Abstract
Results from DNA fingerprint analyses of Mycobacterium tuberculosis complex isolates from tuberculosis (TB) patients diagnosed during 5 years in Denmark are presented. The lack of success in eradicating TB in this low-incidence country may be explained by an unrecognized high frequency of active TB transmission (57%) among native Danes. Only two strains of M. tuberculosis are responsible for 40% of all clustered cases of TB among Danes.
In recent years, DNA fingerprinting of Mycobacterium tuberculosis based on restriction fragment length polymorphism (RFLP) using IS6110 as a probe has been performed on isolates from various parts of the world (1, 7, 12, 19–21). The stability and reproducibility of the technique as well as its usefulness in epidemiological studies has been convincingly demonstrated (8, 16, 17), and new insight into the nature of tuberculosis (TB) transmission has been obtained. Furthermore, RFLP has become an indispensable tool for quality assurance of the processing and culturing of patient samples, since it offers an opportunity to verify suspected cases of cross-contamination (2, 13).
Denmark is a small Scandinavian country with 5 million inhabitants. The incidence of TB in Denmark declined until the middle of 1980s. Since then an increase has been observed, mainly due to immigration. The low but steady occurrence of about 200 cases per year in Danes encompasses a falling incidence in the older adult population and a rising incidence in the younger and middle-aged adult population (10, 11).
The bacterial diagnostics of TB in Denmark, including Greenland, is centralized at the Department of Mycobacteriology at the Statens Serum Institut in Copenhagen. Eighty-three to 91% of all notified TB cases in Denmark are bacteriologically verified (10, 11). In a continuation of a previous study (19, 20), all new isolates of M. tuberculosis complex were analyzed by RFLP by using the standardized procedure described previously (15), and the genotypes were filed together with epidemiological data. The database now comprises information regarding 1,700 TB patients, representing approximately 92% of all culture-positive TB patients from January 1992 to December 1995 and 50% of culture-positive patients from 1996 on. In this paper we present the results of this nationwide DNA fingerprinting of M. tuberculosis complex isolates, with special reference to the molecular epidemiology of TB in the Danish population.
The nationalities of the patients are shown in Table 1. Eleven patients were infected with multidrug-resistant strains. None of these strains were identical, indicating that the current policy of centralized hospitalisation and treatment of patients infected with multidrug-resistant strains in fact prevents secondary transmission of these strains.
TABLE 1.
Origins of TB patients (n = 1,700) included in the study
| Group | n (%) |
|---|---|
| Native Danesa | 761 (45) |
| Inuitsb | 177 (10) |
| Living in Denmark | 32 |
| Living in Greenland | 145 |
| Immigrants | 762 (45) |
| Somalia | 277 |
| Pakistan | 93 |
| Vietnam | 45 |
| Yugoslavia | 37 |
| Turkey | 28 |
| Other nationalities | 282 |
The distinction between native Danes and immigrants is based on information from a central registry.
Natives of Greenland.
One hundred fifty-one of 1,700 patients (9%) were infected with M. tuberculosis complex strains carrying fewer than 5 copies of IS6110 (low-copy-number strains). These patients were excluded from further calculations, since other studies have shown that low-copy-number strains, in spite of identical IS6110 patterns, very often exhibit polymorphic patterns when other genotyping systems are used (3, 4). A majority of the low-copy-number strains were isolated from immigrants, while 41 were cultured from Danes. No low-copy-number strains were cultured from patients from Greenland. Among the Danish low-copy-number strains, 29 of 41 (70%) were identified as Mycobacterium bovis or Mycobacterium bovis BCG. M. bovis and M. bovis BCG were identified on the basis of drug susceptibility analyses, including susceptibility to cycloserine and thiophene-2-carboxylic acid hydrazide. A significant number of low-copy-number strains would limit the value of IS6110 DNA fingerprinting (18), but this is not a major problem in Denmark.
