HWC a substantial challenge for wildlife conservation and human well-being6. In Mozambique, approximately 300 people die annually from crocodile attacks7. Ineffective management of HWC can lead to retaliatory killings of wildlife, particularly in response to human fatalities, undermining efforts to promote coexistence8. Such retaliatory actions are often fueled by the trauma, anger and resentment of vicarious victims of HWC, highlighting the importance of strategies that address post-traumatic disorders from HWC to foster coexistence9,10.
The nature and intensity of HWC-related fatalities vary across regions, influenced by the species involved, cultural attitudes towards wildlife, and the socio-economic conditions of human communities11–15. In Asia, elephants (Elephas maximus) and tigers (Panthera tigris) are frequently implicated in deadly encounters with humans16. Human fatalities from elephants often occur during raids on human agricultural fields17. Although less frequent, tiger attacks occur in areas where human settlements encroach on tiger habitats18. In Africa, HWC is similarly widespread, with megafauna such as African elephants (Loxodonta africana), lions (Panthera leo), hippopotamus (Hippopotamus amphibius), and Nile crocodiles (Crocodylus niloticus) frequently involved in fatal interactions with humans19–22. African elephants, in particular, are known to attack humans, especially in areas with high elephant densities23. Cultural factors often complicate these threats, as traditional practices and beliefs influence how communities perceive and respond to wildlife3,24,25. These conflicts have led to several mitigation strategies, including the construction of physical barriers, relocation or elimination of problematic animals, and community-based conservation programs to reduce human–wildlife encounters26,27. However, these measures only provide temporary relief, as wildlife may adapt, leading to more fatalities.
Zimbabwe’s extensive network of protected areas, including national parks, conservancies and safari areas, continue to support a diverse array of megafaunal species, which are often implicated in HWC27–30. The intensity of HWC in Zimbabwe is driven by changing land use patterns, wildlife population densities, and the socio-economic conditions of local communities31–33. While human populations living adjacent to protected areas frequently experience conflicts with wildlife such as crop destruction, livestock predation, and, in some cases, human fatalities26, it is important to recognize that these interactions are not solely negative. The presence of iconic megafauna like elephants, lions, and buffaloes also offers significant socio-economic benefits, particularly through wildlife-based tourism, which contributes to rural livelihoods and national economies34–36. Additionally, emerging market-based conservation mechanisms, such as biodiversity credits and payments for ecosystem services, hold promise for incentivizing coexistence and habitat protection37. Beyond economic gains, many African communities ascribe deep cultural, spiritual, and symbolic value to wildlife species, which fosters stewardship and positive perceptions of wildlife37,38.
Where human death results from HWC, it often leads to deep emotional trauma to affected families and communities18,39. Despite the devastating nature of such losses, the provision of psychosocial support to victims remains largely neglected18,39. Studies have shown that exposure to traumatic events, particularly the loss of loved ones, can lead to severe psychological distress, including post-traumatic stress disorder (PTSD), depression, and anxiety39–41. However, mental health intervention in the context of HWC-related human fatalities has rarely been prioritized, especially in Zimbabwe, where mental health services have historically been underfunded and underdeveloped42,43. As awareness of mental health issues begins to gain traction across the continent, establishing a baseline for mental health interventions, such as mapping hotspots of human fatalities due to HWC, becomes increasingly crucial. This fosters the crafting of structured psychosocial support in regions where human loss due to HWC is prevalent, and it provides guidance for targeted mental health interventions tailored to these communities.
Community-based natural resource management (CBNRM) programs have made considerable efforts to involve local communities in wildlife conservation and conflict mitigation44–47. However, in other areas, CBNRM programs are not fully implemented, and human settlements continue to expand into wildlife habitats, increasing the frequency of human–wildlife encounters and, consequently, human fatalities. While literature indicates the involvement of megafaunal species such as elephants, lions, buffaloes, hippos, and crocodiles in human fatalities in Zimbabwe48–50, no studies have explicitly quantified their contribution to human fatalities. Previous studies have mostly focused on the economic losses caused by megafaunal species through crop and livestock raids31,51,52. Unlike economic losses, which can be financially compensated, human life cannot be restored. This often results in greater resentment and anger among affected communities, leading to retaliatory killing of wildlife. This study seeks to address this knowledge gap by focusing on the spatial and temporal patterns of fatalities to guide strategies for human–wildlife coexistence. Understanding the dynamics of human fatalities in HWC in Zimbabwe is urgently needed to establish the circumstances under which they occur and to develop targeted measures that ensure providing psychosocial support to affected communities and public safety, reduce retaliatory killing of wildlife and facilitate coexistence. This understanding could also enhance the effectiveness of CBNRM programs by integrating data-driven approaches for problem animal control.
This study aims to contribute to the growing literature on HWC by focusing on human fatalities caused by megafaunal species in Zimbabwe. By analyzing incident records and data on human–wildlife interactions, this research seeks to identify the deadliest species and the spatio-temporal trends of these lethal encounters. The findings will provide valuable insights into species-specific risks and inform the development of effective mitigation strategies to enhance wildlife ownership in communities living in wildlife habitats globally. In addition, this study seeks to identify areas in need for psychosocial support for victims of human loss due to HWC. As mental health issues begin to gather momentum in African countries, the research sets a baseline for mental health interventions by mapping hotspots of human fatalities. By recognizing both the physical and emotional toll of HWC, this work serves as a critical step toward holistic approaches that prioritize both conservation efforts and the well-being of affected communities.
Materials and methods
Study area
The study was conducted in Zimbabwe, which is located in southern Africa. The study area spans approximately 390,757 square kilometres and is bordered by Zambia, Mozambique, South Africa, and Botswana53 (Fig. 1). The country’s diverse landscapes include savannahs, woodlands, and mountainous regions, with a climate that ranges from temperate in the central plateau to hot in the low-lying areas.
Fig. 1.
Study area map showing districts and various wildlife sources including protected areas, conservancies and Campfire areas in Zimbabwe. Map was designed by the first author using ArcGIS Pro 3.2.2 (www.pro.arcgis.com).
Zimbabwe’s biodiversity is mainly concentrated in protected areas, such as Hwange, Mana Pools, Matusadonha, Chizarira, Zambezi and Gonarezhou national parks, and Hurungwe, Charara, Chirisa, Chete, Matetsi safari areas, and Kariba Recreational Park. These protected areas support several megafaunal species including the African elephant, hereafter elephant, lion, spotted hyena (Crocuta crocuta), hippopotamus, hereafter hippo, and Nile crocodile hereafter crocodile. These species are frequently involved in HWC among communities living near wildlife habitats or protected areas5.
Data collection
To understand human and wildlife interactions resulting in HWC, the study utilised the comprehensive HWC databases, which comprise nationwide HWC reports within and outside protected areas consolidated from responsible authorities, including rural district councils and the police. This data was made available by the Zimbabwe Parks and Wildlife Management Authority (ZimParks) for the period covering January 2016 to December 2022. This dataset comprises verified records of HWC incidents, aggregated at the district level on an annual basis. Each record includes details on the species involved, district location, number of people injured or killed (fatalities), and instances of livestock loss. The data is compiled through a structured, cascading reporting system that draws from multiple sources, including communal areas, local authorities, law enforcement agencies, and public health centres. For example, in the event of a fatality in a communal area, the village head reports the incident to the local councillor, who is affiliated with the Rural District Council (RDC). The RDC, in turn, reports such cases along with other HWC-related incidents to ZimParks. Given this coordinated and multi-tiered reporting structure, we consider the dataset to be generally reliable for understanding patterns of HWC in Zimbabwe. To ensure analytical rigor, we included only those records that contained sufficient spatio-temporal detail, specifically, the year and location of each incident, necessary for meaningful analysis. As a result, our study focused on 322 confirmed records of human fatalities involving six megafaunal species: crocodile, elephant, buffalo, lion, hippo, and hyena. This focus reflects both the severity and frequency of fatal encounters with these species, as documented in previous studies22,30,53. We acknowledge, however, that the exclusion of incomplete or inconsistent records may have resulted in the underrepresentation of certain species and regions.
