Abstract
Objective
We aimed to assess the association of early pregnancy isolated maternal hypothyroxinemia (IMH), including persistent IMH, with adverse obstetric and perinatal outcomes.
Methods
This study was a large-scale retrospective cohort study based at a tertiary hospital in Shanghai, China between January 2013 and December 2016. Independent t-tests, chi-square tests, and stratified univariate and multivariate logistic regression analyses were performed to examine the associations between IMH and adverse pregnancy outcomes. Trend chi-square tests were used to evaluate the differences in the incidence of adverse pregnancy outcomes among the euthyroid, transient IMH, and persistent IMH groups.
Results
A total of 37,734 women with singleton pregnancies were classified into the euthyroid group (n = 35,733) and the IMH group (n = 2,001), based on thyroid function test results obtained during early pregnancy (gestational weeks 11–13). After adjusting for confounders, stratified analyses showed that IMH was associated with increased risks of gestational diabetes mellitus (GDM) primarily in women aged < 35 years and those with pre-pregnancy BMI < 24 kg/m². In women aged ≥ 35 years with a BMI ≥ 24 kg/m², IMH was associated with an elevated risk of preeclampsia. Additionally, in women aged ≥ 35 years, IMH was significantly linked to preterm birth and low birth weight (LBW). Fetal distress and macrosomia were consistently associated with IMH across all subgroups. No significant interaction effects were observed between maternal age or BMI and IMH on adverse outcomes. Trend chi-square tests indicated a linear increase in the incidence of preeclampsia, preterm birth, macrosomia, emergency cesarean section, and fetal distress across the euthyroid, transient IMH, and persistent IMH groups.
Conclusion
Early pregnancy IMH is associated with increased risks of several adverse pregnancy outcomes, and stratified analyses suggest that these risks may vary by maternal age and pre-pregnancy BMI. Both transient and persistent IMH were associated with an increased risk of adverse pregnancy outcomes, with persistent IMH related to a broader spectrum of adverse events.
Keywords: Thyroid dysfunction, Isolated maternal hypothyroxinemia, Maternal outcome, Neonatal outcome, Preeclampsia
Introduction
Thyroid hormones play a pivotal role in maintaining normal pregnancy and ensuring optimal fetal neurodevelopment [1, 2]. To meet the increased metabolic demands of gestation, Maternal thyroid hormone production rises by approximately 50% during pregnancy [3]. This heightened requirement may unmask pre-existing thyroid dysfunction in women with limited functional reserve. While universal screening for thyroid disorders in pregnancy remains controversial [4], the rising prevalence has led 21–69% of thyroid association members to advocate for routine screening [5]. Hypothyroidism represents a common endocrine disorder, particularly in iodine-deficient regions [3]. Since the early 2000 s, isolated maternal hypothyroxinemia (IMH) - defined by low free thyroxine (FT4) with normal thyroid-stimulating hormone (TSH) levels - has emerged as a distinct clinical entity [3]. Epidemiological studies demonstrate varying prevalence rates: 1.3–2.3% in iodine-sufficient populations such as in the UK (1.6%) [6] and the US (1.3–2.3%) [7], contrasting sharply with higher rates in iodine-deficient areas (Netherlands: 4.3–8.5% [8]; Thailand: 8.4% [9]; Italy: 3.2–12.7% [10]; Spain: 20.6–26.5% [11]).
The clinical significance of IMH remains debated. While some studies associate it with adverse neurodevelopmental outcomes [12–15] and pregnancy complications including gestational diabetes mellitus [16], preeclampsia [17], preterm delivery [18], and intrauterine growth restriction [19], others report no significant impact on perinatal outcomes or childhood development [20–24]. This study aims to clarify these conflicting findings by systematically evaluating the potential risks associated with IMH, with the ultimate goal of informing evidence-based clinical management strategies.
Methods
Subjects
This is a retrospective cohort study conducted at a tertiary specialized hospital in Shanghai. We retrospectively extracted medical records from the hospital’s electronic medical record system for 52,027 women with singleton pregnancies who had completed both prenatal care and delivery at our hospital between January 2013 and December 2016. After applying inclusion and exclusion criteria, 37,734 eligible participants were included in the final analysis. The study was approved by the hospital’s ethics committee. Since this was a secondary analysis of de-identified data collected for routine clinical care, informed consent was waived by the ethics committee. The study was conducted in accordance with approved institutional and ethical guidelines. The exclusion criteria were as follows: (1) pregnant women who were receiving or had a history of thyroid hormone or antithyroid drug treatment; (2) multiple pregnancy or underwent in vitro fertilization; (3) use of medications or presence of chronic diseases that may affect thyroid function (e.g., cancer, hypertension, heart disease, diabetes, autoimmune diseases); (4) absence of thyroid function test results in early pregnancy or incomplete medical records; and (5) those who did not deliver at our hospital.
Data collection
Fasting blood samples were collected from the median cubital vein during early pregnancy (11–13 weeks) and late pregnancy (32–34 weeks). Serum was separated by centrifugation within 6 h of collection. TSH, FT4, and TPOAb concentrations were measured using the Architect i2000 immunoassay (Abbott, Chicago, USA) according to the manufacturer’s protocol. TPOAb ≥ 5.61 IU/ml was considered positive. Pre-pregnancy information, including maternal age, gravidity, parity, education level, smoking status, and alcohol consumption, was collected through structured interviews at the first clinical visit. Height and weight were also measured to calculate body mass index (BMI). Based on the above electronic medical record system, we conducted a study to assess the risk of IMH and a range of pregnancy complications and neonatal outcomes. Including maternal pregnancy outcomes (GDM, preeclampsia, gestational hypertension, emergency cesarean section, preterm birth, placenta previa, and postpartum hemorrhage), and neonatal outcomes (low Apgar score, fetal distress, premature delivery, low birth weight, macrosomia, and fetal growth and development indicators).
Definition of IMH
IMH is defined as the presence of a low maternal free thyroxine (fT4) level in conjunction with a normal maternal thyroid-stimulating hormone (TSH) level. In our study, we specifically defined IMH as an FT4 concentration below the 5th percentile and a TSH concentration within the 2.5th to 97.5th percentiles, based on institution-specific, gestational age-adjusted reference ranges. These percentiles were derived from a large reference population of pregnant women who delivered at our hospital. IMH was defined and assessed at two gestational time points: early pregnancy (11–13 weeks) and late pregnancy (32–34 weeks), using corresponding gestational age-specific thresholds.
Ultrasound-based fetal biometric measurements
Fetal biometric parameters, including biparietal diameter (BPD), femur length (FL), and abdominal measurements such as transverse abdominal diameter (TAD), anterior-posterior abdominal diameter (APAD), and abdominal circumference (AC) were measured using standardized ultrasound protocols within one week before delivery to ensure accuracy, with all scans performed by certified sonographers using calibrated equipment following strict measurement protocols: BPD was obtained at the thalamic level including the cavum septum pellucidum, FL was measured along the long axis from the greater trochanter to distal condyle, and AC was taken at the umbilical vein level incorporating the stomach bubble and portal sinus. Each measurement was performed three times, with a second expert review if the variation exceeded 5%. Standardized equipment settings, proper fetal positioning, and identification of anatomical landmarks during fetal rest were applied to reduce measurement errors and variability.
