Abstract
Background
Malaria is the most common tropical infection in the UK. Current guidelines suggest that testing on 3 consecutive days is required following an initial negative result. This study aimed to see whether newer diagnostics (loop-mediated amplification assay [LAMP]) had sufficient sensitivity to support a change in diagnostic practice.
Methods
Blood samples from 11 participants who had undergone controlled human malaria infection (CHMI) with Plasmodium falciparum malaria were assessed from day 6 (C+6) for malaria positivity using the Carestart Malaria rapid diagnostic test (RDT) and from C+4 using the Alethia Malaria LAMP assay. Quantitative polymerase chain reaction had been performed twice daily during CHMI follow-up. A retrospective analysis of samples submitted to the Sheffield Teaching Hospitals for malaria testing over a 5-y period was conducted, evaluating the combination of the Carestart RDT alongside blood film analysis, as per UK guidelines.
Results
In CHMI samples, LAMP was positive for all parasitaemias >1000 parasites/ml, whereas RDTs were less reliable (59% positive for parasitaemias >1000 parasites/ml). The combination of RDT and blood films for clinical samples diagnosed most infections, but only a minority of negative samples had subsequent tests.
Conclusions
LAMP has higher sensitivity than current UK recommended methods, with a potential to review the requirement for additional days of testing in the majority of patients.
Keywords: diagnostics, LAMP, malaria, RDT
Introduction
Malaria is the most common imported tropical infection in European travellers with short-term exposure, with 1719 imported infections recorded in the UK in 2019 (data since skewed by the coronavirus disease 2019 [COVID-19] pandemic and changes in travel). The majority of infections were caused by Plasmodium falciparum and occurred in travellers who had visited friends and relatives in sub-Saharan Africa (SSA).1 P. falciparum accounts for >99% of infections in SSA, where 95% of all malaria infections occur.2 The World Health Organization (WHO) recommends that all suspected malaria cases be confirmed by microscopy or with a rapid diagnostic test (RDT) before treatment.3 RDTs are now the most commonly used method for diagnosis globally.4
Guidelines for the diagnosis of malaria in the UK suggest that microscopy should be undertaken on high-quality thick and thin blood films in all cases of suspected malaria. These should be examined by two trained observers, with a minimum of 200 oil immersion fields examined in the thick film. Where a blood film is negative, but there is a strong clinical suspicion of malaria, repeat blood films should be examined 12–24 h after the first and again after a further 24 h.5 The accuracy of microscopy depends on operator experience, with detection thresholds of 4–100 parasites/µl (4000–100 000 parasites/ml) reported.6 RDTs are an alternative method for malaria diagnosis increasingly used worldwide. These tests detect target antigen, which may be pan-Plasmodium species, such as Plasmodium lactate dehydrogenase (pLDH) and aldolase, or species specific, such as histidine-rich protein II (HRP2) for P. falciparum. Large numbers of these tests have been brought to market, with variable performance quality. In 2006 the WHO, in collaboration with the Foundation for Innovative New Diagnostics (FIND), developed a program for evaluating the performance of commercially available RDTs to inform those procuring tests for national malaria programs. This assessed performance at two fixed parasite densities of 200 parasites/µl (200 000 parasites/ml) and 2000 parasites/µl (2 million parasites/ml) using geographically diverse, cryopreserved P. falciparum and Plasmodium vivax clinical samples with consistently comparable concentration ranges of HRP2, pLDH and aldolase determined by quantitative enzyme-linked immunosorbent assay.7 This program provides recommended procurement criteria for countries looking to source RDTs for malaria diagnostics, with a recommendation that tests are able to detect ≥75% at 200 parasites/μl for P. falciparum (and P. vivax, where applicable).
RDTs are only recommended as a method for confirming the presence of P. falciparum on a blood film or where there is insufficient experience for adequate microscopic assessment to be carried out as per current UK guidelines, particularly in an on-call situation when blood film diagnosis may be performed by relatively inexperienced observers or where malaria diagnoses are rare.5 The performance of laboratories in diagnosing malaria and quantifying parasitaemia in the UK is monitored through the National External Quality Assessment Scheme (NEQAS). This demonstrates the challenges facing malaria diagnosis, even in a resource-rich setting. Although malaria was correctly diagnosed by 97% of laboratories, the correct species was reported by 54% (for a blood film demonstrating Plasmodium ovale) and 90% (P. falciparum on thick film) of laboratories in 2016. In this survey, 17% of laboratories reported a false positive result, diagnosing a parasitic infection on a negative blood film,8 although it is likely that this false positive rate will be higher for NEQAS samples than for clinical samples, as laboratories taking part in the NEQAS scheme include many that will see few cases of malaria and there is also an ‘expectation bias’, as a NEQAS sample will more often contain a pathogen rather than be a negative sample. It is clear that there remains room for improvement in malaria diagnostics and in ruling out malaria infection in particular.