Data analysis
Chi-square test
To test the contribution of each species to human fatalities, we calculated the total number of times each species was involved in human fatalities during the study period. The proportional contribution of each species was then assessed as a percentage of the total number of human fatalities due to megafaunal species. The chi-squared test was used to evaluate the statistical significance of the contribution of each species. The test was chosen based on its wide use in evaluating proportional contributions in previous HWC studies54,55 and its appropriateness for categorical data which helps to determine whether the observed distribution of fatalities among species deviates from what would be expected by chance. The test was conducted using the following equation:
| 1 |
where Oi represents the observed frequency of fatalities for each species, and Ei represents the expected frequency under the null hypothesis of equal contribution by all species.
Mann–Kendall test
To analyze temporal trends in human fatalities due to HWC, we used the Mann–Kendall test based on the number of fatalities per year. This non-parametric test is useful for identifying trends in time series data without assuming any specific distribution, making it suitable for our data, which may not follow a normal distribution56,57. The Mann–Kendall test has been widely used to detect trends in numerous HWC studies58,59. This study used the Mann–Kendall test to determine whether there was a statistically significant increase or decrease in human fatalities over the study period, both for individual species and all species combined. The test was conducted using the following equation:
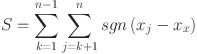 |
2 |
where sgn (xj − xk) represents the sign function that returns 1, 0 or − 1 based on the difference between xj and xk. The test produces a test statistic (S), the corresponding p-value, and Sen’s slope estimator (Q) to quantify the magnitude of the trend. The Sen’s slope estimator provides the rate of change, with a positive value indicating an increasing trend in fatalities over time and a negative value indicating a decreasing trend. A significant p-value (p < 0.05) indicates a statistically significant trend in the number of fatalities over time.
Getis-Ord Gi* statistics
To identify the hotspots of HWC incidents based on the number of events per district, the study employed the Getis-Ord Gi* statistic. This spatial analytical method is effective for detecting statistically significant clusters of high or low HWC events, which is essential for informing targeted mitigation strategies60–63. Its application extends beyond HWC mapping, having been successfully used to identify hotspots of wildlife road mortality64 and wildlife disease outbreaks65, as well as in recent analyses of HWC spatial patterns66. By identifying significant hotspots of fatalities using hotspot analysis, we can better understand the spatial dynamics of HWC and allocate resources more strategically. The Getis-Ord Gi* was conducted using the following equation:
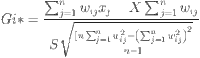 |
where xj is the attribute value (fatalities) at location j, Wij is the spatial weight between locations i and j. X is the mean of the attribute values and S is the standard deviation of the attribute values.
The analysis produces Getis-Ord Gi* statistic (G*) and associated z-scores and p-values. A positive z-score (typically greater than 1.96 for a significance level of 0.05) indicates significant clustering of high values (hotspots) in a given area, while a negative z-score indicates clustering of low values (cold spots). A p-value less than 0.05 supports the identification of statistically significant clustering patterns.
Results
Proportional contributions to fatalities
The results showed a significant disparity in the proportional contributions of different megafaunal species to total human fatalities (p < 0.05). Crocodiles and elephants emerged as the leading contributors, responsible for approximately 83% of the fatalities, with crocodiles accounting for about 51% and elephants for 32%. In contrast, other species, such as lions (3%) and spotted hyenas (2%) were found to contribute significantly less to the overall fatalities (Fig. 2). The chi-squared test confirmed that the observed distribution of fatalities among species is statistically significant (p < 0.05), indicating that crocodiles and elephants are significantly involved in fatal encounters with humans compared to other species.
Fig. 2.
Contribution of megafaunal wildlife species to human fatalities.
Temporal trends in fatalities
The temporal analysis using the Mann–Kendall test indicated a statistically significant increasing trend in fatalities involving crocodiles and elephants over the study period (p < 0.05) (see Fig. 3). For crocodiles, the Sen’s slope estimate was 3.5, indicating an upward trend from 16 fatalities in 2016 to 37 in 2022 (Fig. 3). Similarly, fatalities due to elephants increased from 9 in 2016 to 24 in 2022, with Sen’s slope estimate of 3.0 (Fig. 3). Conversely, other species such as hippos, buffaloes, lions, and hyenas showed insignificant changes in fatality rates over the same period (p > 0.05).
Fig. 3.
Trends in HWC related human fatalities for the period between 2016 and 2022 for (a) crocodile, (b) elephant, (c) buffalo, (d) hippo, (e) lion, (f) hyena. The green line shows the plot based on data values, and the blue dashed line shows the trend line.
Overall, the data reveal a significant upward trend in all HWC-related fatalities across all species, with total fatalities increasing from 17 to 67 per year (Sen’s slope = 7.0, p < 0.05) (See Fig. 4). This trend suggests that without intervention, HWC may escalate, further endangering both human lives and wildlife populations.
Fig. 4.
Trends in the total HWC related human fatalities for the period between 2016 and 2022.
Geographical distribution of fatalities
Results for the Getis-Ord Gi* statistics identified significant hotspots and coldspots for fatalities due to crocodile, elephant, buffalo, lion, hippo and hyena (Fig. 5). Significant hotspots for fatalities due to crocodile, elephant, buffalo, lion and hippo were situated in both the northern and western districts of Zimbabwe, namely Hurungwe, Kariba, Binga, Hwange and Gokwe (North and South) districts. For fatalities due to buffalo and hyena, significant hotpots were observed in the south-eastern districts of Zimbabwe namely Chiredzi, Bikita, Chipinge, Buhera and Beitbridge. All the species had a hotspot for fatalities in the northern districts of Zimbabwe namely Hurungwe and Kariba. On the other hand, coldspots for fatalities were consistently observed in areas stretching from the northeastern districts (~ Centenary, Guruve and Rushinga) through the central districts (~ Kwekwe, Chegutu and Gweru) down to south western districts (~ Mangwe, Matobo, Gwanda) of the country.
Fig. 5.
Spatial distribution of hotspots and cold spots for human fatalities across districts of Zimbabwe due to megafaunal species, namely: (a) crocodile, (b) elephant, (c) buffalo, (d) lion, (e) hippo, (f) hyena. The Gi* statistic is a z-score which indicates the intensity of direction of spatial clustering, where high Gi* values (warm colours) show significant hotspots and low values (cold colours) show significant cold spots. Maps were designed by the first author using R-studio 2024.09.0 (www.posit.co/rstudio-desktop).
Overall, districts located in the northern and western districts of Zimbabwe exhibited significant hotspots for HWC fatalities, particularly involving the five megafaunal species under investigation (See Fig. 6). This suggests a concentrated risk area that may require focused intervention strategies. Notably, these districts have a high concentration of protected areas. In contrast, districts in the northeastern, central, and southwestern regions show significant cold spots of HWC fatalities. These regions are characterized by fewer concentrations of protected areas.