Definitions of adverse pregnancy outcomes
The following definitions of adverse pregnancy outcomes, based on medical guidelines and the reported literature, were used in this study: (1) Gestational age was based on the interval between the last menstrual period and the date of delivery of the baby. When the menstrual estimate of gestational age was inconsistent by 7 or more days within the ultrasound measurement, we used the ultrasound corrected gestational age; (2) Low birth weight (LBW): the newborn birthweight < 2,500 g; (3) Macrosomia: the newborn birthweight ≥ 4,000 g; (4) Preterm birth was defined as births with gestational age less than 37 weeks; (5) GDM was diagnosed by the 75 g oral glucose tolerance test at 24–28 weeks of gestation. Women with a fasting blood glucose level ≥ 5.1 mmol/L, a 1-h blood glucose level ≥ 10.0 mmol/L or a 2-h blood glucose level ≥ 8.5 mmol/L were defined as GDM; (6)Postpartum Hemorrhage was defined as blood loss of more than 500 mL following vaginal delivery or more than 1,000 mL following cesarean delivery; (7) Gestational hypertension was defined as new-onset systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg on at least two occasions, at least 4 h apart, after 20 weeks of gestation in previously normotensive women, without proteinuria or other signs of preeclampsia, and typically resolving within 12 weeks postpartum [25]; (8) Preeclampsia(PE) is defined per ACOG guidelines as meeting either above hypertension criteria with greater than or equal to 300 mg urine protein excretion in a 24-hour period or a protein/creatinine ratio of greater than or equal to 0.3 [25]; (9) Placenta previa is refers to a pathological condition after 28 weeks of gestation in which the placenta is attached to the lower segment of the uterus, with its lower edge reaching or covering the internal cervical os, and positioned below the presenting part of the fetus. (10) Low Apgar score was defined as 1-minute Apgar ≤ 7; (11) Fetal distress is a syndrome in which the fetus is hypoxic and/or acidosis in utero, manifested as an abnormal pattern on cardiography, or umbilical artery blood gas analysis with PH < 7.2 or meconium contamination of amniotic fluid or 1-minute Apgar score < 7 [26].
Statistical analysis
Data were analyzed with SPSS version 21.0 (IBM Corp., Armonk, NY) and R software version 3.6.1 (R Development Core Team, July 2019; http://www.r-project.org). Continuous variables with a normal distribution are expressed as mean ± standard deviation and were compared using the independent samples t-test. Non-normally distributed variables are presented as median (interquartile range) and were compared using the Mann–Whitney U test. Qualitative data were expressed as rates and compared using the chi-square test. Stratified univariable and multivariable logistic regression analyses were performed to compare pregnancy outcomes with IMH, with maternal age and pre-pregnancy BMI as stratification factors. Multivariate regression analysis was used to adjust for confounding variables such as gravidity, parity, and education level. The Enter method (forced entry) was applied to build multivariable models, and interaction effects of maternal age and pre-pregnancy BMI were also evaluated. Odds ratios (ORs) with 95% confidence intervals were calculated to identify risk factors and assess their impact. The forest plot was established based on the results of the multivariate logistic regression analysis using R software. And we used the Chi-square test for trend to compare the differences in the incidence of adverse pregnancy outcomes among the euthyroid group, transient IMH group and persistent IMH group. Statistical significance was set at P < 0.05 (all tests were 2-sided).
Missing data handling
When the missing rate of a variable was below 5%, and missing values were distributed relatively evenly across the sample without obvious systematic bias, Listwise deletion was applied for complete case analysis. For variables with missing rates exceeding 5%, Little’s MCAR test was conducted to assess whether the missing data were missing completely at random (MCAR). If the test indicated data were MCAR, listwise deletion was continued; if not, domain knowledge was used to classify the missing data mechanism as missing at random (MAR) or missing not at random (MNAR). Multiple imputation was applied for MAR to estimate missing values, thereby reducing bias and enhancing the robustness and validity of the analysis. For MNAR data, reasonable interpretations were made based on the specific medical context of this study, and more advanced missing data methods, such as sensitivity analyses using pattern-mixture models, would be applied when necessary.
Results
Handling of missing data
A total of 37,734 pregnant women were included in this study. Among them, 2,001 cases were diagnosed as IMH of early pregnancy, and 35,733 cases were euthyroid. Figure 1 shows a flowchart of participants in the study.
Fig. 1.
Flowchart of participants in the study
In this study, 935 participants were missing thyroid function test results in early pregnancy (11–13 weeks), and an additional 156 participants lacked key covariate information. Overall, 1,091 cases (2.2% of the study population) had incomplete data. Previous methodological literature suggests that when the proportion of missing data is very low (commonly below 5%), the bias introduced is likely negligible, and complete-case analysis (CCA) is generally acceptable [27]. Given the low proportion and dispersed pattern of missingness in our dataset, CCA was employed, as the benefits of multiple imputation in this context would likely be minimal. In contrast, the missing rate for thyroid function data in late pregnancy (32–34 weeks) was relatively higher, primarily due to obstetric complications that resulted in preterm birth and, consequently, missed testing at the scheduled time. To assess the missing data mechanism, we performed Little’s MCAR test, incorporating late-pregnancy TSH and FT4 levels, maternal age, BMI, parity, gravidity, education level, and pregnancy outcomes (including preterm birth, emergency cesarean section, LBW, GDM, macrosomia, preeclampsia, and fetal distress). The test rejected the assumption of MCAR (p < 0.01), suggesting that the missing data pattern was more consistent with MNAR. Considering the potential association between missingness and adverse pregnancy outcomes, we did not perform multiple imputation for late-pregnancy thyroid data. Instead, our main analysis focused on early pregnancy data, which had a lower rate of missingness, to ensure the robustness and validity of the results.
Population characteristics
Table 1 displays the baseline characteristics of pregnant women stratified by first-trimester IMH status. Compared to the euthyroid group, the IMH group had significantly higher maternal age, BMI, gravidity, multiparity rate, and educational level (P < 0.05). In contrast, there were no significant differences between the two groups in terms of fetal sex, smoking status, or alcohol consumption (P > 0.05).
Table 1.
Basic characteristics of pregnant women by first-trimester IMH status
| Basic characteristic | Euthyroid (n = 35,733) |
IMH (n = 2,001) |
P value |
|---|---|---|---|
| Maternal age (y) | 30 (27–32) | 31 (29–34) | < 0.001* |
| Pre-pregnancy BMI (kg/m 2) | 20.6 (19.1–22.3) | 21.8 (20.0-23.7) | < 0.001* |
| Newborn sex, n (%) | |||
| male | 18,669 (52.2%) | 1,044 (52.2%) | 0.963 |
| female | 17,064 (47.8%) | 957 (47.8%) | |
| Gravidity, n (%) | |||
| < 3 | 35,380 (99.0%) | 1,920 (96.0%) | < 0.001* |
| ≥ 3 | 353 (1.0%) | 81 (4.0%) | |
| Parity, n (%) | |||
| Primipara | 29,340 (82.1%) | 1,369 (68.4%) | < 0.001* |
| Multipara | 6,393 (17.9%) | 632 (31.6%) | |
| Educational levels, n (%) | |||
| High school and lower | 8,188 (22.9%) | 396 (19.8%) | < 0.001* |
| Bachelor | 21,047 (58.9%) | 1,215 (60.7%) | |
| Master | 5,990 (16.8%) | 344 (17.2%) | |
| Doctorate and higher | 508 (1.4%) | 46 (2.3%) | |
| Smoke, n (%) | |||
| No | 35,706 (99.9%) | 2,001 (100.0%) | 0.400 |
| Yes | 27 (0.1%) | 0 (0.0%) | |
| Alcohol, n (%) | |||
| No | 35,732 (100%) | 2,001 (100.0%) | 0.999 |
| Yes | 1 (0.0%) | 0 (0.0%) | |
Non-normally distributed variables are presented as median (interquartile range) and compared using non-parametric tests
Abbreviations BMI body mass index, IMH isolated maternal hypothyroxinemia
*P < 0.05 was considered statistically significant
Diagnostic criteria for IMH
In this study, normal reference ranges of TSH and FT4 in women were defined as the 2.5th and 97.5th percentiles. The normal cut-off values in the first trimester (11–13 weeks) were: TSH (0.03–3.65) mIU/L; FT4 (11.70–19.60) pmol/L. The 5th population percentile for FT4 is 12.20 pmol/L. The normal cut-off values in the third trimester (32–34 weeks) were: TSH (0.39–3.67) mIU/L; FT4 (9.10–14.40) pmol/L. The 5th population percentile for FT4 is 9.50 pmol/L. Using these percentile-based cut-offs, IMH was defined as an FT4 concentration below the 5th percentile with a TSH concentration between the 2.5th and 97.5th percentiles. Individuals with both FT4 and TSH concentrations within the 2.5th to 97.5th percentiles were classified as euthyroid. According to whether IMH lasted into the third trimester, they were divided into the euthyroid group (normal in both the first and third trimester) with a total of 31,349 cases, the transient IMH group (IMH in early pregnancy but normal in late pregnancy) with 1,347 cases, and the persistent IMH group (IMH lasted from early to late pregnancy) with 510 cases.
