Abstract
Following the isolation of Mycoplasma pneumoniae from urogenital specimens (M. Goulet, R. Dular, J. G. Tully, G. Billows, and S. Kasatiya, J. Clin. Microbiol. 33:2823–2825, 1995), a study was undertaken to confirm the observations by PCR. Specific primers directed to the P1 adhesin gene of M. pneumoniae were used. A total of 300 genital specimens were tested for M. pneumoniae and Mycoplasma genitalium by culture and PCR. Of these, 15 were positive by culture and 17 were positive by PCR for M. pneumoniae. No M. genitalium was detected in any of the specimens by either method. The present study demonstrates that PCR is sensitive and rapid compared to cumbersome culture methods and can be used to detect M. pneumoniae in urogenital specimens in a routine diagnostic laboratory.
Mycoplasma pneumoniae is a pathogen primarily of the human respiratory tract. However, it has been found to be associated with a varied range of extrapulmonary complications, with mild to severe symptoms (9, 13). Isolation of M. pneumoniae from a tubo-ovarian abscess and from three cases of vaginal infections has been reported previously (19).
The detection of M. pneumoniae by culture methods requires 3 to 4 weeks (15). Routine serological methods for the detection of M. pneumoniae infections are limited by a significant degree of variability and nonspecificity (2). Although immunoglobulin M assays partially overcome these difficulties, they also have limitations (17). In addition, it has been shown that M. pneumoniae has some of the same protein and lipid antigenic determinants as Mycoplasma genitalium (12). Cross-reactive epitopes have been detected among several proteins, including the large (170 kDa) surface membrane protein P1 of M. pneumoniae and the 140-kDa protein of M. genitalium, which are believed to mediate mycoplasma adherence to host cells (14). Although these microorganisms appear to have similar morphologies, limited DNA homology (1.8 to 8% based on total genomic DNA-DNA hybridization tests) exists between M. genitalium and M. pneumoniae (6). These difficulties have led to the development of PCR methods for these fastidious microorganisms (18). Recently, PCR has been chosen to detect M. genitalium (7), Ureaplasma urealyticum, and Mycoplasma hominis (11). Deguchi et al. (7) suggested that PCR is much more sensitive than culture in detecting M. genitalium.
Initially, our interest in this area arose due to a coincidental isolation of M. pneumoniae from a cervical swab submitted to our laboratory by a gynecological clinic for routine isolation of genital mycoplasmas. This rare observation prompted an investigation to determine the presence of M. pneumoniae in urogenital specimens, leading to the isolation of 24 strains of M. pneumoniae (10). M. genitalium has been isolated from the respiratory as well as the genital area (1). Also, M. pneumoniae and M. genitalium were both isolated from synovial fluid (21), suggesting that these two organisms can occur together. These findings and our recent isolation of M. pneumoniae in urogenital specimens (10) led us to initiate a study to detect M. pneumoniae by PCR in urogenital specimens as well as to differentiate M. pneumoniae and M. genitalium directly in urogenital specimens.
M. pneumoniae (ATCC 15531) and M. genitalium (ATCC 33530) were purchased from the American Type Culture Collection (Rockville, Md.). The mycoplasma strains were cultured in SP-4 medium (22) containing 2,000 U of penicillin G ml−1 and 2.5 μg of amphotericin B ml−1. Thallium acetate was not added to the medium, since it can inhibit the growth of M. genitalium (20).
Urogenital specimens were obtained from 300 patients. Most samples were cervical swabs, but pelvic fluid and urine samples were also obtained. A few urethral swabs were obtained from male patients. The ages of the patients ranged from 17 to 51 years. The patients did not report a history of respiratory disease during the 6 months prior to specimen collection, and most patients belonged to a socioeconomic group that, on average, had a low risk of contracting sexually transmitted diseases.
Endocervical swabs were collected and inoculated into a transport medium (8) and then transferred to the laboratory within 24 h. Urethral swabs taken from male patients were also transferred to transport medium for cultivation attempts. The specimens were processed on arrival or after storage at −70°C. A portion of the specimen was processed for culture, while the remaining volume was prepared for PCR as described below.