Fig. 6.
The spatial distribution of hotspots and cold spots of human fatalities from HWC across districts of Zimbabwe. The Gi* statistic is a z-score which indicates the intensity of direction of spatial clustering, where high Gi* values (warm colours) show significant hotspots and low values (cold colours) show significant cold spots. Map was designed by the first author using R-studio 2024.09.0 (www.posit.co/rstudio-desktop).
Discussion
This study offers a comprehensive overview of the role megafaunal species play in human fatalities in Zimbabwe. Our findings reveal that elephants and crocodiles are responsible for over 80% of these fatalities, highlighting the urgent need to reassess and refine current mitigation strategies in areas with elephants and crocodiles. These include implementing more effective deterrent measures, such as electric fences with increased voltage or acoustic deterrents and improving HWC management programs to educate local communities about safe practices and coexistence with these large animals67. Additionally, investing in habitat restoration and maintaining wildlife corridors can help reduce human–wildlife encounters and mitigate the risk of fatalities68. While the involvement of these species in crop raiding and livestock depredation is well documented68–71, our findings also indicate that these species impact human lives. Understanding human fatalities from HWC is crucial, as this often leads to deep-seated anger, resentment, and retaliatory killings within affected communities41. These retaliatory actions not only threaten the survival of the species involved but also undermine broader conservation efforts72. For species such as crocodiles and elephants, which already face significant conservation challenges, the escalation of HWC into retaliatory killings can have dire consequences72–74. While often abundant in certain regions, crocodiles are still vulnerable to overexploitation and habitat loss75. Retaliatory killings can exacerbate these threats, particularly in areas where crocodile populations are already under pressure from human activities such as fishing and habitat encroachment76. Elephants are listed as vulnerable by the International Union for Conservation of Nature (IUCN) due to threats from habitat fragmentation, poaching, and human-elephant conflict77. Zimbabwe’s elephant population, though large, is not exempt from these pressures78,79. Retaliatory killings, driven by the loss of human lives, can contribute to population declines and disrupt social structures within elephant herds, further endangering the species. Therefore, it is imperative to develop and implement targeted interventions that prioritise human safety and wellbeing while safeguarding these key species’ survival. By fostering coexistence, the results from this study can help ensure the long-term conservation of these species and the conservation of the ecosystems they inhabit.
Temporal distribution
Our findings reveal a significant upward trend in fatalities involving crocodiles and elephants over the study period (p < 0.05). Many water bodies (e.g., Lake Kariba and Ruti Dam) in Zimbabwe are home to crocodiles and are situated near communities that rely on these waters for fishing, bathing, washing, and collecting water50,68,78. Community utilisation of these water resources with inadequate safety measures poses a significant risk of human-crocodile encounters and fatalities80. In addition, increasing human population density in this part of the country (See Fig. 7c) could be driving overfishing by local communities. This can decimate fish stocks and drive crocodiles to alternative food sources, particularly increasing the likelihood of fatal encounters81. This is also supported by an observation from a study by Matanzima73, who showed that most crocodile-related fatalities in Kariba district were in the dry season when crocodiles’ natural habitat and prey base was diminished. This underscores the need for sustainable resource management that considers the rising human population to ensure that fish stocks remain sufficient to support crocodile populations82.
Fig. 7.
The human population density distribution for Zimbabwe in the years 2016 (a) and 2020 (b), together with the percentage change in population density during the same period between 2016 and 2020 (c). Maps were designed by the first author using ArcGIS Pro 3.2.2 (www.pro.arcgis.com).
The rising fatalities involving elephants could be related to Zimbabwe’s growing elephant population, now exceeding 83,000, the second largest globally after Botswana83. As elephant populations grow, so does the demand for space, food, and water, often bringing elephants closer to human settlements83,84. This increased interaction increases the risk of conflict, particularly in areas where human activities encroach on traditional elephant ranges or movement corridors19,85. These fatalities often occur when humans attempt to protect their crops from elephants86–88. These fatal encounters also occur during the dry season, when water scarcity drives elephants closer to human settlements in search of water89.
We found no significant trend in human fatalities due to hippo, buffalo, lion and hyenas (p > 0.05). This could be because these species typically do not attack humans unless provoked or threatened. Hippos and buffaloes, for example, are generally known to be aggressive primarily when they feel cornered or perceive a threat to their territory or young89,90. Additionally, the hippo and buffalo population has significantly declined in the past 10 years especially in the northern parts of the country (see Table S1), thereby limiting chances of their encounters with humans. Lions and hyenas are more likely to target livestock or wild prey rather than humans, and attacks on people often occur when humans unknowingly intrude into their habitat91,92. However, some studies have documented increased livestock depredation and non-fatal human encounters with these species93–95. Human fatalities, unlike mere attacks or livestock raids, often involve unavoidable, high-risk situations with profound socio-economic and psychological impacts on communities. Therefore, trends in human fatalities warrant particular attention, necessitating targeted conservation and conflict mitigation efforts that prioritize human safety and wellbeing while addressing broader HWC dynamics96.
Spatial distribution
The study observed hotspots for five megafaunal species (crocodiles, elephants, buffaloes, lions, and hippos) in Zimbabwe’s northern and western regions (Fig. 5). The spatial distribution patterns of these hotspots are likely driven by a combination of connectivity, ecological, environmental and anthropogenic factors96–98. The northern and western regions are characterised by extensive river systems, such as the Zambezi River, which supplies Lake Kariba providing ideal habitats for crocodiles and hippos, including large herbivores like elephants and buffaloes100. These environments support a high density of wildlife and together with a growing human population density which increase the likelihood of human–wildlife interactions, particularly where human activities, such as agriculture and fishing, bring people into proximity to dangerous animals49. Data from the world population hub indicates that the human population density in most of the northern and western parts of the country increased by over 50% (See Fig. 7c) during the period between 2016 (Fig. 7a) and 2020 (Fig. 7b). This certainly increased chances of human–wildlife encounters during this same period which recorded a steep rise in HWC-related fatalities (refer to Fig. 4).
Moreover, these regions are closely connected to the proximity of multiple protected areas within the Kavango-Zambezi Transfrontier Conservation Area (KAZA TFCA). KAZA TFCA is the largest transboundary conservation area in the world, spanning five countries: Angola, Botswana, Namibia, Zambia, and Zimbabwe. This region encompasses numerous national parks and wildlife reserves that serve as critical habitats for lions, elephants, and other megafaunal species101. The KAZA TFCA facilitates wildlife movement across borders through its vast, interconnected network of protected areas, essential for conserving wide-ranging species such as elephants and lions102. However, this connectivity also increases human–wildlife interactions in adjacent human settlements, as elephants and lions often move into areas where human activities have encroached upon traditional wildlife corridors102,103. This overlap can lead to more frequent and sometimes fatal encounters between humans and wildlife, particularly in regions such as western Zimbabwe, where the boundaries between protected and communal lands are not always clearly defined69,104,105. Improving wildlife corridors in the KAZA TFCA, strengthening of CBNRM programs and enhancing early warning systems to alert communities of nearby wildlife movements could help reduce HWC fatalities and foster coexistence between humans and wildlife in this biologically rich but conflict-prone region.
Southeastern Zimbabwe exhibited fewer HWC fatalities involving most species (crocodiles, elephants, buffaloes, lions, and hippos), except for hyenas, which had their hotspots in this area. This variation could be due to different ecological conditions and human settlement patterns that reduce the frequency of encounters with species such as crocodiles, elephants, buffaloes, lions, and hippos106–110. Hyenas, however, are highly adaptable and opportunistic predators that often thrive in areas with high human density due to the availability of anthropogenic food resources111. Their ability to scavenge and exploit human waste may explain their hotspots in this region, where they conflict with humans over resources.