Comparison of pregnancy outcomes
Table 2 shows the maternal and neonatal outcomes according to first-trimester IMH status (IMH vs. euthyroid). The prevalence of premature delivery and emergency CS (cesarean section) was higher in the IMH group than in the euthyroid group (P < 0.05). And the incidences of GDM and preeclampsia were higher in the IMH group with significant differences (P < 0.05). However, there were no significant differences in the incidences of gestational hypertension, placenta previa, and postpartum hemorrhage (P > 0.05). Compared to women with euthyroid status, the incidences of low Apgar score and fetal distress were higher in the IMH group (P < 0.05). And those women with IMH were more likely to give birth to macrosomia and LBW (P < 0.05). In addition, the fetal head circumference (HC), femur length (FL), humerus length (HL), and transverse abdominal diameter (TAD) of the IMH group in late pregnancy were all larger than those of the euthyroid group (P < 0.05). However, there was no significant difference in Biparietal diameter (BPD) between the two groups (P > 0.05).
Table 2.
Comparison of maternal and neonatal outcomes based on first-trimester IMH status
| Outcome | Euthyroid (n = 35,733) |
IMH (n = 2,001) |
P value |
|---|---|---|---|
| Maternal outcomes | |||
| GDM, n (%) | 3,653 (10.2%) | 359 (17.9%) | < 0.001* |
| OGTT (mmol/L) | |||
| 0 h | 4.1 (3.9–4.4) | 4.2 (3.9–4.5) | < 0.001* |
| 1 h | 7.7 (7.1–8.5) | 7.9 (7.4–9.1) | < 0.001* |
| 2 h | 6.2 (5.4–7.1) | 6.5 (5.6–7.5) | < 0.001* |
| Gestational hypertension, n (%) | 1,016 (2.8%) | 63 (3.1%) | 0.411 |
| Preeclampsia, n (%) | 710 (2.0%) | 72 (3.6%) | < 0.001* |
| Delivery outcomes | |||
| Placenta previa, n (%) | 203 (0.6%) | 5 (0.2%) | 0.062 |
| Emergency CS, n (%) | 2,157 (6.0%) | 159 (7.9%) | 0.001* |
| Postpartum hemorrhage, n (%) | 314 (0.9%) | 24 (1.2%) | 0.138 |
| Neonatal outcomes | |||
| Premature delivery, n (%) | 1,684 (4.7%) | 128 (6.4%) | < 0.001* |
| Fetal distress, n (%) | 1,555 (4.3%) | 176 (8.7%) | < 0.001* |
| Low Apgar score, n (%) | 316 (0.9%) | 29 (1.4%) | 0.013* |
| LBW, n (%) | 963 (2.6%) | 72 (3.5%) | 0.022* |
| Macrosomia, n (%) | 2,174 (5.9%) | 208 (10.4%) | < 0.001* |
| Fetal development (mm) | |||
| Biparietal diameter (BPD) | 91.8 ± 4.1 | 91.8 ± 4.4 | 0.464 |
| Head Circumference (HC) | 306.9 ± 38.8 | 311.7 ± 21.5 | < 0.001* |
| Femur Length (FL) | 67.0 (64.0–69.0) | 68.0 (64.0–70.0) | 0.009* |
| Humerus Length (HL) | 59.0 (56.0–61.0) | 59.0 (56.0–61.0) | < 0.001* |
| Transverse Abdominal Diameter (TAD) | 98.0 (92.0-103.0) | 99.0 (93.0-104.0) | 0.002* |
Continuous variables with normal distribution are expressed as mean±SD and compared using the independent samples t-test. Non-normally distributed variables are presented as median (interquartile range) and compared using the Mann-Whitney U test
Abbreviations GDM Gestational Diabetes Mellitus, LBW Low birth weight, CS cesarean section, IMH isolated maternal hypothyroxinemia
*P < 0.05 was considered statistically significant
Association of IMH in the first trimester with adverse pregnancy outcomes
To comprehensively evaluate the association between first-trimester IMH and adverse pregnancy outcomes, we performed stratified analyses based on maternal age and pre-pregnancy BMI (Table 3). Although univariate analyses indicated statistically significant associations between IMH and several adverse outcomes across multiple subgroups, multivariate adjustment for confounders—including pre-pregnancy BMI, age, parity, gravidity, and education level—revealed that fetal distress and macrosomia remained significantly associated with IMH in all stratified subgroups. Other adverse outcomes, however, showed significance only within specific subgroups.
Table 3.
Association of adverse pregnancy outcomes with first-trimester IMH, stratified by maternal age and pre-pregnancy BMI
| Outcome | Stratification | Incidence (%) | Crudea | Adjustedb | P for Interactionc | ||||
|---|---|---|---|---|---|---|---|---|---|
| IMH group (n = 2,001) | Euthyroid (n = 35,733) |
OR (95% CI) | P value | OR (95% CI) | P value | ||||
| Preterm birth | Age | < 35 (y) | 5.4 | 4.5 | 1.21 (0.97 ~ 1.52) | 0.095 | 1.11 (0.87 ~ 1.43) | 0.399 | 0.073 |
| ≥ 35 (y) | 9.7 | 6.3 | 1.61 (1.15 ~ 2.25) | 0.006 * | 1.67 (1.16 ~ 2.40) | 0.006 * | |||
| BMI | < 24 (kg/m2) | 6.0 | 4.6 | 1.29 (1.02 ~ 1.63) | 0.030 * | 1.23 (0.97 ~ 1.55) | 0.084 | 0.725 | |
| ≥ 24 (kg/m2) | 7.9 | 5.8 | 1.37 (0.92 ~ 2.05) | 0.121 | 1.30 (0.87 ~ 1.96) | 0.201 | |||
| GDM | Age | < 35 (y) | 16.5 | 9.2 | 1.94 (1.69 ~ 2.23) | < 0.001 * | 1.42 (1.21 ~ 1.66) | < 0.001 * | 0.146 |
| ≥ 35 (y) | 22.9 | 18.1 | 1.34 (1.06 ~ 1.70) | 0.013 * | 1.14 (0.88 ~ 1.48) | 0.331 | |||
| BMI | < 24 (kg/m2) | 15.4 | 9.0 | 1.66 (1.42 ~ 1.94) | < 0.001 * | 1.40 (1.19 ~ 1.64) | < 0.001 * | 0.260 | |
| ≥ 24 (kg/m2) | 27.0 | 20.1 | 1.29 (1.01 ~ 1.65) | 0.043 | 1.20 (0.93 ~ 1.54) | 0.162 | |||
| Preeclampsia | Age | < 35 (y) | 3.2 | 1.9 | 1.66 (1.24 ~ 2.24) | < 0.001 * | 1.37 (0.99 ~ 1.90) | 0.055 | 0.264 |
| ≥ 35 (y) | 5.1 | 2.5 | 2.12 (1.33 ~ 3.38) | 0.002 * | 1.92 (1.15 ~ 3.20) | 0.013 * | |||