An aliquot (0.2 ml) of the sample in transport medium was inoculated into each of the following media: U9 broth and modified (A7B) agar for Ureaplasma isolation and identification (16), arginine broth (23), SP-4 broth (20), and mycoplasma broth and agar (8) for the isolation of mycoplasmas. All broth cultures were incubated at 37°C and were examined every 3rd day for turbidity and a pH change over an 8-week period. The broth cultures were subcultured onto agar plates at 5-, 10-, and 15-day intervals. Agar plates were incubated at 37°C in a 10% carbon dioxide environment and were examined weekly over an 8-week period for potential M. pneumoniae or M. genitalium colonies and tested for hemadsorption and hemolysis.
DNA from different mycoplasma strains and clinical samples was prepared for PCR as described by Cadieux et al. (3). Briefly, the sample (about 100 μl) was centrifuged at 14,000 rpm for 20 min, washed in phosphate-buffered saline (0.1 M NaCl, 2.5 mM KCl, 10 mM Na2HPO4, 1.5 mM KH2PO4 [pH 7.4]), and resuspended in 60 μl of distilled water. The sample was then boiled for 5 to 10 min and stored at 4°C.
The primers were synthesized on an Applied Biosystems 380A DNA synthesizer by the phosphoramidite method as described previously (3). The pair P4 (P4A, 5′AGG CTC AGG TCA ATC TGG CGT GGA 3′ and P4B, 5′GGA TCA AAC AGA TCG GTG ACT GGG T3′) was specific to the M. pneumoniae P1 adhesin gene and amplified a 345-bp fragment from nucleotides 3947 to 4291 (GenBank accession no. M18639). The pair G3 (G3A, 5′ GCT TTA AAC CTG GTA ACC AGA TTG ACT 3′ and G3B, 5′ GAG CGT TAG AGA TCC CTG TTC TGT TA 3′) was specific to the M. genitalium adhesin gene and amplified a 507-bp fragment from nucleotides 3754 to 4260 (GenBank accession no. M31431). The primers were chosen to have long but equal normalized length (24) to allow the use of a high annealing temperature, which would reduce the possibility of obtaining unwanted bands originating from nonspecific amplification.
PCR amplification and electrophoresis were performed as described previously (3). PCR were performed in a total volume of 50 μl containing 5 μl of 10× PCR buffer (100 mM Tris-HCl [pH 8.3], 500 mM KCl, 27 mM MgCl2, 0.1% gelatin), 250 μM (each) deoxynucleoside triphosphate (Pharmacia Baie D’Urfe, Quebec, Canada), 1.0 μM of P4 primers, 2 U of Taq DNA polymerase (Promega, Madison, Wis.), and 20 μl of the sample to be amplified. The reaction volume was overlaid with 50 μl of paraffin oil to prevent evaporation. The samples were subjected to 30 cycles of amplification. The samples were electrophoresed on a 1.5% (wt/vol) agarose gel (Promega), followed by ethidium bromide staining and photography.
To verify that the fragments amplified by the pairs of primers P4 and G3 were the ones expected, oligonucleotides specific to internal regions of those fragments were synthesized and used as probes to hybridize Southern blots. The probes were synthesized as described previously (3) except that the labelling was done by the nonradioactive digoxigenin method. Probe 4A4B was specific to the 345-bp fragment of M. pneumoniae DNA amplified by the P4 primers and had the sequence 5′ GAT GTT GAT GGT ATT GTA C 3′. Probe 3A3B was specific to the 507-bp fragment of M. genitalium DNA amplified by the G3 primers and had the sequence 5′ CCT TTG ATT GTA ACT GTT C 3′. The probes were labelled at their 3′ end by terminal deoxynucleotidyl transferase (terminal transferase) with the DIG-oligonucleotide 3′ end labelling kit (Boehringer Mannheim, Quebec, Canada).
With the isolation of M. pneumoniae from urogenital specimens in early 1995 (10), reported from our laboratory, we thought it worthwhile to use the newer, faster PCR methodology for a larger study of M. pneumoniae incidence in urogenital specimens. Although PCR has been used successfully to detect M. pneumoniae in respiratory specimens, no PCR study has yet been done to detect M. pneumoniae in urogenital specimens. The PCR protocol described by Cadieux et al. (3) was used to detect M. pneumoniae in urogenital specimens. The guidelines of Chewley (4) were followed to prevent carryover contamination from previously amplified PCR products. Appropriate positive and negative controls were also included.