The variation in hotspots underscores the need for targeted intervention strategies that address the specific dynamics of HWC in different regions of Zimbabwe. The adaptation of these spatial distribution patterns in formulating strategies for providing psychosocial support to vicarious victims of HWC is of major value. The psychological impact of HWC-related human fatalities is an overlooked dimension of conflict mitigation. The trauma experienced by affected families and communities can drive retaliatory actions, exacerbating the conflict. Therefore, it is imperative that counselling and rehabilitation resources and services be deployed to the northern and western hotspots of Zimbabwe. Furthermore, in hotspot areas, efforts could focus on mitigating risks associated with water-based activities and reducing conflicts with large herbivores and predators through community-based management and early warning systems111–113. Conversely, in the southeastern region, interventions could prioritize waste management and securing livestock to reduce hyena-related conflicts. Tailoring strategies to the unique ecological and social contexts of each region will be crucial for effectively reducing HWC fatalities, restoring a sense of wildlife ownership in communities and promoting coexistence between humans and wildlife92. These findings have broader implications for HWC management across Africa, where megafaunal species frequently come into contact with human populations. By developing data-driven, species-specific strategies, other countries facing similar conflicts can benefit from the approaches suggested in this study, ultimately reducing retaliatory killings and enhancing conservation outcomes.
Limitations and recommendations
While this study provides valuable insights into the spatio-temporal dynamics of megafaunal species in Zimbabwe, it lacks an important temporal dimension: the consideration of seasonal variation. Understanding seasonality is crucial for planning and implementing targeted interventions. For example, crocodile attacks often peak during the dry season when water levels are lower, and the water becomes murky and muddy, increasing the likelihood of human encounters with these animals114. Also, elephants and buffaloes are known to exhibit increased aggression during the dry season due to intensified competition for limited water and food resources, potentially skewing conflict data towards certain periods115. Similarly, the movement patterns of lions and hyenas during the wet season when their natural prey may migrate or become less accessible can result in increased proximity to human settlements, leading to higher instances of livestock depredation116. These seasonal dynamics were not explicitly accounted for in the analysis and may affect the interpretation of spatial and temporal HWC patterns.
We could not evaluate the effect of seasonality of these species in our study due to missing records on the place, month and day of each attack. This lack of detailed spatial–temporal data limits our ability to identify patterns that could be critical for mitigating human–wildlife conflict. In the process of cleaning the dataset for analysis, we may have inadvertently excluded relevant records from key regions due to missing or inconsistent information. This may have resulted in underrepresentation of certain areas, particularly the southeastern regions of Zimbabwe, which support significant wildlife populations. Notable among these are communities surrounding Gonarezhou National Park, Save Valley Conservancy, Bubye Valley Conservancy, and Nuanetsi Ranch. The absence of comprehensive data from these areas may have influenced the spatial distribution patterns observed in our analysis. Future studies should include precise spatial–temporal information, such as the specific dates and coordinates of incidents to accurately assess seasonal trends and patterns in human–wildlife conflicts. To address loss of data along bureaucratic protocols followed when reporting HWC-related fatalities, we recommend the development of a national HWC monitoring and reporting system which ensures more centralised, standardized and consistent recording of data. Reliable data would facilitate crafting of more timely and effective interventions. For instance, implementing seasonal warnings, increasing patrolling during high-risk periods, or temporarily restricting high-risk activities like fishing or farming near water bodies during peak conflict times could significantly reduce human fatalities93.
Conclusion
This study concludes that elephants and crocodiles are the leading causes of human fatalities in Zimbabwe, accounting for over 80% of such incidents. By establishing a national baseline for these critical issues, this research paves the way for more effective, targeted interventions to improve human–wildlife coexistence. Specifically, maps for fatality hotspots should guide deployment of psychosocial support services for vicarious victims of HWC. Future strategies should leverage detailed temporal data and address ecological factors to enhance conflict resolution efforts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the directorate at the Zimbabwe Parks and Wildlife Management Authority for approving this study.
Author contributions
BK and KM conceptualized the study. BK curated and analyzed data and wrote the original draft. CM, EG and JM reviewed and edited the manuscript. EG and JM validated and supervised the study.
Funding
No funding was received for this study.
Data availability
The data presented in this study is available on request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carter, N. H. & Linnell, J. D. Co-adaptation is key to coexisting with large carnivores. Trends Ecol. Evol.31(8), 575–578 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Frank, B., Glikman, J. A. & Marchini, S. Human–wildlife interactions: Turning conflict into coexistence, vol. 23. Cambridge University Press, 2019. [Online]. Available: https://books.google.com/books?hl=en&lr=&id=G26MDwAAQBAJ&oi=fnd&pg=PR13&dq=Frank,+B.,+Glikman,+J.+A.,+and+Marchini,+S.+(Eds.).+(2019).+Human%E2%80%93Wildlife+Interactions:+Turning+Conflict+into+Coexistence.+Cambridge+University+Press.&ots=m5t3PUkwAz&sig=-Fwz5Ou3e2f6ZzowbaDdfgWuJw4 Accessed May 01 2025.
- 3.Pooley, S., Bhatia, S. & Vasava, A. Rethinking the study of human–wildlife coexistence. Conserv. Biol.35(3), 784–793. 10.1111/cobi.13653 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrahms, B. et al. Climate change as a global amplifier of human–wildlife conflict. Nat. Clim. Change13(3), 224–234. 10.1038/s41558-023-01608-5 (2023). [Google Scholar]
- 5.Utete, B. & Matanzima, J. Human Wildlife Conflict and Opportunities for Co-existence. In Living Wildl. Zimb. 1 (Springer, 2024). [Google Scholar]
- 6.Dickman, A. J. & Hazzah, L. Money, Myths and Man-Eaters: Complexities of Human–Wildlife Conflict. In Problematic Wildlife (ed. Angelici, F. M.) 339–356 (Springer, 2016). 10.1007/978-3-319-22246-2_16. [Google Scholar]
- 7.FAO. Food and Agricultural Organisation of the United Nations. Human–wildlife conflict in Africa: Causes, consequences and management strategies, Rome, Italy, 2009. [Online]. Available: https://www.fao.org/4/i1048e/i1048e00.htm Accessed 12 Sep 2024.
