Abstract
Background
Primary mitochondrial myopathies (PMM) are disorders that involve defects in oxidative phosphorylation (OXPHOS) and impair mainly, but not exclusively, skeletal muscles. Progressive external ophthalmoplegia (PEO), eyelid ptosis, exercise intolerance and skeletal muscle weakness are the most common symptoms of myopathy in mitochondrial diseases, impairing ocular motility and visual abilities.
Methods
Twenty-five patients underwent complete ophthalmological examination, including best corrected visual acuity (BCVA), ptosis evaluation, dilated fundus examination, and orthoptic examinations, including cover and cover-uncover test, ocular motility analysis, fusional amplitude (FA) vergence for near and for distance, Bagolini striated glasses test (BSGs) and Worth four-dot lights test (WFDT).
Results
Mean age at evaluation was of 47,2 ± 16.07 years. Twenty-two (88%) out of 25 patients had a PEO disease, while three (12%) of them a Kearn-Sayre syndrome (KSS). Ocular motility impairment was found in 92% of the population. Fifteen patients (60%) didn’t complain of double vision in casual seeing condition despite some of them showed manifest strabismus both at far (53%) and at near (60%). A compensation sensorial mechanism, mainly suppression, was detected through sensory tests. The near and distance fusional capabilities in convergence and in divergence (CFAs and DFAs) were absent in 68 and 72% of the whole sample respectively. PEO manifests at an older age than KSS (p = 0.003), diplopia does not correlate with disease duration (p = 0.06) and no predictive factors for diplopia can be identified.
Conclusions
A significant number of patients not complaining of double vision in casual seeing state showed manifest or latent/manifest strabismus at FAoD and NAoD. Most strabismic patients had a monocular suppression or alternate diplopia and suppression at sensory tests (BSGs and WFDT). The pathophysiology of these sensory adaptations in an adult visual system can only be hypothesized. A multidisciplinary approach is essential for proper clinical management and to analyze an understand clinical features pathogenesis.
Keywords: Mitochondrial diseases, Mitochondrial diseases strabismus, Mitochondrial diseases diplopia, Neurological strabismus, Mitochondrial diseases eyelid ptosis, Ophthalmological abnormalities in mitochondrial diseases
Background
Mitochondrial diseases (MDs) are a group of complex and clinically heterogeneous metabolic disorders defined by a genetic defect predominantly affecting mitochondrial OXPHOS, the main source of ATP generation in cells [1]. Among the neurogenetic disorders with the highest prevalence [2, 3], MDs manifest clinically with isolated neuromuscular symptoms or, more commonly, in association with multisystem manifestations due to the characteristic involvement of energy-dependent tissues [1, 4, 5].
The distinctive extraordinary phenotypic variability and the dual genetic control, with nuclear genome (nDNA) and mitochondrial DNA (mtDNA) working in concert, make these disorders a challenge for the clinician [6].
CPEO had a family occurrence in 28% of cases. There are three distinct routes of inheritance: maternal transmission linked to mitochondrial point mutations, as shown in other mitochondrial illnesses, autosomal recessive inheritance, and autosomal dominant inheritance. Unlike occasional instances of single mitochondrial deletions, autosomal inheritance may be linked to multiple deletions of mitochondrial DNA [7].
Recently, a consortium of international experts has defined a specific subgroup of MDs named primary mitochondrial myopathies (PMM) characterized by a predominant, but not exclusively, skeletal muscle involvement [8, 9]. The affection of external eye muscles represents the most common presentation of PMM [10], resulting in a chronic progressive external ophthalmoplegia (CPEO or PEO) with a limitation of extraocular movements and progressive bilateral ptosis [11]. Ocular myopathy can represent the only clinical manifestation (PEO phenotype) or be part of a multisystemic involvement (PEO-plus syndrome). Within this variable clinical spectrum, the Kearn-Sayre syndrome (KSS), a severe phenotype of PEO-associated mitochondrial disease, is defined by a triad of PEO, onset before the age of 20, pigmentary retinopathy plus one of the following conditions: cardiac conduction block, cerebrospinal fluid (CSF) protein greater than 100 mg/dL, cerebellar ataxia [12–15]. Recently, a consensus from the North American Mitochondrial Disease Consortium (NAMDC) proposed to substitute these criteria with a clinical tetrad: PEO, pigmentary retinopathy, cardiac conduction block and skeletal muscle involvement, considering the requirement of age-of-onset before 20 years an arbitrary cut-off not well-supported by data, and CSF uncommon in clinical practice for these patients [11].