Nucleic acid amplification techniques, in particular loop-mediated isothermal amplification (LAMP), have been developed to try to improve sensitivity compared with traditional microscopy or RDTs. The Alethia Malaria (formerly Illumigene Malaria) LAMP assay from Meridian Bioscience (Cincinnati, OH, USA) is reported to have a limit of detection (LoD) of 2 parasites/µl (2000 parasites/ml) for P. falciparum (3D7 clone) and 0.125 parasites/µl (125 parasites/ml) for P. vivax (India VII strain). The newer Alethia Malaria PLUS assay reports a LoD of 0.25 parasites/µl (250 parasites/ml) and 0.063 parasites/µl (63 parasites/ml) for P. falciparum and P. vivax, respectively. This is a marked improvement on the sensitivity of RDTs or traditional microscopy, with a high negative predictive value, and therefore could be used as a more reliable ‘rule-out’ test, meaning that repeated tests may be unnecessary, as is currently advised following an initial negative RDT or thick blood film where there is a clinical suspicion of malaria infection. Unlike quantitative polymerase chain reaction (qPCR), LAMP assays only require a small bench-top machine, there is no extraction step and they require limited technical ability to conduct, meaning that adoption into a clinical laboratory workflow should be straightforward. Due to the relatively high cost of LAMP (compared with blood film or RDT) and low rates of infection, it is likely to be a more appropriate test in the setting of low-endemicity/non-endemic countries than in malaria-endemic areas.9 The potential for use of LAMP assays has been included in the recent update to the British Society for Haematology guidelines for the laboratory diagnosis of malaria,5 but the guidance falls short of specifying where this test might be most useful and highlights its limitations in being unable to differentiate current or recent past infection with a positive result, as assays can remain positive for up to 4 weeks after successful malaria treatment because of residual parasite DNA.
Controlled human malaria infection (CHMI) studies are a useful method for early efficacy assessment of novel malaria vaccines and therapeutics. Infection can be delivered by the bites of infectious mosquitoes, injection of cryopreserved sporozoites or injection of parasitized red blood cells.10 Volunteers are monitored frequently (usually twice daily) and parasitaemias are typically very low at the point of diagnosis. Thick film sensitivity for parasitaemia >10 000 parasites/ml has been reported as 81% (confidence interval [CI] 65–97) following sporozoite CHMI in healthy adult volunteers, but only 29% (CI 19–39) for parasitaemias of 1000–10 000 parasites/ml.11 Most centres conducting CHMI studies use qPCR to measure parasitaemia, which has been demonstrated to be a more sensitive and consistent method for measuring parasite density.11–13 Reference laboratories use qPCR for diagnosis, particularly in low parasitaemia infections, mixed infection or where speciation is difficult by microscopy, but it is not used for routine clinical diagnosis. CHMI therefore provides an opportunity to assess these diagnostics with serial, undiluted clinical samples with very low parasitaemias compared with qPCR.
In addition to the data provided through the CHMI study, the potential utility in a clinical setting was investigated through retrospective analysis of blood film/RDT results over a 5-y period for samples submitted to the Sheffield Teaching Hospitals (STH) NHS Foundation Trust haematology laboratories prior to the COVID-19 pandemic. This aimed to evaluate the efficacy of serial blood film testing (as recommended by national guidelines) in the context of adjunctive RDTs for malaria infection by any species. A study in Queensland, Australia demonstrated that 6.7% of cases were diagnosed on subsequent testing following an initial negative result when thick and thin blood films alone were used for diagnosis. The majority of cases missed on the first blood film were non-falciparum or mixed infections (82%).14 A study in Melbourne, Australia found that combining blood film and the BinaxNOW RDT diagnosed malaria cases in 96.5% of cases in a study of 255 cases tested with both blood films and RDT. Of those infections missed on the initial test, the majority were due to P. vivax (seven of nine cases) and all except one were diagnosed on the second set of tests.15 These previous studies suggest that although current widely used techniques are generally effective for excluding malaria infection, there is room for improvement and a more sensitive assay could save time for patients and clinical and laboratory staff if malaria could be reliably excluded on an initial test. The Alethia Malaria LAMP assay has been assessed in both endemic and non-endemic settings and has consistently demonstrated high sensitivity and specificity for malaria infection, with similar accuracy to real-time PCR but providing a faster result and with relatively little laboratory skill required.16,17 Our study aimed to demonstrate that at very low parasitaemias, well below the levels seen with symptomatic malaria, LAMP reliably detected P. falciparum infection and could therefore be considered as a ‘rule-out’ test for patients with suspected infection, reducing the burden on clinical and laboratory services as well as patients.