- 8.Felix, N., Kissui, B. M., Munishi, L. & Treydte, A. C. Retaliatory killing negatively affects African lion (Panthera leo) male coalitions in the Tarangire-Manyara Ecosystem, Tanzania. PLoS ONE17(8), e0272272. 10.1371/journal.pone.0272272 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merson, S. D., Dollar, L. J., Johnson, P. J. & Macdonald, D. W. Retaliatory killing and human perceptions of Madagascar’s largest carnivore and livestock predator, the fosa (Cryptoprocta ferox). PLoS ONE14(3), e0213341 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viollaz, J. S., Thompson, S. T. & Petrossian, G. A. When human–wildlife conflict turns deadly: Comparing the situational factors that drive retaliatory leopard killings in South Africa. Animals11(11), 3281 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anand, S. & Radhakrishna, S. Investigating trends in human–wildlife conflict: Is conflict escalation real or imagined?. J. Asia-Pac. Biodivers.10(2), 154–161. 10.1016/j.japb.2017.02.003 (2017). [Google Scholar]
- 12.Dickman, A. J., Hazzah, L., Carbone, C. & Durant, S. M. Carnivores, culture and ‘contagious conflict’: Multiple factors influence perceived problems with carnivores in Tanzania’s Ruaha landscape. Biol. Conserv.178, 19–27. 10.1016/j.biocon.2014.07.011 (2014). [Google Scholar]
- 13.Galley, W. & Anthony, B. P. Beyond crop-raiding: Unravelling the broader impacts of Human–Wildlife conflict on rural communities. Environ. Manag.74(3), 590–608. 10.1007/s00267-024-02018-9 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahler, J. S. & Gore, M. L. Local perceptions of risk associated with poaching of wildlife implicated in human–wildlife conflicts in Namibia. Biol. Conserv.189, 49–58. 10.1016/j.biocon.2015.02.001 (2015). [Google Scholar]
- 15.Su, K., Zhang, H., Lin, L., Hou, Y. & Wen, Y. Bibliometric analysis of human–wildlife conflict: From conflict to coexistence. Ecol. Inform.68, 101531. 10.1016/j.ecoinf.2021.101531 (2022). [Google Scholar]
- 16.Acharya, K. P., Paudel, P. K., Neupane, P. R. & Köhl, M. Human–wildlife conflicts in Nepal: Patterns of human fatalities and injuries caused by large mammals. PLoS ONE11(9), e0161717. 10.1371/journal.pone.0161717 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kr, S. & Das and S. Chattopadhyay,. Human fatalities from wild elephant attacks—A study of fourteen cases. J. Forensic Leg. Med.18(4), 154–157. 10.1016/j.jflm.2011.01.017 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Gulati, S., Karanth, K. K., Le, N. A. & Noack, F. Human casualties are the dominant cost of human–wildlife conflict in India. Proc. Natl. Acad. Sci.118(8), e1921338118. 10.1073/pnas.1921338118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoare, R. Lessons from 20 years of human–elephant conflict mitigation in Africa. Hum. Dimens. Wildl.20(4), 289–295 (2015). [Google Scholar]
- 20.Nguinguiri J. C. et al. Managing human–wildlife conflicts in central and southern Africa, 2017. [Online]. Available: https://agritrop.cirad.fr/591095/1/Unasylva249-2017-nguinguiri-trebuchon-lebel-cornelis-.pdf Accessed 25 Aug 2024.
- 21.Seoraj-Pillai, N. & Pillay, N. A meta-analysis of human–wildlife conflict: South African and global perspectives. Sustainability9(1), 34. 10.3390/su9010034 (2017). [Google Scholar]
- 22.Zvidzai, M. et al. Application of maximum entropy (MaxEnt) to understand the spatial dimension of human–wildlife conflict (HWC) risk in areas adjacent to Gonarezhou National Park of Zimbabwe. Ecol. Soc.10.5751/ES-14420-280318 (2023). [Google Scholar]
- 23.Sitati, N. W., Walpole, M. J., Smith, R. J. & Leader-Williams, N. Predicting spatial aspects of human–elephant conflict. J. Appl. Ecol.40(4), 667–677. 10.1046/j.1365-2664.2003.00828.x (2003). [Google Scholar]
- 24.Castillo-Huitrón, N. M., Naranjo, E. J., Santos-Fita, D. & Estrada-Lugo, E. The importance of human emotions for wildlife conservation. Front. Psychol.11, 1277 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sillero-Zubiri, C., Sukumar, R. & Treves, A. Living with wildlife: the roots of conflict and the solutions. In Key Topics in Conservation Biology 266–272 (Blackwell Publishing, 2007). [Google Scholar]
- 26.Distefano, E. Human–Wildlife Conflict worldwide: collection of case studies, analysis of management strategies and good practices. Food Agric. Organ. U. N. FAO Sustain. Agric. Rural Dev. Initiat. SARDI Rome Italy Available FAO Corp. Doc. Repos. Httpwww Fao Orgdocuments, 2005, [Online]. Available: https://www.fao.org/3/au241e/au241e.pdf Accessed 25 Aug 2024.
- 27.Dube, K. R. & Kavhu, B. Opportunities and challenges of human–python conflict intervention in local communities adjacent to Nyanga National Park, Zimbabwe. Conserv. Sci. Pract.4(1), e589. 10.1111/csp2.589 (2022). [Google Scholar]
- 28.Gandiwa, E., Gandiwa, P. & Muboko, N. Living with wildlife and associated conflicts in a contested area within the Northern Gonarezhou National Park, Zimbabwe. J. Sustain. Dev. Afr.14(6), 252–260 (2012). [Google Scholar]
- 29.Gonhi, P., Chibememe, G., Utete, B., Mahakata, I. & Madamombe, H. Dynamics and determinants of human–hyaena conflicts in the surroundings of a protected area. Afr. J. Ecol.62(1), e13221. 10.1111/aje.13221 (2024). [Google Scholar]
- 30.Jani, V., De Wit, A. H. & Webb, N. L. Conflict over wildlife conservation in the Mbire District, Northern Zimbabwe. Afr. J. Wildl. Res.49(1), 137. 10.3957/056.049.0137 (2019). [Google Scholar]
- 31.Matseketsa, G., Muboko, N., Gandiwa, E., Kombora, D. M. & Chibememe, G. An assessment of human–wildlife conflicts in local communities bordering the western part of Save Valley Conservancy, Zimbabwe. Glob. Ecol. Conserv.20, e00737 (2019). [Google Scholar]
- 32.Chakuya, J., Chikara, M. & Gandiwa, E. Living with wildlife and associated conflicts in areas adjacent to protected areas, Northern Zimbabwe. Integr. Conserv.3(1), 12–21. 10.1002/inc3.39 (2024). [Google Scholar]
- 33.Dhliwayo, I. Emerging threats and the socio-ecological resilience of local communities, South-East Zimbabwe. In PhD Thesis, Chinhoyi University of Technology, 2023. [Online]. Available: https://ir.cut.ac.zw/xmlui/handle/123456789/395 Accessed 25 Aug 2024.
- 34.Mudimba, T. Perceptions of local residents and authorities on human–wildlife coexistence in Zimbabwe. University of Johannesburg (South Africa), 2019. [Online]. Available: https://search.proquest.com/openview/14d81f2ee6ebf755100c7cb4a81c0101/1?pq-origsite=gscholar&cbl=2026366&diss=y Accessed 25 Aug 2024.
- 35.Lindsey, P. et al. Conserving Africa’s wildlife and wildlands through the COVID-19 crisis and beyond. Nat. Ecol. Evol.4(10), 1300–1310 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Spenceley, A. et al. Tourism in protected and conserved areas amid the COVID-19 pandemic. Parks27, 103–118 (2021). [Google Scholar]
- 37.Snyman, S. et al. State of the wildlife economy in Africa. 2021. [Online]. Available: https://policycommons.net/artifacts/1817148/state-of-the-wildlife-economy-in-africa/2554072/ Accessed 01 May 2025.