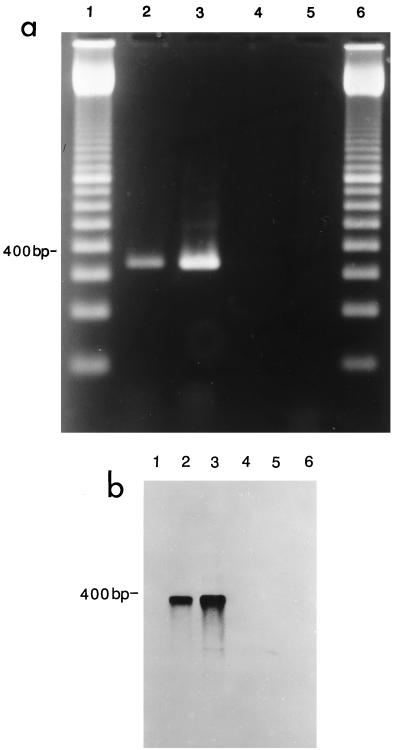
The results shown in Fig. 1a indicate that an M. pneumoniae-positive control (lane 2) and a culture-positive specimen (lane 3) generated the expected 345-bp amplified DNA, while DNA from a negative specimen (lane 4) and from M. genitalium (lane 5) were not amplified. Figure 1b confirms the specificity of amplification through the use of a Southern blot, as previously shown by Cadieux et al. (3).
FIG. 1.
(a) Agarose gel electrophoresis showing specificity of P4 primers tested with M. pneumoniae and M. genitalium. Lane 1, 100-bp ladder (Pharmacia); lane 2, DNA from M. pneumoniae ATCC 15531 amplified with P4 primers; lane 3, DNA from a clinical isolate positive for M. pneumoniae; lane 4, DNA from a clinical isolate negative for M. pneumoniae; lane 5, DNA from M. genitalium ATCC 33530 amplified with P4 primers; lane 6, 100-bp ladder (Pharmacia). (b) Southern blot of the gel shown in Fig. 1a hybridized with the probe 4A4B specific for M. pneumoniae. Lanes 2 and 3, the probe hybridized to the 345-bp M. pneumoniae-specific fragment only.
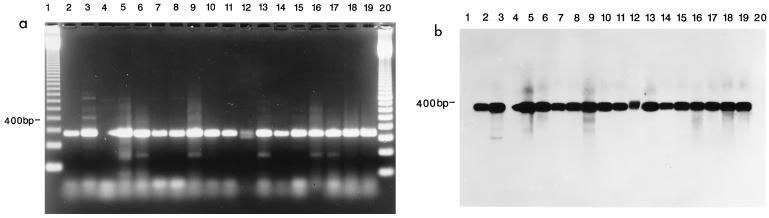
A total of 300 specimens were analyzed by culture and PCR for the presence of M. pneumoniae and M. genitalium. We obtained 17 samples positive by PCR for M. pneumoniae; of these, 15 were also positive by culture (Table 1). M. genitalium was not detected in any of the samples in this survey. Figure 2a shows typical bands obtained for some representative positive specimens after PCR amplification and stained by ethidium bromide, while Fig. 2b shows the Southern probe confirmation results for these positive samples. Other organisms commonly found included U. urealyticum (130 single isolates plus 16 cocultures with M. hominis and 2 cocultures with M. pneumoniae). M. hominis also appeared alone in 14 specimens and in coculture with M. pneumoniae in 3 specimens. The PCR was completely specific, giving a positive result for M. pneumoniae cultures.
TABLE 1.
Comparison of results obtained for M. pneumoniae detection by culture and PCR for 300 urogenital specimensa
| M. pneumoniae culture result | No. of samples with PCR result indicated
|
|
|---|---|---|
| + | − | |
| + | 15b | 0 |
| − | 2 | 283 |
Tested simultaneously for Mycoplasma genitalium.
Three of these samples were also positive for M. hominis, and two were also positive for U. urealyticum.
FIG. 2.