Methods
Study design and approvals
This was a diagnostic accuracy study of commercially available malaria diagnostics (LAMP and RDT) compared with the gold standard of qPCR in serial samples from individuals known to have P. falciparum infection. Use of CHMI samples enabled assessment of truly low parasitaemias without dilution of samples (as is done for RDT assessment in the FIND program).
The VAC063C study (NCT03906474) was a clinical study to assess the safety of primary, secondary and tertiary blood-stage controlled human P. falciparum malaria infection in healthy UK adults and to characterize parasite growth dynamics.18 This was a follow-on study from the VAC063 open-label phase I/IIa clinical trial (NCT02927145) of the novel blood-stage P. falciparum candidate vaccine RH5.1/AS01B, which was the first study to show a significant in vivo impact on the rate of parasite growth in malaria-naïve adults undergoing CHMI.19
VAC063C received ethical approval from the UK NHS Research Ethics Service (Oxfordshire Research Ethics Committee A, Ref 18/SC/0521). The trial was conducted according to the principles of the current revision of the Declaration of Helsinki 2008 and in full conformity with the International Council for Harmonisation Guideline for Good Clinical Practice.
Participants
Volunteers were healthy males and non-pregnant females ages 18–50 y who were invited to participate in VAC063C having previously undergone primary or secondary homologous CHMI in the VAC063 trial, but who had been malaria-naïve prior to enrolment in VAC063. In addition, healthy malaria-naïve males and non-pregnant females ages 18–50 y were invited to take part in a primary CHMI in VAC063C. All volunteers gave written informed consent prior to participation. A total of 11 volunteers were recruited at the Centre for Clinical Vaccinology and Tropical Medicine, University of Oxford, Oxford, UK.
P. falciparum blood-stage CHMI
Blood-stage P. falciparum infection was administered intravenously with approximately 750 infected red blood cells.18 The inoculum used for CHMI was originally produced at QIMR Berghofer Medical Research Institute in Brisbane, Australia in 1994 and consists of aliquots of P. falciparum (clone 3D7)-infected erythrocytes taken from a single donor.20–22 More than 500 volunteers have been challenged with the inoculum since 1997 (84 in Oxford to date) and the estimated number of infected erythrocytes has varied from 30 to 6000. CHMI of malaria-naïve adult individuals using this inoculum has always resulted in parasitaemia as detected by qPCR and/or microscopy.20,21 The inoculum was thawed and prepared under strict aseptic conditions as previously described.23
Participants were undergoing tertiary (n=6), secondary (n=2) or primary (n=3) CHMI with a homologous inoculum (Table 1). The demographics of the VAC063C participants are shown in Table 2. Participants were monitored twice daily for symptoms, with blood taken to assess parasitaemia by qPCR at each visit and were treated with either artemether/lumefantrine (Riamet) (n=9) or atovaquone/proguanil (Malarone) (n=2) once they had parasitaemia of >5000 parasites/ml if symptomatic or >10 000 parasites/ml if asymptomatic.
Table 1 .
VAC063C study groups.
| VAC063C group | Group size | VAC063 | VAC063C | |
|---|---|---|---|---|
| 1 (tertiary) | 6 | Primary CHMI | Secondary CHMIa | Tertiary CHMIb |
| 2 (secondary) | 2 | N/A | Primary CHMI | Secondary CHMIb |
| 3 (primary) | 3 | N/A | N/A | Primary CHMI |
Approximate 4-month interval between primary and secondary CHMI.
Approximate 8-month interval between preceding CHMI and VAC063C study.
Table 2 .