Among the 1,549 patients infected with high-copy-number strains, 49% were infected with M. tuberculosis strains which were part of a cluster (defined as at least two patients with strains exhibiting identical RFLP patterns) while 51% were infected with unique strains. Among immigrants, clustered strains comprised only 30%. Immigrants arrive from different parts of the world, where different RFLP patterns are endemic. TB infections among immigrants in Denmark are most often the result of reactivation of strains with which they were infected in their countries of origin. In Greenland, the cluster frequency is extremely high, 79%. This is in accordance with the results obtained in previous studies (19). The explanation can probably be found both in the very high TB incidence in Greenland (at least 15 to 20 times as high as that in Denmark and increasing) and in the fact that Greenland is a geographically isolated country where exchange of TB strains from other areas has been and still is limited.
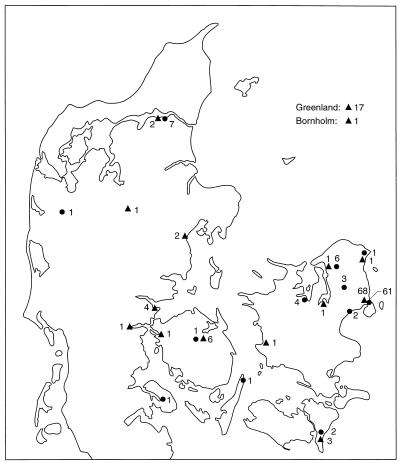
In the Danish population, 57% of the patients were infected with an M. tuberculosis strain which was part of a cluster. The frequency of M. tuberculosis strain clustering was calculated for different age groups, by gender, and by residence in or outside central Copenhagen. As shown in Fig. 1, cluster frequency in age groups up to 50 to 60 years is even higher than 57%. It is higher for males than for females and higher for patients living in central Copenhagen than for patients living outside central Copenhagen. Figure 2 shows the number of patients in the database and the incidence of notified cases. The distribution of clustered Danish TB patients on small clusters (2 to 3 patients with identical strains), medium-size clusters (4 to 18 patients with identical strains), and the two largest clusters was calculated. As shown in Table 2, approximately 40% of all clustered Danish TB patients and 55% of the clustered Danish TB patients living in central Copenhagen belonged to one of two large clusters designated cluster 1 and cluster 2. The geographical distribution of these clusters is shown in Fig. 3. These strains are mainly found in Copenhagen, but microepidemics in other cities have been observed. Cluster 1, which carries 13 copies of IS6110, consists of 110 patients: 72 Danes, 33 patients from Greenland (16 living in Denmark), and 5 immigrants (2 from Finland, 1 from Yugoslavia, 1 from Germany, and 1 from Morocco). Cluster 2, which carries 11 copies of IS6110, consists of 90 patients: 88 Danes and 2 immigrants (1 from Sierra Leone and 1 from Syria). Cluster 1 was previously reported to be related to a chain of TB transmission from Greenland to Denmark (19). The transmission was by then thought to be related to a certain area of Copenhagen with known social and health problems. Transmission of this strain is still believed to be associated with this specific area of Copenhagen. Results from a recent study of patients coinfected with human immunodeficiency virus and TB in Denmark indicate that this strain is also related to drug addict circles (5). The presence of immigrants in these two clusters is likely to be due to transmission of TB from Danes to immigrants, since both clusters were observed among a large number of Danish TB patients before any patients of foreign origin were observed. Transmission from immigrants to Danes is not a problem of concern according to our data, since only a few cases of clustering between Danes and immigrants have been found.
FIG. 1.
Frequency of case clustering among Danes, grouped by age and gender (A) or by age and residence (B), from 1992 to 1996.
FIG. 2.
Number of patients with RFLP-analyzed TB isolates and incidence of notified cases.
TABLE 2.
Distribution on cluster size of all clustered Danish TB patients and of clustered Danish TB patients living in central Copenhagen
| Cluster sizea | % of patients in each cluster size
|
|
|---|---|---|
| All patients | Central Copenhagen patients | |
| 2–3 | 27 | 14 |
| 4–18 | 33 | 31 |
| 110 (cluster 1) | 18 | 27 |
| 90 (cluster 2) | 22 | 28 |
Number of patients.
FIG. 3.