- 38.Infield, M., Entwistle, A., Anthem, H., Mugisha, A. & Phillips, K. Reflections on cultural values approaches to conservation: Lessons from 20 years of implementation. Oryx52(2), 220–230 (2018). [Google Scholar]
- 39.Kideghesho, J. R. The potentials of traditional African cultural practices in mitigating overexploitation of wildlife species and habitat loss: Experience of Tanzania. Int. J. Biodivers. Sci. Manag.5(2), 83–94. 10.1080/17451590903065579 (2009). [Google Scholar]
- 40.Barua, M., Bhagwat, S. A. & Jadhav, S. The hidden dimensions of human–wildlife conflict: Health impacts, opportunity and transaction costs. Biol. Conserv.157, 309–316 (2013). [Google Scholar]
- 41.Blackie, I. R. Posttraumatic stress and psychological impacts of human wildlife conflict on victims, their families and caretakers in Botswana. Hum. Dimens. Wildl.28(3), 248–264. 10.1080/10871209.2022.2036394 (2023). [Google Scholar]
- 42.Bond, J. & Mkutu, K. Exploring the hidden costs of human–wildlife conflict in northern Kenya. Afr. Stud. Rev.61(1), 33–54 (2018). [Google Scholar]
- 43.Hendler, R. et al. ‘We are not really marketing mental health’: Mental Health Advocacy in Zimbabwe. PLoS ONE11(9), e016186. 10.1371/journal.pone.0161860 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Musebenzi, D. A Critical Examination of the Realisation of the Right to Mental Health for Children with Mental Disabilities in Zimbabwe, 2023. [Online]. Available: http://ir.gzu.ac.zw:8080/xmlui/handle/123456789/695 Accessed 20 Oct 2024.
- 45.Gandiwa, E., Heitkönig, I. M., Lokhorst, A. M., Prins, H. H. & Leeuwis, C. CAMPFIRE and human–wildlife conflicts in local communities bordering northern Gonarezhou National Park, Zimbabwe. Ecol. Soc.10.5751/ES-05817-180407 (2024). [Google Scholar]
- 46.Mutandwa, E. & Gadzirayi, C. T. Impact of community-based approaches to wildlife management: Case study of the CAMPFIRE programme in Zimbabwe. Int. J. Sustain. Dev. World Ecol.14(4), 336–344. 10.1080/13504500709469734 (2007). [Google Scholar]
- 47.Phiri, K. et al. Knowledge, Attitudes and Perceptions of Communities Towards the CAMPFIRE: A Case Study of Selected Districts of Matabeleland. In Living with Wildlife in Zimbabwe: Navigating Conflict and Co-existence 89 (Springer, 2024). [Google Scholar]
- 48.Tchakatumba, P. K., Gandiwa, E., Mwakiwa, E., Clegg, B. & Nyasha, S. Does the CAMPFIRE programme ensure economic benefits from wildlife to households in Zimbabwe?. Ecosyst. People15(1), 119–135. 10.1080/26395916.2019.1599070 (2019). [Google Scholar]
- 49.Marowa, I., Matanzima, J. & Nhiwatiwa, T. Interactions between humans, crocodiles, and hippos at Lake Kariba, Zimbabwe. Human-Wildlife Interact.15(1), 25 (2021). [Google Scholar]
- 50.Matanzima, J. & Marowa, I. Human–Wildlife Conflict and Precarious Livelihoods of the Tonga-Speaking People of North-Western Zimbabwe. In Livelihoods of Ethnic Minorities in Rural Zimbabwe (eds Helliker, K. et al.) 107–122 (Springer, 2022). 10.1007/978-3-030-94800-9_6. [Google Scholar]
- 51.Utete, B. Human–Wildlife-Water Conflicts. In Living with Wildlife in Zimbabwe: Navigating Conflict and Co-existence 173 (Springer, 2024). [Google Scholar]
- 52.Musiwa, A. R. & Mhlanga, W. Human–wildlife conflict in Mhokwe Ward, Mbire District, North-East Zimbabwe. Afr. J. Ecol.58(4), 786–795. 10.1111/aje.12774 (2020). [Google Scholar]
- 53.Mutasa, C. Zimbabwe’s Climate: Past, Present and Future Trends. In Climate Change Law in Zimbabwe: Concepts and Insights, 11–47 (2019).
- 54.Zhou, Z. Wildlife Tourism and Human–Wildlife Conflicts in Zimbabwe: Causes, Compensation, and Community Perceptions. In Wildlife Tourism Dynamics in Southern Africa: Contemporary, Issues Challenges and Prospects for Sustainable Development (eds Stone, L. S. & Stone, M. T.) 15–29 (Springer, 2024). 10.1007/978-3-031-57252-4_2. [Google Scholar]
- 55.Esmaeili, S., Hemami, M.-R. & Goheen, J. R. Human dimensions of wildlife conservation in Iran: Assessment of human–wildlife conflict in restoring a wide-ranging endangered species. PLoS ONE14(8), e0220702. 10.1371/journal.pone.0220702 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Espinosa, S. & Jacobson, S. K. Human–wildlife conflict and environmental education: Evaluating a community program to protect the Andean Bear in Ecuador. J. Environ. Educ.43(1), 55–65. 10.1080/00958964.2011.579642 (2012). [Google Scholar]
- 57.Hamed, K. H. Exact distribution of the Mann-Kendall trend test statistic for persistent data. J. Hydrol.365(1–2), 86–94 (2009). [Google Scholar]
- 58.Sen, P. K. Estimates of the regression coefficient based on Kendall’s tau. J. Am. Stat. Assoc.63(324), 1379–1389 (1968). [Google Scholar]
- 59.Ji, Y., Wei, X., Liu, F., Li, D. & Li, J. Spatial-temporal patterns of human–wildlife conflicts under coupled impact of natural and anthropogenic factors in Mt. Gaoligong, western Yunnan, China. Glob. Ecol. Conserv.40, e02329 (2022). [Google Scholar]
- 60.Pérez-Flores, J., Mardero, S., López-Cen, A. & Contreras-Moreno, F. M. Human–wildlife conflicts and drought in the greater Calakmul Region, Mexico: Implications for tapir conservation. Neotropical Biol. Conserv.16(4), 539 (2021). [Google Scholar]
- 61.Betty, E. L. et al. Using emerging hot spot analysis of stranding records to inform conservation management of a data-poor cetacean species. Biodivers. Conserv.29(2), 643–665. 10.1007/s10531-019-01903-8 (2020). [Google Scholar]
- 62.Galinskaitė, L. et al. The influence of landscape structure on wildlife-vehicle collisions: Geostatistical analysis on hot spot and habitat proximity relations. ISPRS Int. J. Geo-Inf.11(1), 63 (2022). [Google Scholar]
- 63.Ruda, A., Kolejka, J. & Silwal, T. GIS-assisted prediction and risk zonation of wildlife attacks in the Chitwan National Park in Nepal. ISPRS Int. J. Geo-Inf.7(9), 369 (2018). [Google Scholar]
- 64.Garrah, E., Danby, R. K., Eberhardt, E., Cunnington, G. M. & Mitchell, S. Hot spots and hot times: Wildlife road mortality in a regional conservation corridor. Environ. Manag.56(4), 874–889. 10.1007/s00267-015-0566-1 (2015). [DOI] [PubMed] [Google Scholar]
- 65.Barro, A. S. et al. Identifying hotspots of human anthrax transmission using three local clustering techniques. Appl. Geogr.60, 29–36 (2015). [Google Scholar]
- 66.Yang, N. et al. Mapping potential human-elephant conflict hotspots with UAV monitoring data. Glob. Ecol. Conserv.43, e02451. 10.1016/j.gecco.2023.e02451 (2023). [Google Scholar]
- 67.Rusoke, T. Improving livelihoods of smallholder farmers adjacent to wildlife protected areas to mitigate Human-wildlife conflicts in Uganda. 2024, [Online]. Available: https://www.researchgate.net/profile/Taddeo-Rusoke/publication/383123890_Improving_Livelihoods_of_Smallholder_Farmers_Adjacent_to_Wildlife-Protected_Areas_to_Mitigate_Human-Wildlife_Conflicts_in_Uganda_A_Case_Study_of_Selected_Parishes_Bordering_Kibale_National_Park/links/66bde986145f4d35535cbbc5/Improving-Livelihoods-of-Smallholder-Farmers-Adjacent-to-Wildlife-Protected-Areas-to-Mitigate-Human-Wildlife-Conflicts-in-Uganda-A-Case-Study-of-Selected-Parishes-Bordering-Kibale-National-Park.pdf Accessed 12 Sep 2024.