VAC063C demographic information for study participants.
| Characteristics | VAC063C | |||
|---|---|---|---|---|
| Primary CHMI | Secondary CHMI | Tertiary CHMI | ||
| Participants, n | 3 | 2 | 6 | |
| Female, n (%) | 2 (67) | 2 (100) | 2 (33) | |
| Age (years) | Median | 25 | 26 | 29 |
| Range | 23–50 | 23–29 | 23–34 | |
| Body mass index (kg/m2) | Median | 29.8 | 19.3 | 24.6 |
| Range | 24.4–31.5 | 19.1–19.5 | 19.0–33.0 | |
| Ethnicity, n (%) | White British | 2 (67) | 2 (100) | 4 (67) |
| White other | 0 (0) | 0 (0) | 1 (17) | |
| Asian | 0 (0) | 0 (0) | 1 (17) | |
| Other | 1 (33) | 0 (0) | 0 (0) | |
| Smoking, n (%) | Unknown | 1 (50) | 1 (17) | |
| Alcohol excess, n (%) | Unknown | 0 (0) | 0 (0) | |
qPCR for P. falciparum
qPCR was performed on whole blood samples collected in tubes containing ethylenediaminetetraacetic acid (EDTA) as previously described,24 with the following modifications. Blood was collected at baseline and at clinical protocol–defined time points following CHMI for qPCR in 2.0-ml tubes containing EDTA. DNA was extracted from 0.4-ml EDTA whole blood using a QIAsymphony SP robot, utilizing the Qiagen DSP Blood Mini Kit and the pre-loaded Blood 400 v6 extraction protocol (Qiagen, Hilden, Germany), with a 100-μl elution in ATE buffer selected. A total of 5 μl of each extraction was used per assay well and was run in triplicate for qPCR (equivalent to 60 μl of blood directly assessed). Parasites per millilitre equivalent mean values were generated by a standard Taqman absolute quantitation, against a defined standard curve of diluted P. falciparum 3D7 DNA, qualified against DNA from counted parasites in whole blood, previously extracted by the same method. qPCR was conducted on an ABI StepOne Plus machine with v2.3 software, using default Universal qPCR and QC settings, apart from the use of 45 cycles and 25-μl reaction volume. The lower limit of detection (LLOD) of the assay is 20 parasites/ml (0.02 parasites/µl) and the process used has since been formally validated as suitable for diagnostic purposes (NJE, personal communication).
RDT and LAMP assays
Based on previous data and qPCR results, blood samples were assessed from day 6 post-CHMI (C+6) for malaria positivity using the Carestart Malaria (Pan) RDT and from C+4 using the Illumigene Malaria LAMP assay (now the Alethia Malaria assay). Results from a previous study indicated that RDTs were consistently negative prior to C+8 (unpublished data), so day C+6 was chosen as the date to commence RDT testing to ensure all positive results were captured while minimizing resource waste. The assessment of the LAMP assay was conducted following the end of the CHMI follow-up period, once all samples were available, as a separate project using surplus frozen EDTA whole blood. The assay has been validated on frozen samples, so use of frozen rather than fresh samples was not expected to influence the results.25,26 Given the higher sensitivity of LAMP, and based on qPCR data from previous CHMIs, an earlier time point of C+4 was chosen as the first time point to commence LAMP assays. Time points prior to C+4 were not considered necessary given that parasitaemias in this very reproducible model are not detectable above the qPCR LLOD until after C+4.19,23
RDTs were carried out using whole blood in real time during the VAC063C CHMI trial according to the manufacturer’s instructions daily from C+6. The results of the RDTs were not made available to the clinical team and were not used for diagnosis. The Alethia LAMP assays were carried out as per the manufacturer's instructions26 after completion of CHMI participant follow-up on 50-µl thawed stored blood samples from samples collected daily (morning visits) from each participant from C+4 until the day of diagnosis (range C+8–C+16). Diagnosis occurred when the protocol defined thresholds of parasitaemia of >5000 parasites/ml if symptomatic or >10 000 parasites/ml if asymptomatic were reached. Samples from the day prior to CHMI (C−1) were also processed as negative controls for all participants (all confirmed negative for P. falciparum by qPCR), in addition to the external controls provided with the LAMP kit.
Retrospective analysis of clinical malaria testing in Sheffield
A service evaluation of samples submitted to the STH NHS Foundation Trust haematology laboratories over a 5-y period (2013–2018) was carried out using pseudo-anonymized data extracted from the Apex laboratory results system. The STH haematology laboratories provide diagnostic services to primary and secondary care within Sheffield, as well as to district general hospitals within the region. Standard testing algorithms for malaria testing include use of the CareStart Malaria immunochromatographic RDT as well as preparation of thick and thin Giemsa-stained blood films. Following a positive malaria test (either RDT or blood film), samples are sent to the malaria reference laboratory for confirmation, where identification by blood film (and PCR, where necessary) is carried out. Tests conducted within a 96-h period on the same patient were considered serial tests relating to the same clinical episode. Samples sent post-treatment only were excluded from analysis, as RDTs can remain positive for many days despite adequate treatment. As this was a service evaluation, ethical approval was not required, but the results were reported and presented to the Trust Clinical Effectiveness Unit as well as local departments.