Map of Denmark showing the geographical distribution of patients infected with M. tuberculosis strains belonging to the two most frequent clusters. Triangles, cluster 1; circles, cluster 2. Numbers represent cases.
Neither of the two patterns of clusters 1 and 2 is found in the international database now comprising 6,000 isolates (14). However, in a pilot study in which 30 M. tuberculosis strains isolated from TB patients living in the southern part of Sweden were analyzed, two strains were found to be identical to the strains in cluster 1. Two strains exhibited genotypes very similar to that of cluster 2, indicating that these strains may have expanded predominantly in the northern hemisphere.
It was previously predicted (9) that TB would be eradicated among Danes by the middle of the next century. Good case finding, effective treatment, and contact tracing would keep the rate of recently infected patients as low as 33% of reactivated cases. This study is in agreement with the general opinion that TB in a low-incidence country such as Denmark is mostly due to reactivation. However, as described in previous studies (20), a high proportion of TB cases appear to be due to recent infection. Especially the two very large clusters described above indicate that active transmission of TB, probably in certain marginalized subpopulations, is still a considerable problem despite a good national TB program. Ongoing studies with the purpose of obtaining detailed information on patients in the large clusters from medical records and interviews will hopefully reveal topics of interest for further contact tracing.
It is possible that the very frequent occurrence of the two strains of clusters 1 and 2 could be explained by higher virulence of these strains favoring transmission. This hypothesis is at the moment being investigated by virulence testing of the strains by a recently described method (6).
The Danish RFLP database has provided new knowledge of TB epidemiology in Denmark and has raised questions concerning the traditional concept of TB transmission. We are currently trying to utilize the results of the RFLP analyses in clinical work by making reports to the responsible physicians. Despite careful contract tracing, unrecognized TB transmission is obviously going on. Hopefully, molecular epidemiological analyses such as that presented here will help reveal how efforts to eradicate TB in Denmark should be intensified.
REFERENCES
- 1.Alland D, Kalkut G E, Moss A R, McAdam R A, Hahn J A, Bosworth W, Drucker E, Bloom B R. Transmission of tuberculosis in New York City. An analysis by DNA fingerprinting and conventional epidemiologic methods. N Engl J Med. 1994;330:1710–1716. doi: 10.1056/NEJM199406163302403. [DOI] [PubMed] [Google Scholar]
- 2.Bauer J, Thomsen V O, Poulsen S, Andersen A B. False-positive results from cultures of Mycobacterium tuberculosis due to laboratory cross-contamination confirmed by restriction fragment length polymorphism. J Clin Microbiol. 1997;35:988–991. doi: 10.1128/jcm.35.4.988-991.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burman W J, Reves R R, Hawkes A P, Rietmeijer C A, Yang Z, el Hajj H, Bates J H, Cave M D. DNA fingerprinting with two probes decreases clustering of Mycobacterium tuberculosis. Am J Respir Crit Care Med. 1997;155:1140–1146. doi: 10.1164/ajrccm.155.3.9117000. [DOI] [PubMed] [Google Scholar]
- 4.Chaves F, Yang Z H, El-Hajj H, Alonso M, Burman W J, Eisenach K D, Dronda F, Bates J H, Cave M D. Usefulness of the secondary probe pTBN12 in DNA fingerprinting of Mycobacterium tuberculosis. J Clin Microbiol. 1996;34:1118–1123. doi: 10.1128/jcm.34.5.1118-1123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dragsted, U. B. 1997. Unpublished data.