Although ocular involvement represents one of the peculiar clinical manifestations of MDs, there are few published papers in which these aspects are documented in depth and in a systematic way [16].
The purpose of this manuscript is to assess the eye, eyelid and extraocular muscles involvement with an analysis of the sensorimotor status in a well characterized mitochondrial cohort of patients through a prospective multidisciplinary approach.
Methods
Patient population
This is a prospective observational study performed at the Ophthalmology Unit and Neurophysiopatology Unit of the Fondazione Policlinico Universitario Agostino Gemelli IRCCS between November 1 st, 2020, and February 28th, 2023.
Following genetic, biochemical, and histochemical diagnosis of MD, patients were categorized into PEO/PEOplus or KSS. In each patient, the diagnosis was confirmed by mitochondrial DNA analysis and muscle biopsy and genetic data were collected retrospectively from patients'hospital data. A neurologist with expertise in mitochondrial medicine and an ophthalmologist evaluated the patients. All subjects were assessed using the NMDAS, a semiquantitative rating scale used to monitor a patient’s disease status in relation to other organ systems commonly involved in mitochondrial disease [17].
Patients who did not wish to participate in the study and patients with either missing or incomplete records were excluded.
Standard protocol approvals, registrations, and patient consents
This research study was conducted in accordance with the ICH Guidelines for Good Clinical Practice, in accordance with the Declaration of Helsinki (1991), and with the approval of the Fondazione Policlinico Universitario Agostino Gemelli IRCCS Institutional Review Board, approval reference number 13764. A written informed consent for data collection and analysis was obtained from every patient. Photographs were obtained in selected cases upon patients’ permission.
Procedures
All patients underwent a complete orthoptic and ophthalmological assessment.
The ophthalmological examination included best-corrected visual acuity (BCVA) measurement, ptosis evaluation with margin-reflex distance (MRD) and levator function (LF) measurements, tonometry, lens status examination and dilated fundus examination.
Best correct visual acuity (BCVA) was assessed for right and left eye using ETDRS charts. MRD were measured holding an examination torch about half a meter away from the patients and asking them to look it inside. With a ruler held against the upper eyelid, was then measured the distance between the light reflex on pupillary foramen and the superior eyelid margin (MRD). When pupillary foramen was covered by the upper eyelid, making so impossible the visualization of a visible light reflex, MRD showed a negative value, with its absolute value measured starting from the superior lid margin and then rising it up with a finger till to pupillary foramen. For analytical purposes, a value of 0 was assigned when the upper lid margin covered the center of the pupil; whereas if it outstripped the inferior pupil margin, a value equal to – 1 was assigned. LF was assessed asking to patients to looking downgaze and then upgaze, with the eyebrow kept fixed with the examiner hand, and measuring the excursion in millimeters of the upper eyelid between the two gaze positions.
The orthoptic examination included near (33 cm) and far (6 m) prism and alternating cover test and ocular motility evaluation. Divergence and convergence fusional amplitudes (FAs) were measured at distance (6 m) and at near (33 cm) fixation through the full optical correction, when possible, using an accommodative target, first at distance and then at near. Starting from the base-out or base-up or down prisms totally compensating the deviation, base-out prisms of decreasing power were used to measure divergence FA, whereas prisms of increasing power were used to measure convergence FA. The examination of ocular motility was performed assessing the amplitude of ductions (monocular eye movements during occlusion of the other eye) and versions (binocular eye movements in the same direction). Ductions and versions are tested by asking the patients to look at eight positions of gaze Versions were graded taking into account basic anatomical landmarks such as the position of the limbus in relation to the medial and lateral canthus (horizontal versions) and the excursion beyond the primary gaze position (vertical versions). We used a scale from 1 to 5 to qualify versions hypofunction (1-normal, 2-mild, 3-moderate, 4-severe, 5-complete ophthalmoplegia).