Results
LAMP and RDT assays on serial CHMI samples
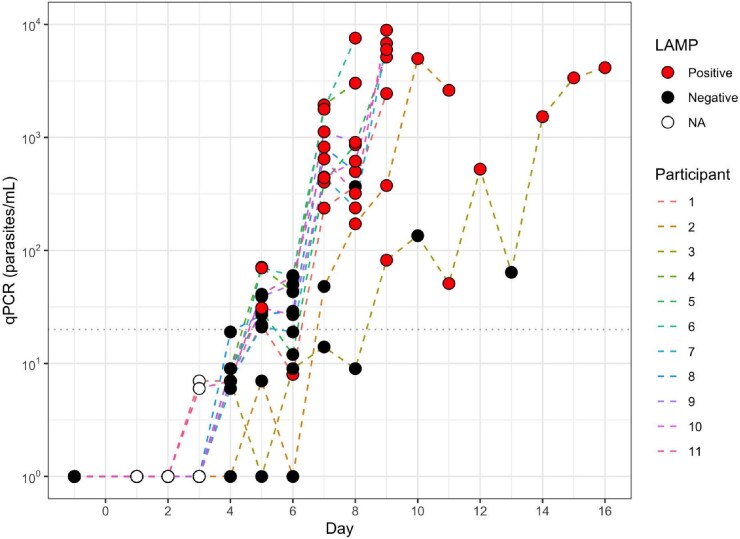
Daily samples were tested for the 11 participants until the predefined qPCR thresholds for treatment were reached. LAMP assay positivity in relation to parasitaemia by qPCR is shown in Figure 1. The LAMP assay was reliably positive at a parasitaemia of 1000 parasites/ml (i.e. 1 parasite/µl) in this population, with positive results as early as C+5 (100% positive for parasitaemias >1000 parasites/ml; 22/22 samples). Below this parasitaemia there were some false negative results documented (88.2% positive for parasitaemia in the 100–1000 parasites/ml range; 15/17 samples). The sensitivity of the test at >1000 parasites/ml (i.e. lower than the published LoD) was therefore 100%, with a negative predictive value of 100%. The LAMP assay was positive prior to the diagnosis time point for all participants. All samples tested prior to CHMI were negative by LAMP assay and qPCR. Specificity of the LAMP assay was 100% in our study.
Figure 1.
Parasites/ml with corresponding LAMP result in serial samples from malaria-infected individuals. Dotted line shows qPCR limit of quantification (20 parasites/ml).
There were no positive RDT results before C+9, despite parasitaemias of up to 7567 parasites/ml by qPCR on C+8. The RDT was also negative at D10 for one of the participants diagnosed at that point, with a parasitaemia by qPCR of 47 131 parasites/ml. Interpretation was difficult in some cases, with only a faint line detectable to indicate a positive result (Figure 2). RDTs were positive at the diagnosis time point (i.e. when the diagnosis threshold was reached) in 9 of 11 participants (median 15 518 parasites/ml [range 4141–61 760]). The sensitivity of the RDT assay at >1000 parasites/ml (i.e. >1 parasite/µl) was 45% (specificity 100%).
Figure 2.
Example of RDT results. Samples from C+9. All samples were positive by qPCR and LAMP at this time point (median 6394 parasites/ml [range 82–33 190]; n=10, as 1 participant had reached diagnosis criteria on C+8.
RDT and blood film results from clinical samples
A total of 1429 patient episodes were included in the final analysis, from which there were 101 initial positive tests. Of these, 99 were confirmed malaria infections (two patients had false positive RDT tests). Of the 1328 episodes that were initially negative, 599 patients (45.1%) had only a single malaria test, 243 (18.3%) had two tests and 486 (36.6%) hade a total of three or more serial tests. Of these samples, only one patient had a true positive test following an initial negative test. As these were pseudo-anonymized data, we were unable to determine if the reason for not repeating the test was due to an alternative diagnosis being made, low clinical suspicion for malaria or whether these were missed tests. The majority of cases were due to P. falciparum (76.5%), following travel to SSA, consistent with national data. The sensitivity of the combination of blood film and Carestart RDT in this study was 95.88% (95% CI 91.92 to 99.83).