- 68.Perera, B. The human-elephant conflict: A review of current status and mitigation methods. Gajah30(2009), 41–52 (2009). [Google Scholar]
- 69.Chihona, S. The impact of Nile crocodile (Crocodylus niloticus) on the communal livelihoods: A case study of areas surrounding Ruti Dam in Gutu and Buhera districts in Zimbabwe. Masters, University of South Africa, 2014. Accessed 25 Aug 2024. [Online]. Available: http://thesisbank.jhia.ac.ke/1127/
- 70.Mpakairi, K., Ndaimani, H., Vingi, K., Madiri, T. H. & Nekatambe, T. Ensemble modelling predicts human carnivore conflict for a community adjacent to a protected area in Zimbabwe. Afr. J. Ecol.56(4), 957–963. 10.1111/aje.12526 (2018). [Google Scholar]
- 71.Parker, G. E. & Osborn, F. V. Investigating the potential for chilli Capsicum spp. to reduce human–wildlife conflict in Zimbabwe. Oryx40(3), 343–346 (2006). [Google Scholar]
- 72.WWF SARPO. Human wildlife conflict manual. Harare Zimb. WWF South. Afr. Reg. Programme Off. SARPO, 2005.
- 73.Matanzima, J. Negative Human–Wildlife Interactions at Lake Kariba: Emphasis on Crocodile and Hippo Attacks on People. In The Materiality of Lake Kariba 135–172 (Springer, 2024). 10.1007/978-981-99-9573-8_5. [Google Scholar]
- 74.Sadie, Y. Human–wildlife conflict and wildlife conservation: Attitudes of the Ovahimbas in Namibia. Confl. Trends2019(3), 38–46 (2019). [Google Scholar]
- 75.Bishop, J. M., Leslie, A. J., Bourquin, S. L. & O’Ryan, C. Reduced effective population size in an overexploited population of the Nile crocodile (Crocodylus niloticus). Biol. Conserv.142(10), 2335–2341. 10.1016/j.biocon.2009.05.016 (2009). [Google Scholar]
- 76.Mosse, M. N., Odadi, W. O. & Kibue, G. W. Anthropogenic threats to crocodiles, and the level and sociodemographic determinants of their utilization in lower River Tana Basin, Kenya. Trop. Conserv. Sci.17, 19400829241241456. 10.1177/19400829241241457 (2024). [Google Scholar]
- 77.Ngcobo, J. N., Nedambale, T. L., Nephawe, K. A., Sawosz, E. & Chwalibog, A. The future survival of African elephants: Implications for conservation. Int. J. Avian Wildl. Biol.3(5), 379–384 (2018). [Google Scholar]
- 78.Ngorima, P. et al. Trends in elephant poaching in the Mid-Zambezi Valley, Zimbabwe: Lessons learnt and future outlook. Afr. J. Ecol.60(4), 1342 (2022). [Google Scholar]
- 79.Muguti, T. Human–Wildlife Conflicts and Livelihoods in Binga District, Zimbabwe: Local Communities’ Lived Experiences. In Living with Wildlife in Zimbabwe (eds Matanzima, J. & Utete, B.) 41–57 (Springer, 2024). 10.1007/978-3-031-66060-3_3. [Google Scholar]
- 80.Simakani, A., Mashapa, C., Muboko, N., Mutanga, C. N. & Gandiwa, E. Trends and local perceptions of human-crocodile conflicts in Kariba town, northern Zimbabwe. Hum. Dimens. Wildl.29(4), 432–440. 10.1080/10871209.2023.2243970 (2024). [Google Scholar]
- 81.Shankar, V. S., Purti, N. & Prabakaran, N. Snowballing trends of saltwater crocodile conflicts in Andaman Islands: A mounting concern for conservation and sustainable co-existence. Eur. J. Wildl. Res.70(4), 67. 10.1007/s10344-024-01818-y (2024). [Google Scholar]
- 82.Utete, B. A review of the conservation status of the Nile crocodile (Crocodylus niloticus Laurenti, 1768) in aquatic systems of Zimbabwe. Glob. Ecol. Conserv.29, e01743. 10.1016/j.gecco.2021.e01743 (2021). [Google Scholar]
- 83.Chakanyuka, T. L. CITES and the African Elephant. Chin. J. Environ. Law.10.1163/24686042-12340049 (2020). [Google Scholar]
- 84.Long, H. et al. Patterns of human–wildlife conflict and management implications in Kenya: A national perspective. Hum. Dimens. Wildl.25(2), 121–135. 10.1080/10871209.2019.1695984 (2020). [Google Scholar]
- 85.Nicole, B.-F. An assessment of the human–wildlife conflict across Africa. In Wildlife Population Monitoring 1–10 (IntechOpen, 2019). [Google Scholar]
- 86.Tiller, L. N. et al. Changing seasonal, temporal and spatial crop-raiding trends over 15 years in a human-elephant conflict hotspot. Biol. Conserv.254, 108941 (2021). [Google Scholar]
- 87.dos Santos, J. Long-range movements of three savannah Elephants (Loxodonta Africana Africana) within a transfrontier conservation area (TFCA)–Adaptive Management Implications. Elephant Mov. Hum.-Elephant Confl. Transfront. Conserv. Area. 11. [Online]. Available: https://scholar.sun.ac.za/bitstreams/aac5137c-daf5-4286-a9c2-f0022bee2a9f/download#page=27 Accessed 25 Aug 2024.
- 88.Gross, E. M. et al. Exploring routes to coexistence: Developing and testing a human-elephant conflict-management framework for African elephant-range Countries. Diversity14(7), 525. 10.3390/d14070525 (2022). [Google Scholar]
- 89.Loarie, S. R., Aarde, R. J. V. & Pimm, S. L. Fences and artificial water affect African savannah elephant movement patterns. Biol. Conserv.142(12), 3086–3098. 10.1016/j.biocon.2009.08.008 (2009). [Google Scholar]
- 90.Liebenberg, L. A field guide to the animal tracks of southern Africa. New Africa Books, 1990. [Online]. Available: https://books.google.com/books?hl=en&lr=&id=oEFz4cEv01UC&oi=fnd&pg=PP13&dq=African+Buffaloes+are+generally+known+to+be+aggressive+primarily+when+they+feel+cornered+or+perceive+a+threat+to+their+territory+or+young+&ots=lFlghd55sG&sig=R5zTCe37MGE97kqLHrAzgkgFIJo Accessed 12 Sep 2024.