(a) Agarose gel electrophoresis showing detection of M. pneumoniae in clinical specimens by PCR. Lanes 1 and 20, 100-bp ladder (Gibco BRL); lane 2, DNA from M. pneumoniae ATCC 15531 amplified with P4 primers; Lanes 3 to 19, DNA from clinical isolates amplified by P4 primers. (b) Southern blot of gel shown in panel a hybridized with probe 4A4B specific for M. pneumoniae. The probe hybridized to the 345-bp M. pneumoniae-specific fragment only in lanes 2 to 19.
The female patients in this study reported symptoms of cervicitis, unusual discharge, chronic abdominal pain, vulvitis, unusual urinary symptoms, infertility, recurrent abortions, stillbirths, abdominal pain in pregnancy, and abnormal bleeding, while the male patients reported prostatic or urethral pain, recurrent urethritis, and in some cases, infertility. The age group in which M. pneumoniae was isolated ranged from 19 to 49 years. Most of the positive specimens were cervical swabs while in one case it was an urethral swab. Symptoms for the female patients from which M. pneumoniae was isolated were cervicitis, urethritis, and abdominal pain, while the male patient’s symptom was urethritis.
In conclusion, our study clearly demonstrates that the PCR method is specific, sensitive, and rapid; that it clearly distinguishes between M. pneumoniae and M. genitalium; that it can be used in a routine clinical laboratory; and that it should be considered the method of choice to detect M. pneumoniae in urogenital specimens. Our study also confirms our earlier report on the significant frequency of occurrence of M. pneumoniae in the urogenital tract. Considering the known pathogenicity of M. pneumoniae to human epithelial tissue as well as the clinical symptoms found in the female patients in this study, further work is clearly needed to determine the role of M. pneumoniae in urogenitary infections.
REFERENCES
- 1.Baseman J B, Dallo S F, Tully J G, Rose D L. Isolation and characterization of Mycoplasma genitalium strains from the human respiratory tract. J Clin Microbiol. 1988;26:2266–2269. doi: 10.1128/jcm.26.11.2266-2269.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunner M, Prescott B, Greenberg H, James W D, Horswood R L, Channock R M. Unexpectedly high frequency of antibody to Mycoplasma pneumoniae in human sera as measured by sensitive techniques. J Infect Dis. 1977;135:524–530. doi: 10.1093/infdis/135.4.524. [DOI] [PubMed] [Google Scholar]
- 3.Cadieux N, Lebel P, Brousseau R. Use of a triplex polymerase chain reaction for the detection and differentiation of Mycoplasma pneumoniae and Mycoplasma genitalium in the presence of human DNA. J Gen Microbiol. 1993;139:2431–2437. doi: 10.1099/00221287-139-10-2431. [DOI] [PubMed] [Google Scholar]
- 4.Chewley J P. The polymerase chain reaction. A review of the practical limitations for human immunodeficiency virus diagonosis. J Virol Methods. 1989;25:179–188. doi: 10.1016/0166-0934(89)90031-1. [DOI] [PubMed] [Google Scholar]
- 5.Csŭtőrtoki V, Stipkovits L. Nachweis von Mycoplasma pneumoniae Infektionen in weiblichen Geschlechtsorganen. Zentralbl Gynäkol. 1977;99:877–879. [PubMed] [Google Scholar]
- 6.Dallo S F, Chavoya A, Su C J, Baseman J B. DNA and protein sequence homologies between the adhesins of Mycoplasma genitalium and Mycoplasma pneumoniae. Infect Immun. 1989;57:1059–1065. doi: 10.1128/iai.57.4.1059-1065.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deguchi T, Gilroy C B, Taylor-Robinson D. Comparison of two PCR-based assays for detecting Mycoplasma genitalium in clinical specimens. Eur J Clin Microbiol Infect Dis. 1995;14:629–631. doi: 10.1007/BF01690742. [DOI] [PubMed] [Google Scholar]
- 8.Dular R, Lambert M, Bruce B W, Phipps P M, Rossier E, Kasatiya S. Mycoplasma pneumoniae infections in a rural setting in Canada. Can Med Assoc J. 1987;136:1271–1273. [PMC free article] [PubMed] [Google Scholar]