| BMI | < 24 (kg/m2) | 2.7 | 1.7 | 1.54 (1.09 ~ 2.17) | 0.014 * | 1.36 (0.96 ~ 1.93) | 0.084 | 0.617 | |
| ≥ 24 (kg/m2) | 6.8 | 4.2 | 1.62 (1.05 ~ 2.49) | 0.030 * | 1.72 (1.11 ~ 2.67) | 0.016 * | |||
| Emergency CS | Age | < 35 (y) | 8.1 | 6.2 | 1.34 (1.11 ~ 1.62) | 0.002 * | 1.02 (0.83 ~ 1.24) | 0.873 | 0.165 |
| ≥ 35 (y) | 7.3 | 4.7 | 1.58 (1.08 ~ 2.32) | 0.020 * | 1.40 (0.94 ~ 2.09) | 0.095 | |||
| BMI | < 24 (kg/m2) | 8.1 | 6.0 | 1.13 (0.93 ~ 1.37) | 0.234 | 1.11 (0.91 ~ 1.35) | 0.315 | 0.432 | |
| ≥ 24 (kg/m2) | 7.3 | 6.4 | 0.90 (0.60 ~ 1.35) | 0.609 | 0.94 (0.62 ~ 1.41) | 0.747 | |||
| Fetal distress | Age | < 35 (y) | 8.8 | 4.4 | 2.11 (1.75 ~ 2.53) | < 0.001 * | 1.49 (1.23 ~ 1.81) | < 0.001 * | 0.590 |
| ≥ 35 (y) | 8.8 | 4.2 | 2.23 (1.56 ~ 3.20) | < 0.001 * | 1.81 (1.25 ~ 2.64) | 0.002 * | |||
| BMI | < 24 (kg/m2) | 8.3 | 4.2 | 1.62 (1.34 ~ 1.97) | < 0.001 * | 1.51 (1.24 ~ 1.84) | < 0.001 * | 0.933 | |
| ≥ 24 (kg/m2) | 10.4 | 5.5 | 1.54 (1.09 ~ 2.17) | 0.013 * | 1.62 (1.15 ~ 2.30) | 0.006 * | |||
| LBW | Age | < 35 (y) | 3.0 | 2.5 | 1.18 (0.87 ~ 1.59) | 0.291 | 1.11 (0.79 ~ 1.55) | 0.545 | 0.081 |
| ≥ 35 (y) | 4.6 | 2.9 | 1.60 (1.00 ~ 2.58) | 0.051 | 1.91 (1.16 ~ 3.15) | 0.011 * | |||
| BMI | < 24 (kg/m2) | 3.0 | 2.6 | 1.15 (0.83 ~ 1.58) | 0.403 | 1.20 (0.87 ~ 1.66) | 0.266 | 0.291 | |
| ≥ 24 (kg/m2) | 4.5 | 2.8 | 1.59 (0.94 ~ 2.68) | 0.084 | 1.55 (0.91 ~ 2.64) | 0.105 | |||
| Macrosomia | Age | < 35 (y) | 10.0 | 5.9 | 1.79 (1.51 ~ 2.13) | < 0.001 * | 1.52 (1.26 ~ 1.84) | < 0.001 * | 0.883 |
| ≥ 35 (y) | 10.6 | 6.4 | 1.74 (1.25 ~ 2.40) | < 0.001 * | 1.53 (1.06 ~ 2.20) | 0.023 * | |||
| BMI | < 24 (kg/m2) | 8.4 | 5.2 | 1.73 (1.42 ~ 2.12) | < 0.001 * | 1.46 (1.19 ~ 1.80) | < 0.001 * | 0.872 | |
| ≥ 24 (kg/m2) | 16.3 | 11.5 | 1.56 (1.16 ~ 2.09) | 0.003 * | 1.52 (1.13 ~ 2.05) | 0.006 * | |||
Abbreviations GDM Gestational Diabetes Mellitus IMH isolated maternal hypothyroxinemia, CS cesarean section, LBW low birth weight, OR Odds Ratio, CI Confidence Interval
*P < 0.05 was considered statistically significant
aUnivariate logistic regression was performed using the euthyroid group as the reference
b Estimates were obtained using multivariate logistic regression models, adjusted for maternal age, pre-pregnancy BMI, gravidity, parity, and educational level
c P values for interaction evaluated the modifying effects of maternal age and BMI on the association between IMH and pregnancy outcomes
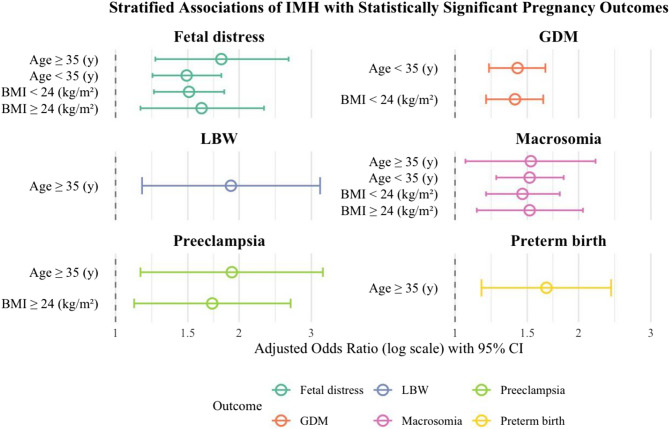
To focus on the most clinically relevant findings, Fig. 2 displays forest plots of only those stratified associations between first-trimester IMH and adverse pregnancy outcomes that reached statistical significance, thereby highlighting key subgroup-specific risks and emphasizing the most relevant associations.
Fig. 2.
Forest plot of multivariate logistic regression analysis for the association between IMH in the first trimester and adverse pregnancy outcomes. Forest plot showing stratified associations between first-trimester IMH and adverse pregnancy outcomes. Colored points represent adjusted ORs for each outcome stratified by maternal age and pre-pregnancy BMI categories. Vertical Lines extending from the points indicate the 95% CIs. The dashed vertical reference line at OR = 1 indicates no association. Only statistically significant associations are displayed. Different colors correspond to different pregnancy outcomes as indicated in the legend, facilitating comparison across outcomes and subgroups. Adjustment variables included maternal age, pre-pregnancy BMI, parity, gravidity, and education level. Abbreviations: IMH, isolated maternal hypothyroxinemia; GDM, gestational diabetes mellitus; CS, cesarean section; LBW, low birth weight; aOR, adjusted odds ratio; CI, confidence interval
Specifically, in the Subgroup of women younger than 35 years, IMH was significantly associated with an increased risk of gestational diabetes mellitus (GDM) (adjusted OR = 1.42, P < 0.001), fetal distress (adjusted OR = 1.49, P < 0.001), and macrosomia (adjusted OR = 1.52, P < 0.001). In women aged 35 years and older, IMH significantly elevated the risk of preterm birth (adjusted OR = 1.67, P = 0.006), preeclampsia (adjusted OR = 1.92, P = 0.013), fetal distress (adjusted OR = 1.81, P = 0.002), LBW (adjusted OR = 1.91, P = 0.011), and macrosomia (adjusted OR = 1.53, P = 0.023). Among women with pre-pregnancy BMI < 24 kg/m², IMH was significantly associated with higher risks of GDM (adjusted OR = 1.40, P < 0.001), fetal distress (adjusted OR = 1.51, P < 0.001), and macrosomia (adjusted OR = 1.46, P < 0.001). In contrast, in the subgroup with BMI ≥ 24 kg/m², IMH was significantly linked to increased risks of preeclampsia (adjusted OR = 1.72, P = 0.016), fetal distress (adjusted OR = 1.62, P = 0.006), and macrosomia (adjusted OR = 1.52, P = 0.006). Despite these significant associations within specific subgroups, no statistically significant interaction effects were observed between maternal age or pre-pregnancy BMI and IMH on any adverse pregnancy outcome (P for interaction > 0.05), suggesting a limited moderating role of these factors in the relationship between IMH and adverse pregnancy outcomes.