- 91.Tefera, G. G., Tessema, T. H., Bekere, T. A. & Gutema, T. M. Human-common hippo (Hippopotamus amphibius)-conflict in the Dhidhessa Wildlife Sanctuary and its surrounding, Southwestern Ethiopia. PLoS ONE19(5), e0303647 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dickman, A. J. Complexities of conflict: The importance of considering social factors for effectively resolving human–wildlife conflict. Anim. Conserv.13(5), 458–466. 10.1111/j.1469-1795.2010.00368.x (2010). [Google Scholar]
- 93.Treves, A. & Karanth, K. U. Human-carnivore conflict and perspectives on carnivore Management Worldwide. Conserv. Biol.17(6), 1491–1499. 10.1111/j.1523-1739.2003.00059.x (2003). [Google Scholar]
- 94.Loveridge, A. J. et al. Bells, bomas and beefsteak: Complex patterns of human-predator conflict at the wildlife-agropastoral interface in Zimbabwe. PeerJ5, e2898 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matindike, S., Chibememe, G. & Mahakata, I. Livestock losses and predation crisis in communal areas around Sengwa Wildlife Research Area (SWRA), Zimbabwe. Open Access Libr. J.10(4), 1–13 (2023). [Google Scholar]
- 96.Woodroffe, R., Thirgood, S. & Rabinowitz, A. People and wildlife, conflict or co-existence?, vol. 9. Cambridge University Press, 2005. [Online]. Available: https://books.google.com/books?hl=en&lr=&id=HXMl0C3cHJwC&oi=fnd&pg=PR8&dq=Woodroffe,+R.,+Thirgood,+S.,+%26+Rabinowitz,+A.+(2005).+People+and+Wildlife,+Conflict+or+Co-existence%3F.+Cambridge+University+Press.&ots=IKjdoMNCDR&sig=iBq4JtQcaStLPqOc8vjva7q-frw Accessed 25 Aug 2024.
- 97.Buchholtz, E. K., Stronza, A., Songhurst, A., McCulloch, G. & Fitzgerald, L. A. Using landscape connectivity to predict human–wildlife conflict. Biol. Conserv.248, 108677 (2020). [Google Scholar]
- 98.König, H. J. et al. Integrated framework for stakeholder participation: Methods and tools for identifying and addressing human–wildlife conflicts. Conserv. Sci. Pract.3(3), e399. 10.1111/csp2.399 (2021). [Google Scholar]
- 99.Teixeira, L. et al. Linking human and ecological components to understand human–wildlife conflicts across landscapes and species. Conserv. Biol.35(1), 285–296. 10.1111/cobi.13537 (2021). [DOI] [PubMed] [Google Scholar]
- 100.Chase, M. J. et al. Continent-wide survey reveals massive decline in African savannah elephants. PeerJ4, e2354 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stoldt, M., Göttert, T., Mann, C. & Zeller, U. Transfrontier conservation areas and human–wildlife conflict: The case of the Namibian component of the Kavango-Zambezi (KAZA) TFCA. Sci. Rep.10(1), 7964 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cushman, S. A. et al. Prioritizing core areas, corridors and conflict hotspots for lion conservation in southern Africa. PLoS ONE13(7), e0196213 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schlossberg, S., Chase, M. J. & Griffin, C. R. Poaching and human encroachment reverse recovery of African savannah elephants in south-east Angola despite 14 years of peace. PLoS ONE13(3), e0193469 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Van Aarde, R. J. & Jackson, T. P. Megaparks for metapopulations: Addressing the causes of locally high elephant numbers in southern Africa. Biol. Conserv.134(3), 289–297 (2007). [Google Scholar]
- 105.Bauer, D. Landscape-scale conservation of lions in the Kavango-Zambezi Transfrontier Conservation Area. In PhD Thesis, Staats-und Universitätsbibliothek Hamburg Carl von Ossietzky, 2020. [Online]. Available: https://ediss.sub.uni-hamburg.de/handle/ediss/8819 Accessed 25 Aug 2024.
- 106.Munthali, S. M., Smart, N., Siamudaala, V., Mtsambiwa, M. & Harvie, E. Integration of ecological and socioeconomic factors in securing wildlife dispersal corridors in the Kavango-Zambezi transfrontier conservation area, Southern Africa. Sel. Stud. Biodivers.181, 2018. [Online]. Available: https://books.google.com/books?hl=en&lr=&id=UvyPDwAAQBAJ&oi=fnd&pg=PA181&dq=As+elephants+and+lions+traverse+these+landscapes,+they+often+move+into+areas+where+human+activities,+such+as+agriculture+and+settlement,+have+encroached+upon+traditional+wildlife+corridors+%2B+western+zimbabwe&ots=SiQGHBJE8A&sig=dO9HZ60VvbqNGhAGa9MuJWhQiEA Accessed 25 Aug 2024.
- 107.Chomba, C., Senzota, R., Chabwela, H., Mwitwa, J. & Nyirenda, V. Patterns of human–wildlife conflicts in Zambia, causes, consequences and management responses. J. Ecol. Nat. Environ.4(12), 303–313 (2012). [Google Scholar]
- 108.Hemson, G. A. The ecology of conservation of lions: Human wildlife conflict in semi-arid Botswana. In PhD Thesis, University of Oxford, 2004. [Online]. Available: http://www.carnivoreconservation.org/files/thesis/hemson_2003_phd.pdf Accessed 12 Sep 2024.
- 109.Mupunga, P. & Shoko, J. Local community perceptions on human wildlife interactions in the face of climate variability. A case of Nyaminyami community, Zimbabwe. Front. Sustain. Tour.3, 1328510 (2024). [Google Scholar]
- 110.Pozo, R. A. et al. A multispecies assessment of wildlife impacts on local community livelihoods. Conserv. Biol.35(1), 297–306. 10.1111/cobi.13565 (2021). [DOI] [PubMed] [Google Scholar]
- 111.Kolowski, J. M. & Holekamp, K. E. Spatial, temporal, and physical characteristics of livestock depredations by large carnivores along a Kenyan reserve border. Biol. Conserv.128(4), 529–541 (2006). [Google Scholar]
- 112.Bel, S. L., Chavernac, D., Mapuvire, G. & Cornu, G. FrontlineSMS as an early warning network for Human–Wildlife mitigation: Lessons learned from tests conducted in Mozambique and Zimbabwe. Electron. J. Inf. Syst. Dev. Ctries.60(1), 1–13. 10.1002/j.1681-4835.2014.tb00427.x (2014). [Google Scholar]
- 113.Gargallo, E. Human–Wildlife Conflict in a ‘successful’ Community Conservation Programme: Economic and territorial impacts on Namibia’s conservancies. J. Arid Environ.193, 104591. 10.1016/j.jaridenv.2021.104591 (2021). [Google Scholar]
- 114.Matanzima, J., Marowa, I. & Nhiwatiwa, T. Negative human–crocodile interactions in Kariba, Zimbabwe: Data to support potential mitigation strategies. Oryx57(4), 452–456. 10.1017/S003060532200014X (2023). [Google Scholar]
- 115.Warner, M. Z. Examining human-elephant conflict in southern Africa: causes and options for coexistence. Master Stud. Capstone Proj. Depatment Earth Envirionment Sci., 2008, [Online]. Available: https://www.researchgate.net/profile/Zoe-Warner-3/publication/268338685_Examining_Human-Elephant_Conflict_in_Southern_Africa_Causes_and_Options_for_Coexistence/links/5c6197a892851c48a9cd324a/Examining-Human-Elephant-Conflict-in-Southern-Africa-Causes-and-Options-for-Coexistence.pdf Accessed 26 Aug 2024.
- 116.Packer, C., Ikanda, D., Kissui, B. & Kushnir, H. Conservation biology: Lion attacks on humans in Tanzania. Nature436(7053), 927 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study is available on request from the corresponding author.









