Bacillus subtilis is a gram-positive, aerobic, spore-forming soil bacterium ubiquitous in the environment. The beneficial effects of B. subtilis spores on the balance of the intestinal microflora is the rationale for its general use as a probiotic preparation in the treatment or prevention of intestinal disorders. B. subtilis spores are available in Italy as a pharmaceutical preparation for oral use. Each dose contains a mixture of 109 spores of four distinct antibiotic-resistant derivatives of ATCC 9799 (Enterogermina; distributed by Sanofi Winthrop, Milan, Italy) (1, 4) per vial. The pathogenic potential of B. subtilis is generally described as low or absent (2). Data on the general importance of infections due to B. subtilis are incomplete, since it is a general practice of most microbiological laboratories to discard these strains or to report them as contaminants. Also, in the cause-of-death statistics of the World Health Organization no data on B. subtilis infections are present since, even if reported, they would be “invisible” at the international comparative level due to the coding used for classification of death causes (2a). In the literature, only a few cases of infections due to B. subtilis are reported (3, 6–8, 10) and only one retrospective study describes the isolation of antibiotic-resistant strains of B. subtilis (6).
The subject of our report is a 73-year-old male with chronic lymphocytic leukemia (leukocyte count, 46,000/mmc with 4% segmented forms, 92% lymphocytes, and 4% monocytes) who was admitted to the hospital because of high fever (40°C), mental confusion, and diarrhea (through the period of hospitalization, the patient had no central line in place). Prehospitalization treatment (over a month) with B. subtilis spores (Enterogermina) (EG) was discontinued upon the patient’s admission to the hospital. On physical examination, the patient showed hepatosplenomegaly and multiple pulmonary thickenings were visible on the chest X ray. He had sluggish mentation and speech but no focal neurological deficits. Blood cultures performed in triplicate (on day 1) were positive for B. subtilis. Treatment with imipenem (days 1 to 16) apparently resolved the infectious episode, although mild fever persisted, possibly due to the lymphoproliferative disorder. After 2 weeks of hospitalization, the patient presented again with a high fever and mental confusion. Blood cultures were repeated (days 16 and 19), and B. subtilis was present in both cultures. Combined antibiotic therapy (ceftazidime, amikacin, and vancomycin, to all of which both strains were susceptible) was started, together with intravenously administered immunoglobulins, and the fever rapidly declined. Nevertheless, the patient showed progressive deterioration of his mental condition, still without focal neurological signs. At this stage, lymphoid cells were detected in the cerebrospinal fluid (cerebrospinal fluid was not cultured), and the patient died within a few days (day 25), probably due to central nervous system involvement.
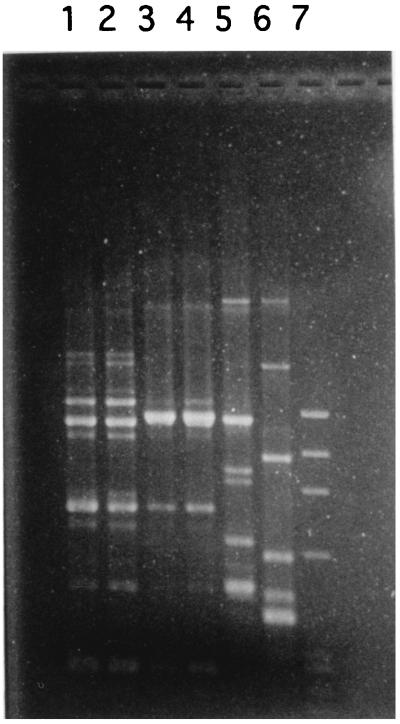
The B. subtilis strains isolated during fever episodes on days 1 and 19 showed resistance to penicillin, erythromycin, rifampin, and novobiocin. The isolate from the blood culture on day 16 differed, being susceptible to rifampin and novobiocin and resistant to chloramphenicol. Due to the unusual pattern of resistance to antimicrobial agents (5), the three isolates were compared to the B. subtilis strains isolated from EG. Strains isolated on days 1 and 19 showed an antibiotic resistance profile identical to that of the rifampin- and novobiocin-resistant EG isolate (EG-RN), while the strain isolated at day 16 showed a resistance profile identical to that of the chloramphenicol-resistant EG strain (EG-CM). Strain typing by antibiogram was confirmed by two other lines of evidence. Biochemical profiles (API 50CH and API 20E; bioMérieux) distinguished the rifampin- and novobiocin-resistant clinical isolate and the EG-RN strain (gelatinase negative) from the chloramphenicol-resistant clinical isolate and the EG-CM strain (gelatinase positive). The randomly amplified polymorphic DNA technique (9) clearly confirmed the clonal difference, evidencing distinctive DNA amplification patterns for the chloramphenicol-resistant strains and for the rifampin- and novobiocin-resistant strains (Fig. 1).