Association of persistent IMH with adverse pregnancy outcomes
As shown in Table 4, trend chi-square tests indicated a linear increase in the incidence of preeclampsia, preterm birth, macrosomia, emergency cesarean section, and fetal distress across the euthyroid, transient IMH, and persistent IMH groups. Compared with the euthyroid group, transient IMH was significantly associated with increased risks of GDM (adjusted OR = 1.41, 95% CI: 1.20–1.66), macrosomia (aOR = 1.52, 95% CI: 1.24–1.87), and fetal distress (aOR = 1.42, 95% CI: 1.15–1.76), but not with preeclampsia, preterm birth, emergency CS, or LBW. Persistent IMH was significantly associated with higher odds of preeclampsia (aOR = 2.52, 95% CI: 1.67–3.80), preterm birth (aOR = 1.54, 95% CI: 1.07–2.23), macrosomia (aOR = 1.77, 95% CI: 1.33–2.37), and fetal distress (aOR = 1.72, 95% CI: 1.28–2.32) compared with euthyroid women. No statistically significant association was observed for GDM, emergency CS, or LBW in this group. Direct comparison between persistent and transient IMH showed that persistent IMH carried a significantly higher risk only for preeclampsia (aOR = 2.33, 95% CI: 1.34–4.05), whereas differences in other outcomes were not statistically significant.
Table 4.
Comparison of pregnancy outcomes among euthyroid, transient IMH, and persistent IMH groups using trend Chi-square test and multivariate logistic regression
| Outcome | Incidence (%) | P-value for trend a | Transient vs. Euthyroid | Persistent vs. Transient | Persistent vs. Euthyroid | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Euthyroid (N = 31,349) |
Transient IMH (N = 1,347) |
Persistent IMH (N = 510) |
OR (95% CI) | p-value b | OR (95% CI) | p-value c | OR (95% CI) | p-valueb | ||
| GDM | 9.9 | 18.3 | 16.3 | < 0.001 * | 1.41 (1.20–1.66) | < 0.001 * | 0.86 (0.64–1.16) | 0.325 | 1.24 (0.96–1.60) | 0.093 |
| Preeclampsia | 1.8 | 2.4 | 5.7 | < 0.001 * | 1.11 (0.75–1.65) | 0.603 | 2.33 (1.34–4.05) | 0.003 * | 2.52 (1.67–3.80) | < 0.001 * |
| Preterm birth | 4.0 | 4.8 | 6.7 | 0.001 * | 1.09 (0.82–1.45) | 0.541 | 1.46 (0.93–2.30) | 0.102 | 1.54 (1.07–2.23) | 0.021 * |
| Macrosomia | 5.9 | 9.9 | 11.6 | < 0.001 * | 1.52 (1.24–1.87) | < 0.001 * | 1.15 (0.82–1.62) | 0.418 | 1.77 (1.33–2.37) | < 0.001 * |
| Emergency CS | 6.1 | 7.4 | 10.2 | < 0.001 * | 1.02 (0.82–1.28) | 0.865 | 1.26 (0.87–1.82) | 0.227 | 1.29 (0.95–1.75) | 0.103 |
| Fetal distress | 4.3 | 7.9 | 10.4 | < 0.001 * | 1.42 (1.15–1.76) | 0.001 * | 1.20 (0.84–1.71) | 0.320 | 1.72 (1.28–2.32) | < 0.001 * |
| LBW | 1.9 | 1.8 | 3.1 | 0.167 | 0.91 (0.58–1.44) | 0.686 | 1.91 (0.96–3.39) | 0.064 | 1.63 (0.96–2.76) | 0.071 |
Abbreviations GDM Gestational Diabetes Mellitus, IMH isolated maternal hypothyroxinemia, CS cesarean section, LBW low birth weight, OR Odds Ratio, CI Confidence Interval
a P-value for trend across categories of different groups
b P values from multivariable logistic regression (reference: euthyroid group), adjusted for maternal age, pre-pregnancy BMI, gravidity, parity, and educational level
c The p-values taken from multivariable logistic regression comparing the persistent IMH group with the transient IMH group
* P < 0.05 was considered statistically significant
Discussion
Main findings
This study demonstrated that IMH in the first trimester is significantly associated with increased risks of adverse maternal and neonatal outcomes compared to euthyroid pregnancies. The study population was stratified by maternal age and pre-pregnancy BMI, and univariate and multivariate logistic regression analyses were performed within these strata to evaluate the association between IMH and adverse pregnancy outcomes. After adjusting for confounders, fetal distress and macrosomia remained significantly associated with IMH across all subgroups, whereas other adverse outcomes reached significance only within specific age or BMI strata. Notably, no significant interaction effects were observed between maternal age or pre-pregnancy BMI and IMH, indicating a limited moderating influence of these factors. This suggests that the impact of IMH on adverse pregnancy outcomes is largely independent. Furthermore, both transient and persistent IMH were associated with an increased risk of adverse pregnancy outcomes, with persistent IMH generally related to a broader spectrum of adverse events, particularly a higher incidence of preeclampsia compared with transient IMH.
Etiology of IMH
Understanding the causes of IMH is essential for optimizing treatment. Iodine deficiency remains the most common cause, as the thyroid shifts from T4 to T3 production to conserve iodine, thereby reducing circulating T4 levels [3]. While the implementation of universal salt iodization in 1995 has led to a marked improvement in iodine status among pregnant women in Shanghai, mild iodine deficiency remains an issue in specific groups, warranting continued attention [28, 29]. Overweight and obesity are additional risk factors; increased subcutaneous fat in euthyroid adults correlates with lower FT4 and higher TSH levels [30]. Obesity may enhance peripheral deiodinase activity, elevating T3 levels, while weight loss has been shown to reduce FT3 concentrations [31]. Other contributing factors include iron deficiency and exposure to environmental pollutants, which may disrupt thyroid function [32–34].
Thresholds for IMH diagnosis
Accurate assessment of thyroid function during pregnancy requires trimester-specific reference ranges, as serum TSH and FT4 levels vary across different institutions and gestational stages due to physiological and methodological differences [35, 36]. Factors such as human chorionic gonadotropin (hCG) and thyroxine-binding globulin (TBG) contribute to dynamic fluctuations in thyroid hormone levels. Given that fetal thyroid function begins around the 20th week of gestation, Maternal thyroid hormones are critical for early fetal brain development. Consequently, evaluating thyroid function at 11–13 weeks provides a clearer understanding of maternal thyroid status before fetal thyroid autonomy [37]. International guidelines emphasize the importance of establishing laboratory- and population-specific reference ranges for TSH and FT4 to prevent misdiagnosis [37]. Variations in assay methodologies [38] and patient demographics [39, 40] further underscore the need for locally validated thresholds. Both the Endocrine Society and the American Thyroid Association recommend against using general population ranges for pregnant women, advocating instead for trimester-specific benchmarks [41]. In our study, we defined IMH as an FT4 concentration below the 5th percentile with normal TSH levels, using institution-specific reference ranges for early (11–13 weeks) and late pregnancy (32–34 weeks). Among 45,171 unselected women, the prevalence of IMH was 4.43% in early pregnancy, aligning with findings from a similar study in Shandong Province, China [42].
Association of IMH in the first trimester with adverse pregnancy outcomes
This study places greater emphasis on IMH in early pregnancy because this period is critical for the development of the fetal central nervous system and the formation of the placenta. During this stage, the fetus depends almost entirely on maternal thyroid hormones. If maternal thyroid dysfunction occurs at this time, it may have lasting and profound effects on fetal growth and development. Therefore, from the perspective of prevention and early risk identification, IMH in early pregnancy holds greater value for both research and clinical intervention.