- 9.Fleischhauer P, Huben U, Mertens M. Nachweis von Mycoplasma pneumoniae in Liquor bei akutes polineuritis. Dtsch Med Wochenschr. 1972;97:678–682. doi: 10.1055/s-0028-1107421. [DOI] [PubMed] [Google Scholar]
- 10.Goulet M, Dular R, Tully J G, Billows G, Kasatiya S. Isolation of Mycoplasma pneumoniae from the human genital tract. J Clin Microbiol. 1995;33:2823–2825. doi: 10.1128/jcm.33.11.2823-2825.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horn M A, Wolff C, Dressel P, Zimmermann A, Ruckdeschel G. Polymerase chain reaction versus culture for detection of Ureaplasma urealyticum and Mycoplasma hominis in the urogenital tract of adults and the respiratory tract of newborns. Eur J Clin Microbiol Infect Dis. 1996;15:595–598. doi: 10.1007/BF01709369. [DOI] [PubMed] [Google Scholar]
- 12.Lind K, Lindhardt B Ø, Schütten H J, Blom J, Christiansen C. Serological cross-reactions between Mycoplasma genitalium and Mycoplasma pneumoniae. J Clin Microbiol. 1984;20:1036–1043. doi: 10.1128/jcm.20.6.1036-1043.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meseguer M A, De Rafael L, Vidal M L. Stevens-Johnson’s syndrome with isolation of Mycoplasma pneumoniae from skin lesions. Eur J Clin Microbiol. 1986;5:167–168. doi: 10.1007/BF02013977. [DOI] [PubMed] [Google Scholar]
- 14.Morrison-Plummer J, Jones D H, Daly K, Tully J G, Taylor-Robinson D, Baseman J B. Molecular characterization of Mycoplasma genitalium species-specific and cross reactive determinants: identification of an immunodominant protein of M. genitalium. Isr J Med Sci. 1987;23:453–457. [PubMed] [Google Scholar]
- 15.Razin S, Tully J G. Methods in mycoplasmology. 1. Mycoplasma characterization. New York, N.Y: Academic Press; 1983. [Google Scholar]
- 16.Shepard M C. Culture media for ureaplasmas. Methods Mycoplasmol. 1983;1:137–146. [Google Scholar]
- 17.Sillis M. The limitations of IgM assays in the serological diagnosis of Mycoplasma pneumoniae infections. J Med Microbiol. 1990;33:253–258. doi: 10.1099/00222615-33-4-253. [DOI] [PubMed] [Google Scholar]
- 18.Skakni L, Sordet A, Just J, Parker J L, Costil J, Moniot-ville N, Baricout F, Chenon A G. Detection of Mycoplasma pneumoniae in clinical samples from pediatric patients by polymerase chain reaction. J Clin Microbiol. 1992;30:2638–2643. doi: 10.1128/jcm.30.10.2638-2643.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas M, Jones M, Ray S, Andrews B. Mycoplasma pneumoniae in a tubo-ovarian abcess. Lancet. 1979;ii:774–775. doi: 10.1016/s0140-6736(75)90765-5. [DOI] [PubMed] [Google Scholar]
- 20.Tully J G, Rose D L, Whitcomb R F, Wenzel R P. Enhanced isolation of Mycoplasma pneumoniae from throat washings with a newly modified culture medium. J Infect Dis. 1979;139:478–482. doi: 10.1093/infdis/139.4.478. [DOI] [PubMed] [Google Scholar]
- 21.Tully J G, Rose D L, Baseman J B, Dallo S F, Lazzell A L, Davis C P. Mycoplasma pneumoniae and Mycoplasma genitalium mixture in synovial fluid isolate. J Clin Microbiol. 1995;33:1851–1855. doi: 10.1128/jcm.33.7.1851-1855.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tully J G, Whitcomb R F, Clark H F, Williamson D L. Pathogenic mycoplasmas: cultivation and vertebrate pathogenicity of a new spiroplasma. Science. 1977;195:892–894. doi: 10.1126/science.841314. [DOI] [PubMed] [Google Scholar]
- 23.Velleca W M, Bird B R, Forrester F T. Laboratory diagnosis of mycoplasma infections. Atlanta, Ga: Centers for Disease Control; 1980. [Google Scholar]
- 24.Wu D Y, Ugozzoli L, Pal B K, Qian J, Wallace R B. The effect of temperature and oligonucleotide primer length on the specificity and efficiency of amplification by the polymerase chain reaction. DNA Cell Biol. 1991;10:233–238. doi: 10.1089/dna.1991.10.233. [DOI] [PubMed] [Google Scholar]