A separate local validation analysis of the Alethia LAMP assay compared with RDT by the haematology laboratory as part of the required assessment for consideration of implementing the technology within the clinical service demonstrated a sensitivity of 100% (95% CI 90.26 to 100) for the Alethia LAMP assay compared with 94.44% (95% CI 81.34 to 99.32) for the Carestart RDT, consistent with our service evaluation data. This evaluation was carried out on a total of 47 samples including NEQAS samples (n=3) and patient samples (n=44, from 38 patients; serial samples were included for 3 patients with malaria). There were 11 negative samples (1 NEQAS, 10 patient samples). Blood samples were collected in EDTA tubes and tests were performed on fresh samples or following storage at −20°C (as per the assay validation). Cleared parasitaemias with known recent infection were considered ‘positive’, as both RDT and LAMP assays are known to remain positive for up to several weeks post-treatment (personal communication; unpublished data).
Discussion
To our knowledge, this is the first time the Alethia Malaria assay has been assessed using serial samples from infected individuals at very low parasitaemias. Our data show that in this setting the assay is 100% sensitive for P. falciparum parasitaemias >1000 parasites/ml—well below the published detection limit of 2000 parasites/ml (2 parasites/µl). LAMP performed better than the Carestart Malaria (Pan) RDT and is more sensitive than the published sensitivity for thick blood film malaria diagnosis, although it remains less sensitive than qPCR. The Carestart RDT had a sensitivity of 45% in samples with >1000 parasites/ml, but we note that the WHO assessment parasitaemia for RDTs is 200 parasites/µl (i.e. 200 000/ml), which was higher than the parasitaemias reached in the CHMI follow-up. This RDT has been shown elsewhere to have an LLOD of 49 000 parasites/ml in dilutions of cultured P. falciparum parasites.27 Similar to an RDT, LAMP does not require a high skill level to operate, unlike blood film microscopy, and provides a result in <1 h. In the CHMI setting, the LAMP assay was positive in all participants prior to the onset of symptoms, unlike the RDT.
There are limitations for diagnosis by LAMP, in that the Alethia Malaria assay does not provide a value for parasitaemia, cannot differentiate Plasmodium infections and is a more expensive test than microscopy or an RDT. A positive test requires a blood film for speciation and parasitaemia calculation, and tests may remain positive for up to 4 weeks following infection due to circulating residual parasite DNA.5 However, given its high sensitivity, it is more reliable for ruling out malaria infection, e.g. in a returning traveller, meaning that the repeat blood films after 24 and 48 h that are currently recommended are no longer needed, leading to reduced overall costs in the majority of cases. Interestingly, the combination of blood film and RDT as used in the STH laboratory was also very sensitive (with similar sensitivity to the data reported by Pasricha et al.15) and the addition of serial days testing was rarely required to make a diagnosis. In this setting, LAMP was also reported to have higher sensitivity (100%) and specificity (100%) by the laboratory team.
Limitations to this study were that for the CHMI LAMP analysis only a single strain of P. falciparum was assessed in a small number of healthy volunteers, limiting the generalizability of our findings. However, a prospective study of samples conducted at the London School of Hygiene and Tropical Medicine from returning travellers with signs and symptoms of malaria, which also included retrospective positive malaria samples, found 100% sensitivity of the same LAMP assay after discrepant resolution, with cases of all species of malaria causing human disease included.28 From these data, the group recommended that a novel algorithm could be introduced that would remove the requirement for additional visits if the first malaria test (by LAMP) was negative. A positive result would lead to an initial provisional report being published and followed up with a subsequent microscopy result (or PCR if microscopy negative) to provide a final report with speciation±parasitaemia. This process was estimated to save US$13 per malaria test in a non-endemic setting with low levels of malaria, such as the UK, compared with current laboratory practices.
Conclusions
In non-endemic settings, malaria is a relatively rare diagnosis, although the most common imported tropical infection in the UK. Our data demonstrate that the current guidelines for repeat testing are not always followed, but additionally, for the most common and most severe malaria (P. falciparum), parasitaemia can be detected by LAMP prior to the onset of symptoms. This provides reassurance that in a symptomatic individual with a negative test it is extremely unlikely that symptoms are due to malaria and an alternative diagnosis should be sought. Data from others also demonstrate diagnosis of all malaria species in symptomatic returning travellers. This suggests that adoption of the LAMP assay by clinical laboratories as a ‘rule-out’ single test would be a safe and effective measure, saving time for clinicians, laboratory staff and patients in not having to do repeat testing on subsequent days for a negative result (i.e. in the majority of cases). However, without this option being provided in national guidance, laboratories may be more reluctant to change practice.