Accumulating evidence suggests that IMH may contribute to adverse pregnancy outcomes such as macrosomia and gestational diabetes mellitus (GDM). A Chinese cohort study reported a 1.22-fold higher risk of macrosomia in women with hypothyroxinemia [43], while other studies, including those by Furnica et al. [12] and Haddow et al. [44], support a similar association. Van et al. further identified a correlation between hypothyroxinemia and increased fetal head circumference [45], while Chen et al. reported that FT4 levels were negatively associated with birthweight [24]. Although multivariate analyses often support these links, some studies reported non-significant results after adjusting for confounders [46]. Mechanistically, thyroid hormones regulate fetal metabolism, oxygen consumption, and the insulin-like growth factor axis, thereby influencing fetal growth [47, 48]. Persistently low FT4 levels in early and late pregnancy may promote excess fat accumulation and higher birthweight, contributing to macrosomia [43]. Additionally, lower FT4 levels are associated with increased maternal insulin resistance [49] and adverse metabolic profiles [47], predisposing women to GDM and fetal overgrowth. Emerging studies continue to emphasize the role of thyroid hormones in maintaining metabolic homeostasis during pregnancy [50], and even small fluctuations in hormone levels have been linked to significant metabolic disturbances [51, 52]. Our findings are consistent with these observations, further highlighting the importance of assessing maternal thyroid function as part of risk stratification for adverse pregnancy outcomes.
Fetal distress and low Apgar scores are recognized contributors to long-term neurological sequelae, including cerebral palsy and autism spectrum disorders [53]. Maternal hypothyroidism has been associated with increased risk of fetal distress and suboptimal neonatal adaptation, as shown in both Chinese and international studies [43, 54, 55]. Thyroid hormones are vital for fetal tissue growth and differentiation, supporting critical postnatal functions such as pulmonary gas exchange, thermoregulation, gluconeogenesis, and cardiac output. Impaired thyroid hormone availability in early pregnancy may compromise placental and fetal development, impairing stress tolerance and increasing the likelihood of intrapartum fetal distress.
The consistent associations between IMH and adverse outcomes, particularly fetal distress and macrosomia, across all subgroups suggest a potential mechanistic pathway independent of maternal age or pre-pregnancy BMI. These observations support the role of IMH as an independent risk factor for poor perinatal outcomes. Clinically, this highlights the importance of incorporating thyroid function testing into routine antenatal risk assessment. Tailored monitoring and timely intervention strategies may facilitate early identification of at-risk pregnancies and contribute to improved maternal and neonatal outcomes.
Impact of persistent IMH
In this study, IMH was categorized as persistent if present in both early and late pregnancy, transient if confined to early pregnancy, and euthyroid if thyroid function remained normal throughout gestation. Prior studies have shown that only 8.4–24.8% of early pregnancy thyroid abnormalities persist into late gestation [56], and our cohort showed a comparable rate of persistent IMH. This pattern Suggests that most thyroid dysfunctions in early pregnancy are transient, Likely reflecting increased Maternal thyroid hormone demands due to fetal utilization, elevated TBG, type 3 deiodinase activity, and hCG-driven thyroid stimulation [1]. Despite the transient nature of many IMH cases, our findings revealed a linear trend in the incidence of GDM, preeclampsia, and macrosomia across euthyroid, transient IMH, and persistent IMH groups, with persistent IMH showing the highest risk. These observations point to the need for early and ongoing thyroid function monitoring. Although universal screening in pregnancy could enable timely detection and management, its long-term clinical utility requires validation through prospective longitudinal studies [57].
Strengths and limitations
Our study has several strengths. First, we used institution-specific, trimester-adjusted reference ranges for TSH and FT4, avoiding the pitfalls of generalized thresholds. Second, given concerns about the potential adverse effects of early pregnancy IMH [58], we rigorously evaluated its association with pregnancy outcomes while adjusting for confounders, including maternal BMI, age, parity, and thyroid autoantibody status. This approach minimized bias from autoimmune thyroid disease. Third, the exclusion of levothyroxine-treated IMH cases eliminated confounding by treatment effects. Fourth, our large sample size enhanced statistical robustness. Finally, our inclusion of late-pregnancy thyroid function assessments provided novel insights into the effects of persistent versus transient IMH.
Limitations must also be acknowledged. First, although Shanghai is generally not classified as iodine-deficient due to longstanding universal salt iodization policies [59], regional and individual variations in iodine status may still exist [60]. Insufficient iodine intake can reduce thyroid hormone production, contributing to IMH, which may affect adverse pregnancy outcomes by altering maternal thyroid function. Unfortunately, our retrospective cohort lacked routine iodine status assessments (e.g., urinary iodine concentration), limiting our ability to adjust for this potential confounder. This absence may introduce residual confounding, as some observed associations between IMH and adverse outcomes could be partially mediated or confounded by underlying iodine deficiency. Therefore, future prospective studies incorporating iodine biomarker measurements are necessary to better control for this confounding factor and clarify the complex interplay between iodine status, IMH, and pregnancy outcomes. Second, our complete-case analysis assumes that data were missing at random; however, unmeasured confounding cannot be excluded. Moreover, thyroid function data in late pregnancy were missing for some participants, likely due to adverse outcomes such as preterm birth rather than random omission. Given the potential association between missingness and poor outcomes, simple imputation methods may be inappropriate. Thus, future studies should consider advanced modeling approaches, such as pattern-mixture models, to better address the impact of MNAR data and enhance the robustness of findings. Finally, the retrospective design underrepresented early adverse outcomes (e.g., miscarriage, fetal anomalies). Hence, future studies should enroll women from conception, irrespective of delivery location, to comprehensively capture these important endpoints.
Conclusion
IMH diagnosed in the first trimester is an independent risk factor for various adverse pregnancy outcomes, with persistent IMH associated with particularly elevated risks. These findings underscore the importance of routine thyroid function screening in early pregnancy to facilitate the timely identification of IMH and enable more precise risk stratification for individualized prenatal care. Although current guidelines do not recommend routine follow-up or treatment for early IMH, our results suggest that selective monitoring of affected women may be warranted. Prospective interventional studies are needed to determine whether closer surveillance or management of early and persistent IMH can improve maternal and neonatal outcomes.
Acknowledgements
The authors express sincere thanks to all the participants and the staff of the International Peace Maternity and Child Health Hospital for their contributions.
Authors’ contributions
All the authors contributed to the work. This study was designed by YM. MX performed the statistical analysis, and MS wrote the manuscript. YM reviewed and edited the manuscript. All authors read and approved the final version of this manuscript.
Funding
Not applicable.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the International Peace Maternity and Child Health Hospital, School of Medicine, Shanghai Jiaotong University (No. GKLW2019-16). Since this was a secondary analysis of de-identified data collected for routine clinical care, informed consent was waived by the ethics committee. The data analysis procedures followed the guidelines in the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mengfan Song and Mingtao Xiong contributed equally to this work.