- 6.Ehrt S, Gunzburg S, Choi M, MacMicking J, Nathan C, Riley L W. Reactive nitrogen intermediate resistance factor of Mycobacterium tuberculosis. 1996. Poster presentation at the Third International Conference on the Pathogenesis of Mycobacterial Infections, Stockholm, Sweden. [Google Scholar]
- 7.Hermans P W, Messadi F, Guebrexabher H, van Soolingen D, de Haas P E, Heersma H, de Neeling H, Ayoub A, Portaels F, Frommel D, et al. Analysis of the population structure of Mycobacterium tuberculosis in Ethiopia, Tunisia, and The Netherlands: usefulness of DNA typing for global tuberculosis epidemiology. J Infect Dis. 1995;171:1504–1513. doi: 10.1093/infdis/171.6.1504. [DOI] [PubMed] [Google Scholar]
- 8.Hermans P W, van Soolingen D, Dale J W, Schuitema A R, McAdam R A, Catty D, van Embden J D. Insertion element IS986 from Mycobacterium tuberculosis: a useful tool for diagnosis and epidemiology of tuberculosis. J Clin Microbiol. 1990;28:2051–2058. doi: 10.1128/jcm.28.9.2051-2058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kok-Jensen A. When can tuberculosis be eradicated in Denmark? Ugeskr Laeg. 1995;157:273–279. [PubMed] [Google Scholar]
- 10.Poulsen S, Bennedsen J. EPI-NEWS. 34. Tuberculosis 1994. Copenhagen, Denmark: Statens Serum Institut; 1995. [Google Scholar]
- 11.Poulsen S, Miörner H. EPI-NEWS. 36. Tuberculosis 1995. Copenhagen, Denmark: Statens Serum Institut; 1996. [Google Scholar]
- 12.Small P M, Hopewell P C, Singh S P, Paz A, Parsonnet J, Ruston D C, Schecter G F, Daley C L, Schoolnik G K. The epidemiology of tuberculosis in San Francisco. A population-based study using conventional and molecular methods. N Engl J Med. 1994;330:1703–1709. doi: 10.1056/NEJM199406163302402. [DOI] [PubMed] [Google Scholar]
- 13.Small P M, McClenny N B, Singh S P, Schoolnik G K, Tompkins L S, Mickelsen P A. Molecular strain typing of Mycobacterium tuberculosis to confirm cross-contamination in the mycobacteriology laboratory and modification of procedures to minimize occurrence of false-positive cultures. J Clin Microbiol. 1993;31:1677–1682. doi: 10.1128/jcm.31.7.1677-1682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Embden, J. (National Institute of Public Health and Environmental Protection, Bilthoven, The Netherlands). 1997. Personal communication.
- 15.van Embden J D A, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Soolingen D, de Haas P E, Hermans P W, Groenen P M, van Embden J D. Comparison of various repetitive DNA elements as genetic markers for strain differentiation and epidemiology of Mycobacterium tuberculosis. J Clin Microbiol. 1993;31:1987–1995. doi: 10.1128/jcm.31.8.1987-1995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Soolingen D, Hermans P W, de Haas P E, Soll D R, van Embden J D. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991;29:2578–2586. doi: 10.1128/jcm.29.11.2578-2586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Soolingen D, Qian L, de Haas P E W, Douglas J T, Traore H, Portaels F, Qing H Z, Enkhsaikan D, Nymadawa P, van Embden J D A. Predominance of a single genotype of Mycobacterium tuberculosis in countries of east Asia. J Clin Microbiol. 1995;33:3234–3238. doi: 10.1128/jcm.33.12.3234-3238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z H, de Haas P E W, van Soolingen D, van Embden J D A, Andersen A B. Restriction fragment length polymorphism of Mycobacterium tuberculosis strains isolated from Greenland during 1992: evidence of tuberculosis transmission between Greenland and Denmark. J Clin Microbiol. 1994;32:3018–3025. doi: 10.1128/jcm.32.12.3018-3025.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Z H, de Haas P E, Wachmann C H, van Soolingen D, van Embden J D, Andersen A B. Molecular epidemiology of tuberculosis in Denmark in 1992. J Clin Microbiol. 1995;33:2077–2081. doi: 10.1128/jcm.33.8.2077-2081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Z H, Mtoni I, Chonde M, Mwasekaga M, Fuursted K, Askgard D S, Bennedsen J, de Haas P E W, van Soolingen D, van Embden J D A, Andersen Å B. DNA fingerprinting and phenotyping of Mycobacterium tuberculosis isolates from human immunodeficiency virus (HIV)-seropositive and HIV-seronegative patients in Tanzania. J Clin Microbiol. 1995;33:1064–1069. doi: 10.1128/jcm.33.5.1064-1069.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]