Acknowledgements
The authors are grateful to Julie Furze, Raquel Lopez Ramon, Megan Baker, Fernando Ramos-Lopez, Ian Poulton, Alison Lawrie, Jee-Sun Cho and Fay Nugent for assistance, and Richard Morter, Amy Flaxman and Duncan Bellamy for qPCR support (Jenner Institute, 490 University of Oxford) , James McCarthy for providing the 3D7 inoculum (QIMR, Herston, QLD, Australia), Ben Orrah and Chris Robson for providing the data for the clinical LAMP service evaluation (Sheffield Teaching Hospitals NHS Foundation Trust) and all the study volunteers.
Contributor Information
Ruth O Payne, The Jenner Institute, University of Oxford, Old Road Campus Research Building, Roosevelt Drive, Oxford OX3 7DQ, UK; Division of Clinical Medicine, School of Medicine and Population Health, University of Sheffield, Sheffield S10 2RX, UK; Laboratory Medicine, Sheffield Teaching Hospitals NHS Foundation Trust, Northern General Hospital, Herries Road, Sheffield, S5 7AU, UK.
Nick J Edwards, The Jenner Institute, University of Oxford, Old Road Campus Research Building, Roosevelt Drive, Oxford OX3 7DQ, UK.
Yrene Themistocleous, The Jenner Institute, University of Oxford, Old Road Campus Research Building, Roosevelt Drive, Oxford OX3 7DQ, UK.
Sarah E Silk, The Jenner Institute, University of Oxford, Old Road Campus Research Building, Roosevelt Drive, Oxford OX3 7DQ, UK.
Jordan R Barrett, The Jenner Institute, University of Oxford, Old Road Campus Research Building, Roosevelt Drive, Oxford OX3 7DQ, UK.
Thomas A Rawlinson, The Jenner Institute, University of Oxford, Old Road Campus Research Building, Roosevelt Drive, Oxford OX3 7DQ, UK.
Ian W Lim, Division of Clinical Medicine, School of Medicine and Population Health, University of Sheffield, Sheffield S10 2RX, UK.
Simon J Draper, The Jenner Institute, University of Oxford, Old Road Campus Research Building, Roosevelt Drive, Oxford OX3 7DQ, UK.
Angela M Minassian, The Jenner Institute, University of Oxford, Old Road Campus Research Building, Roosevelt Drive, Oxford OX3 7DQ, UK.
Authors’ contributions
ROP and SJD conceived the study. ROP, NJE, SES and JRB conducted the assays. YT, TAR and AMM conducted the clinical aspects of the VAC063C trial. IWL and ROP analysed data for the service evaluation. ROP drafted the manuscript. AMM and SJD critically revised the manuscript. All authors read and approved the final manuscript.
Funding
The VAC063 clinical trial was funded by the Office of Infectious Diseases, Bureau for Global Health, USAID, under the terms of Malaria Vaccine Development Program (contract AID-OAA-C-15-00071), for which Leidos was the prime contractor. The opinions expressed here are those of the authors and do not necessarily reflect the views of USAID. This work was also supported in part by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre. ROP also held an NIHR Academic Clinical Lecturer award (CL-2016-04-502). The views expressed are those of the authors and not necessarily those of the NIHR, National Health Service or the UK Department of Health and Social Care. TAR held a Wellcome Trust Research Training Fellowship (108734/Z/15/Z). SJD is a Jenner Investigator and held a Wellcome Trust Senior Fellowship (106917/Z/15/Z).
Competing interests
None declared.