References
- 1.Korevaar TIM, Medici M, Visser TJ, Peeters RP. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nat Rev Endocrinol. 2017;13(10):610–22. 10.1038/nrendo.2017.93. [DOI] [PubMed] [Google Scholar]
- 2.Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31(5):702–55. 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- 3.K A, N P, A B, et al. 2017 guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. https://home.liebertpub.com/thy. Published Online March 1, 2017. 10.1089/thy.2016.0457 [DOI] [PubMed]
- 4.Stagnaro-Green A. Screening pregnant women for overt thyroid disease. JAMA. 2015;313(6):565–6. 10.1001/jama.2014.17226. [DOI] [PubMed] [Google Scholar]
- 5.Azizi F, Amouzegar A, Mehran L, Alamdari S, Subekti I, Vaidya B, et al. Screening and management of hypothyroidism in pregnancy: results of an Asian survey. Endocr J. 2014;61(7):697–704. 10.1507/endocrj.EJ14-0083. [DOI] [PubMed] [Google Scholar]
- 6.Vaidya B, Anthony S, Bilous M, Shields B, Drury J, Hutchison S, et al. Detection of thyroid dysfunction in early pregnancy: universal screening or targeted high-risk case finding? J Clin Endocrinol Metab. 2007;92(1):203–7. 10.1210/jc.2006-1748. [DOI] [PubMed] [Google Scholar]
- 7.Cleary-Goldman J, Malone FD, Lambert-Messerlian G, Sullivan L, Canick J, Porter TF et al. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol. 2008;112(1):85-92. 10.1097/AOG.0b013e3181788dd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henrichs J, Bongers-Schokking JJ, Schenk JJ, Ghassabian A, Schmidt HG, Visser TJ, et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab. 2010;95(9):4227–34. 10.1210/jc.2010-0415. [DOI] [PubMed] [Google Scholar]
- 9.Kominiarek MA, Chauhan SP. Overweight increases risk of first trimester hypothyroxinaemia in iodine-deficient pregnant women. Am J Perinatol. 2016;33(5):433–41. 10.1055/s-0035-1567856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moleti M, Lo Presti VP, Mattina F, Mancuso A, De Vivo A, Giorgianni G, et al. Gestational thyroid function abnormalities in conditions of mild iodine deficiency: early screening versus continuous monitoring of maternal thyroid status. Eur J Endocrinol. 2009;160(4):611–7. 10.1530/EJE-08-0709. [DOI] [PubMed] [Google Scholar]
- 11.Berbel P, Mestre JL, Santamaría A, Palazón I, Franco A, Graells M, et al. Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: the importance of early iodine supplementation. Thyroid. 2009;19(5):511–9. 10.1089/thy.2008.0341. [DOI] [PubMed] [Google Scholar]
- 12.Furnica RM, Gruson D, Lazarus JH, Maiter D, Bernard P, Daumerie C. First trimester isolated maternal hypothyroxinaemia: adverse maternal metabolic profile and impact on the obstetrical outcome. Clin Endocrinol (Oxf). 2017;86(4):576–83. 10.1111/cen.13301. [DOI] [PubMed] [Google Scholar]
- 13.Gong X, Liu A, Li Y, Sun H, Li Y, Li C, et al. The impact of isolated maternal hypothyroxinemia during the first and second trimester of gestation on pregnancy outcomes: an intervention and prospective cohort study in China. J Endocrinol Invest. 2019;42(5):599–607. 10.1007/s40618-018-0960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korevaar TI, Schalekamp-Timmermans S, de Rijke YB, Visser WE, Visser W, de Keizer-Schrama M. Hypothyroxinemia and TPO-antibody positivity are risk factors for premature delivery: the generation R study. J Clin Endocrinol Metab. 2013;98(11):4382–90. 10.1210/jc.2013-2855. [DOI] [PubMed] [Google Scholar]
- 15.Finken MJJ, van Eijsden M, Loomans EM, Vrijkotte TGM, Rotteveel J. Maternal hypothyroxinemia in early pregnancy predicts reduced performance in reaction time tests in 5- to 6-year-old offspring. J Clin Endocrinol Metab. 2013;98(4):1417–26. 10.1210/jc.2012-3389. [DOI] [PubMed] [Google Scholar]
- 16.Olivieri A, Valensise H, Magnani F, Medda E, De Angelis S, D’Archivio M, et al. High frequency of antithyroid autoantibodies in pregnant women at increased risk of gestational diabetes mellitus. Eur J Endocrinol. 2000;143(6):741–7. 10.1530/eje.0.1430741. [DOI] [PubMed] [Google Scholar]
- 17.Sardana D, Nanda S, Kharb S. Thyroid hormones in pregnancy and preeclampsia. J Turk Ger Gynecol Assoc. 2009;10(3):168–71. [PMC free article] [PubMed] [Google Scholar]
- 18.Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105(2):239. 10.1097/01.AOG.0000152345.99421.22. [DOI] [PubMed] [Google Scholar]
- 19.Chan SY, Franklyn JA, Pemberton HN, Bulmer JN, Visser TJ, McCabe CJ, et al. Monocarboxylate transporter 8 expression in the human placenta: the effects of severe intrauterine growth restriction. J Endocrinol. 2006;189(3):465–71. 10.1677/joe.1.06582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf). 2003;59(3):282–8. 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 21.Craig WY, Allan WC, Kloza EM, Pulkkinen AJ, Waisbren S, Spratt DI, et al. Mid-gestational maternal free thyroxine concentration and offspring neurocognitive development at age two years. J Clin Endocrinol Metab. 2012;97(1):E22–28. 10.1210/jc.2011-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casey BM, Dashe JS, Spong CY, McIntire DD, Leveno KJ, Cunningham GF. Perinatal significance of isolated maternal hypothyroxinemia identified in the first half of pregnancy. Obstet Gynecol. 2007;109(5):1129–35. 10.1097/01.AOG.0000262054.03531.24. [DOI] [PubMed] [Google Scholar]
- 23.Chen L, Yang H, Ye E, Lin Z, Peng M, Lin H, et al. Insignificant effect of isolated hypothyroxinemia on pregnancy outcomes during the first and second trimester of pregnancy. Front Endocrinol. 2020;11:528146. 10.3389/fendo.2020.528146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai C, Chen W, Vinturache A, Hu P, Lu M, Gu H, et al. Thyroid hormone concentrations in second trimester of gestation and birth outcomes in Shanghai, China. J Matern Fetal Neonatal Med. 2021;34(12):1897–905. 10.1080/14767058.2019.1651273. [DOI] [PubMed] [Google Scholar]
- 25.Gestational Hypertension and Preeclampsia. ACOG practice bulletin summary, number 222. Obstet Gynecol. 2020;135(6):1492–5. 10.1097/AOG.0000000000003892. [DOI] [PubMed] [Google Scholar]
- 26.Xie X, Kong B, Duan T. Obstetrics and gynecology (the 9th Version). Beijing: People ’ s Medical Publishing House; 2018. pp. 138–40. [Google Scholar]
- 27.Madley-Dowd P, Hughes R, Tilling K, Heron J. The proportion of missing data should not be used to guide decisions on multiple imputation. J Clin Epidemiol. 2019;110:63–73. 10.1016/j.jclinepi.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma ZF. A comparative study of iodized salt programs. Shanghai and Switzerland. Biol Trace Elem Res. 2019;189(2):586. 10.1007/s12011-018-1478-8. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Liu P, Su X, Zou S, Song J, Liu S. A comparison of iodine status in children and pregnant women after a policy change in the iodized salt standard in Shanghai, China. Biol Trace Elem Res. 2018;185(2):275–81. 10.1007/s12011-018-1257-6. [DOI] [PubMed] [Google Scholar]
- 30.Alevizaki M, Saltiki K, Voidonikola P, Mantzou E, Papamichael C, Stamatelopoulos K. Free thyroxine is an independent predictor of subcutaneous fat in euthyroid individuals. Eur J Endocrinol. 2009;161(3):459–65. 10.1530/EJE-09-0441. [DOI] [PubMed] [Google Scholar]