FIG. 1.
Ethidium bromide-stained agarose gel of randomly amplified fragments of B. subtilis chromosomal DNA. Amplification was performed with primer GTTTCCGCCC as described in reference 9. Lane 1, rifampin- and novobiocin-resistant clinical isolate; lane 2, EG-RN strain; lane 3, chloramphenicol-resistant clinical isolate; lane 4, EG-CM strain; lane 5, B. subtilis ATCC 6633; lane 6, B. subtilis reference strain Marburg; lane 7, molecular weight standard (phage ΦX174 cut with restriction enzyme HaeIII).
The described recovery of two different Bacillus strains from the same probiotic preparation in distinct septicemic episodes is indicative both of the severe immunodeficiency of the patient and of a persistence of the microorganism in the intestinal tract, a property already described for strain EG-RN (4). This report, as does a report describing typing of strains by antibiogram only (6), documents the high risk to which severely immunocompromised patients can be exposed when treated with pharmaceutical products based on live microorganisms. We conclude that, even if the septicemia due to the probiotic strains of B. subtilis could not be related directly to the patient’s death, high numbers of viable microorganisms (especially if polyantibiotic resistant) should not be given to any patient with severe immunodeficiency.
REFERENCES
- 1.Ciffo F. Determination of the spectrum of antibiotic resistance of the Bacillus subtilis strains of Enterogermina. Chemioterapia. 1984;3:45–52. [PubMed] [Google Scholar]
- 2.de Boer A S, Diderichsen B. On the safety of Bacillus subtilis and B. amyloliquefaciens: a review. Appl Microbiol Biotechnol. 1991;36:1–4. doi: 10.1007/BF00164689. [DOI] [PubMed] [Google Scholar]
- 2a.Frank, O. (World Health Organization). Personal communication.
- 3.Kiss T, Gratwohl A, Frei R, Osterwalder B, Tichelli A, Speck B. Bacillus subtilis infections. Schweiz Rundsch Med Prax. 1988;77:1219–1223. [PubMed] [Google Scholar]
- 4.Mazza P, Zani F, Martelli P. Studies on the antibiotic resistance of Bacillus subtilis strains used in oral bacteriotherapy. Boll Chim Farm. 1992;131:401–408. [PubMed] [Google Scholar]
- 5.Reva O N, Vyunitskaya V A, Reznik S R, Kozachko L A, Smirnov V V. Antibiotic susceptibility as a taxonomic characteristic of the genus Bacillus. Int J Syst Bacteriol. 1995;45:409–411. doi: 10.1099/00207713-45-2-409. [DOI] [PubMed] [Google Scholar]
- 6.Richard V, Van der Auwera P, Snoeck R, Daneau D, Meunier F. Nosocomial bacteremia caused by Bacillus species. Eur J Clin Microbiol Infect Dis. 1988;7:783–785. doi: 10.1007/BF01975049. [DOI] [PubMed] [Google Scholar]
- 7.Thomas M, Whittet H. Atypical meningitis complicating a penetrating head injury. J Neurol Neurosurg Psychiatry. 1991;54:92–93. doi: 10.1136/jnnp.54.1.92-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velasco E, De Sousa Martins C A, Tabak D, Bouzas L F. Bacillus subtilis infection in a patient submitted to a bone marrow transplantation. Rev Paul Med. 1992;110:116–117. [PubMed] [Google Scholar]
- 9.Vettori C, Paffetti D, Pietramellara G, Stotzky G, Gallori E. Amplification of bacterial DNA bound on clay minerals by the random amplified polymorphic DNA (RAPD) technique. FEMS Microbiol Ecol. 1996;20:251–260. [Google Scholar]
- 10.Wallet F, Crunelle V, Roussel-Delvallez M, Furchard A, Saunier P, Courcol R J. Bacillus subtilis as a cause of cholangitis in polycystic kidney and liver disease. Am J Gastroenterol. 1996;91:1477–1478. [PubMed] [Google Scholar]