Ethical approval
VAC063C received ethical approval from the UK NHS Research Ethics Service (Oxfordshire Research Ethics Committee A, Ref 18/SC/0521). All volunteers gave written informed consent prior to participation. No ethical approval was required for the study done in the STH, as it was conducted as a service evaluation, but the results were reported and presented to the Trust Clinical Effectiveness Unit as well as local departments.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
- 1. Public Health England . Malaria imported into the United Kingdom: 2019. London: Public Health England; 2019. [Google Scholar]
- 2. World Health Organization . World malaria report 2022. Geneva: World Health Organization; 2022. [Google Scholar]
- 3. World Health Organization . World malaria report 2017. Geneva: World Health Organization; 2017. [Google Scholar]
- 4. Aidoo M, Incardona S. Ten years of universal testing: how the rapid diagnostic test became a game changer for malaria case management and improved disease reporting. Am J Trop Med Hyg. 2021;106(1):29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rogers CL, Bain BJ, Garg M et al. British Society for Haematology guidelines for the laboratory diagnosis of malaria. Br J Haematol. 2022;197(3):271–82. [DOI] [PubMed] [Google Scholar]
- 6. Wongsrichanalai C, Barcus MJ, Muth S et al. A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT). Am J Trop Med Hyg. 2007;77(6 Suppl):119–27. [PubMed] [Google Scholar]
- 7. World Health Organization . Malaria rapid diagnostic test performance: results of WHO product testing of malaria RDTs: round 7 (2015–2016). Geneva: World Health Organization; 2017. [Google Scholar]
- 8. UK NEQAS Haematology . Annual report to participants January—December 2016. Watford: UK NEQAS Haematology; 2017. [Google Scholar]
- 9. Morris U, Aydin-Schmidt B. Performance and application of commercially available loop-mediated isothermal amplification (LAMP) kits in malaria endemic and non-endemic settings. Diagnostics (Basel). 2021;11(2):336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stanisic DI, McCarthy JS, Good MF. Controlled human malaria infection: applications, advances, and challenges. Infect Immun. 2018;86(1):e00479–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bejon P, Andrews L, Hunt-Cooke A et al. Thick blood film examination for Plasmodium falciparum malaria has reduced sensitivity and underestimates parasite density. Malar J. 2006;5:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walk J, Schats R, Langenberg MC et al. Diagnosis and treatment based on quantitative PCR after controlled human malaria infection. Malar J. 2016;15(1):398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kamau E, Alemayehu S, Feghali KC et al. Measurement of parasitological data by quantitative real-time PCR from controlled human malaria infection trials at the Walter Reed Army Institute of Research. Malar J. 2014;13:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Makhija KS, Maloney S, Norton R. The utility of serial blood film testing for the diagnosis of malaria. Pathology (Phila). 2015;47(1):68–70. [DOI] [PubMed] [Google Scholar]
- 15. Pasricha JM, Juneja S, Manitta J et al. Is serial testing required to diagnose imported malaria in the era of rapid diagnostic tests? Am J Trop Med Hyg. 2013;88(1):20–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lucchi NW, Gaye M, Diallo MA et al. Evaluation of the Illumigene Malaria LAMP: a robust molecular diagnostic tool for malaria parasites. Sci Rep. 2016;6:36808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ljolje D, Abdallah R, Lucchi NW. Detection of malaria parasites in samples from returning US travelers using the Alethia® Malaria Plus LAMP assay. BMC Res Notes. 2021;14(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salkeld J, Themistocleous Y, Barrett JR et al. Repeat controlled human malaria infection of healthy UK adults with blood-stage Plasmodium falciparum: safety and parasite growth dynamics. Front Immunol. 2022;13:984323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Minassian AM, Silk SE, Barrett JR et al. Reduced blood-stage malaria growth and immune correlates in humans following RH5 vaccination. Med. 2021;2(6):701–19.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Engwerda CR, Minigo G, Amante FH et al. Experimentally induced blood stage malaria infection as a tool for clinical research. Trends Parasitol. 2012;28(11):515–21. [DOI] [PubMed] [Google Scholar]
- 21. Duncan CJ, Draper SJ. Controlled human blood stage malaria infection: current status and potential applications. Am J Trop Med Hyg. 2012;86(4):561–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheng Q, Lawrence G, Reed C et al. Measurement of Plasmodium falciparum growth rates in vivo: a test of malaria vaccines. Am J Trop Med Hyg. 1997;57(4):495–500. [DOI] [PubMed] [Google Scholar]
- 23. Payne RO, Milne KH, Elias SC et al. Demonstration of the blood-stage controlled human malaria infection model to assess efficacy of the Plasmodium falciparum AMA1 vaccine FMP2.1/AS01. J Infect Dis. 2016;213(11):1743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hodgson SH, Ewer KJ, Bliss CM et al. Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals. J Infect Dis. 2015;211(7):1076–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McGinnis E, Chan G, Hudoba M et al. Malaria screening using front-line loop-mediated isothermal amplification: fourteen-month experience in a nonendemic regional hub-and-spoke laboratory setting. Am J Clin Pathol. 2020;155(5):690–7. [DOI] [PubMed] [Google Scholar]
- 26. Meridian Biosciences . Product Brochure Alethia® Malaria. https://www.meridianbioscience.com/uploads/MBI_Alethia_Malaria_SS_2019-04.pdf?country=GB
- 27. Tan AF, Sakam SSB, Rajahram GS et al. Diagnostic accuracy and limit of detection of ten malaria parasite lactate dehydrogenase-based rapid tests for Plasmodium knowlesi and P. falciparum. Front Cell Infect Microbiol. 2022;12:1023219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheaveau J, Nguyen H, Chow B et al. Clinical validation of a commercial LAMP test for ruling out malaria in returning travelers: a prospective diagnostic trial. Open Forum Infect Dis. 2018;5(11):ofy260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.