- 31.Agnihothri RV, Courville AB, Linderman JD, Smith S, Brychta R, Remaley A, et al. Moderate weight loss is sufficient to affect thyroid hormone homeostasis and inhibit its peripheral conversion. Thyroid®. 2014;24(1):19–26. 10.1089/thy.2013.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi X, Han C, Li C, Mao J, Wang W, Xie X, et al. Optimal and safe upper limits of iodine intake for early pregnancy in iodine-sufficient regions: a cross-sectional study of 7190 pregnant women in China. J Clin Endocrinol Metab. 2015;100(4):1630–8. 10.1210/jc.2014-3704. [DOI] [PubMed] [Google Scholar]
- 33.Babić Leko M, Gunjača I, Pleić N, Zemunik T. Environmental factors affecting thyroid-stimulating hormone and thyroid hormone levels. Int J Mol Sci. 2021;22(12):6521. 10.3390/ijms22126521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghassabian A, Pierotti L, Basterrechea M, Chatzi L, Estarlich M, Fernández-Somoano A, et al. Association of exposure to ambient air pollution with thyroid function during pregnancy. JAMA Netw Open. 2019;2(10):e1912902. 10.1001/jamanetworkopen.2019.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang C, Wu Y, Chen L, Yuan Z, Yang S, Liu C. Establishment of assay method- and trimester-specific reference intervals for thyroid hormones during pregnancy in Chengdu, China. J Clin Lab Anal. 2021;35(5):e23763. 10.1002/jcla.23763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mumtaz A, Sadiq F, Zaki S, Batool H, Ibrahim M, Khurram M, et al. Trimester-specific reference ranges for thyroid hormones of pregnant females at tertiary care hospitals in Lahore, Pakistan. BMC Pregnancy Childbirth. 2021;21(1):717. 10.1186/s12884-021-04200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korevaar TIM. Evidence-based tightrope, walking. The 2017 guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid®. 2017;27(3):309–11. 10.1089/thy.2017.29040.tko. [DOI] [PubMed] [Google Scholar]
- 38.Ayres PJ, Barlow J, Garrod O, Tait SA, Tait JF, Walker G. Correlation of adrenal steroid concentrations and changes in electrolyte metabolism. Scand J Clin Lab Invest. 1958;10(Suppl 31):29–49. [PubMed] [Google Scholar]
- 39.Pop VJ, Biondi B, Wijnen HA, Kuppens SM, LVader H. Maternal thyroid parameters, body mass index and subsequent weight gain during pregnancy in healthy euthyroid women. Clin Endocrinol (Oxf). 2013;79(4):577–83. 10.1111/cen.12177. [DOI] [PubMed] [Google Scholar]
- 40.Andersen SL, Andersen S, Carlé A, Christensen PA, Handberg A, Karmisholt J, et al. Pregnancy week-specific reference ranges for thyrotropin and free thyroxine in the North Denmark region pregnancy cohort. Thyroid. 2019;29(3):430–8. 10.1089/thy.2018.0628. [DOI] [PubMed] [Google Scholar]
- 41.De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, et al. Management of thyroid dysfunction during pregnancy and postpartum: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(8):2543–65. 10.1210/jc.2011-2803. [DOI] [PubMed] [Google Scholar]
- 42.Chen L, Ye E, Sun M, Lin H, Yu L, Lin Z, et al. Association between third trimester maternal isolated hypothyroxinemia and adverse pregnancy outcomes. Endocr J. 2023;70(6):611–8. 10.1507/endocrj.EJ22-0528. [DOI] [PubMed] [Google Scholar]
- 43.Zhang C, Yang X, Zhang Y, Guo F, Yang S, Peeters RP, et al. Association between maternal thyroid hormones and birth weight at early and late pregnancy. J Clin Endocrinol Metab. 2019;104(12):5853–63. 10.1210/jc.2019-00390. [DOI] [PubMed] [Google Scholar]
- 44.Haddow JE, Craig WY, Palomaki GE, et al. Impact of adjusting for the reciprocal relationship between maternal weight and free thyroxine during early pregnancy. Thyroid. 2013;23(2):225–30. 10.1089/thy.2012.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Mil NH, Steegers-Theunissen RP, Bongers-Schokking JJ, El Marroun H, Ghassabian A, Hofman A, et al. Maternal hypothyroxinemia during pregnancy and growth of the fetal and infant head. Reprod Sci. 2012;19(12):1315–22. 10.1177/1933719112450338. [DOI] [PubMed] [Google Scholar]
- 46.Li P, Cui J, Li L, Chen X, Ouyang L, Fan J, et al. Association between isolated maternal hypothyroxinemia during the first trimester and adverse pregnancy outcomes in Southern Chinese women: a retrospective study of 7051 cases. BMC Pregnancy Childbirth. 2022;22(1):866. 10.1186/s12884-022-05194-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bassols J, Prats-Puig A, Soriano-Rodríguez P, García-González MM, Reid J, Martínez-Pascual M, et al. Lower free thyroxin associates with a less favorable metabolic phenotype in healthy pregnant women. J Clin Endocrinol Metab. 2011;96(12):3717–23. 10.1210/jc.2011-1784. [DOI] [PubMed] [Google Scholar]
- 48.Roos A, Bakker SJL, Links TP, Gans ROB, Wolffenbuttel BHR. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab. 2007;92(2):491–6. 10.1210/jc.2006-1718. [DOI] [PubMed] [Google Scholar]
- 49.Roef GL, Rietzschel ER, Van Daele CM, Taes YE, De Buyzere ML, Gillebert TC, et al. Triiodothyronine and free thyroxine levels are differentially associated with metabolic profile and adiposity-related cardiovascular risk markers in euthyroid middle-aged subjects. Thyroid. 2014;24(2):223–31. 10.1089/thy.2013.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Longhi S, Radetti G. Thyroid function and obesity. J Clin Res Pediatr Endocrinol. 2013;5(Suppl 1):40–4. 10.4274/jcrpe.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johns LE, Ferguson KK, McElrath TF, Mukherjee B, Meeker JD. Associations between repeated measures of maternal urinary phthalate metabolites and thyroid hormone parameters during pregnancy. Environ Health Perspect. 2016;124(11):1808–15. 10.1289/EHP170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor PN, Razvi S, Pearce SH, Dayan CM. Clinical review: a review of the clinical consequences of variation in thyroid function within the reference range. J Clin Endocrinol Metab. 2013;98(9):3562–71. 10.1210/jc.2013-1315. [DOI] [PubMed] [Google Scholar]
- 53.Osredkar D, Verdenik I, Gergeli AT, Gersak K, Lucovnik M. Apgar score and risk of cerebral palsy in preterm infants: a population-based cohort study. Neuropediatrics. 2021;52(4):310–5. 10.1055/s-0041-1729181. [DOI] [PubMed] [Google Scholar]
- 54.Su PY, Huang K, Hao JH, Xu YQ, Yan SQ, Li T, et al. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab. 2011;96(10):3234–41. 10.1210/jc.2011-0274. [DOI] [PubMed] [Google Scholar]
- 55.Goel P, Radotra A, Devi K, Malhotra S, Aggarwal A, Huria A. Maternal and perinatal outcome in pregnancy with hypothyroidism. Indian J Med Sci. 2005;59(3):116–7. [PubMed] [Google Scholar]
- 56.Fan J, Zhang Y, Zhang C, Barjaktarovic M, Yang X, Peeters RP, et al. Persistency of thyroid dysfunction from early to late pregnancy. Thyroid. 2019;29(10):1475–84. 10.1089/thy.2019.0115. [DOI] [PubMed] [Google Scholar]
- 57.La Verde M, De Franciscis P, Molitierno R, Caniglia FM, Fordellone M, Braca E, et al. Thyroid hormones in early pregnancy and birth weight: a retrospective study. Biomedicines. 2025;13(3):542. 10.3390/biomedicines13030542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J. 2014;3(2):76–94. 10.1159/000362597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian W, Yan W, Liu Y, Zhou F, Wang H, Sun W. The status and knowledge of iodine among pregnant women in Shanghai. Biol Trace Elem Res. 2021;199(12):4489–97. 10.1007/s12011-021-02587-4. [DOI] [PubMed] [Google Scholar]
- 60.Yu Z, Zheng C, Zheng W, Wan Z, Bu Y, Zhang G, et al. Mild-to-moderate iodine deficiency in a sample of pregnant women and salt iodine concentration from Zhejiang province, China. Environ Geochem Health. 2020;42(11):3811–8. 10.1007/s10653-020-00640-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.