The sensory status was evaluated by striated glasses test of Bagolini (BSGs) and Worth’s four lights test (WFDT)..The Bagolini Striated Glasses Test (BSG) is utilized to assess retinal correspondence and binocular vision. The patient utilizes spectacles featuring small striations at specific angles [18]. When observing a point light source, each eye perceives a line that is perpendicular to the striations. Normal binocular vision, or harmonic correspondence, is evidenced when both lines are perceived as creating a cross. Absence or disruption of lines indicates potential suppression or atypical communication. The Worth's Four Lights Test (W4LT) assesses fusion and suppression capabilities. The patient is observed with red-green spectacles while examining four lights: red, green, and white [19]. The examiner can determine whether the patient exhibits normal fusion, suppression of one eye, or diplopia—double vision—by analyzing the number and color range reported by the patient.
Statistical analysis
Descriptive analyses were performed using frequencies and percentages for categorical data and mean and standard deviation (SD) for continuous data. Skewness and Kurtosis were used to investigate the distribution of the collected quantitative data and the Saphiro-Francia test was used to investigate normal distribution. To assess the impact of age on the phenotype, T-test was applied to compare the variance of the means between KSS and PEO phenotype. The test was conducted on both the age at onset and at assessment as well as on elapsed time to avoid confounding. The chi-square test or Fisher's exact test were used to analyze the correlation between diplopia and clinicopathological features [20]. The analysis of the determinants of diplopia in myopathic patients was conducted by developing a multivariable logistic regression model.
The covariates included in the model were: gender, age at onset and evaluation, the time elapsed between evaluation and onset, strabismus, ptosis, ocular motility and data derived from WFDT, BSGs, FAs, angle of deviation (AoD), both far and near point. Multivariable logistic regression models were constructed using the strategy suggested by Hosmer and Lemeshow [21].
Each variable was examined by univariable analysis and was included in the multivariable logistic model when p-values were < 0.20.
The influence of the independent variables on each binary outcome examined was expressed as odds ratio (or) and 95% confidence interval (CI). Statistical significance was set at p < 0.05.
Statistical analysis was performed with Stata 17.0 software (Stata Corporation, College Station, TX, USA).
Results
Twenty-five patients, 15 males and 10 females, with confirmed MDs and ocular involvement were enrolled in this study. Mean age at evaluation was of 47,2 ± 16.07 years (range 20–71 years), while mean age at disease onset was 22,56 ± 9.90 years (range 8–40 years). Mean time of disease was 24.64 ± 13.16 years. Twenty-two (88%) out of 25 patients had a PEO/PEO plus phenotypes, three (12%) of them a KSS. Regarding the genotype we found the following data: 10 patients (40%) reported single mtDNA deletion, 7 (28%) POLG variants [22], 5 (20%), SLC25A4 variants[23], 2 (8%) m.3243A > G [24] and 1 (4%) patient associated with TOP3A variant. [25]. As major ocular history not directly associated to the mitochondrial myopathy, a 22 years-old patient underwent cataract surgery in both eye at age of 10 for bilateral congenital cataract.
Visual acuity
Mean BCVA was 0.092 ± 0.125 LogMAR in right eye and 0.096 ± 0.149 LogMAR in left eye.
Lens and fundus evaluation
In 2/25 patients (8%) a cortico-nuclear cataract was detected, while 2/25 (8%) were pseudophakic.
Only one (4%) patient, affected by KSS, showed abnormalities of the macular RPE.
Ocular motility and angle of deviation
An exodeviation was found for far in 21 (84%) patients, 3 (12%) had a vertical deviation and 1 (4%) an esodeviation. Likewise, an exodeviation for near was found in 21 (84%) patients, 3 (12%) had a vertical deviation and 1 (4%) an esodeviation. In particular, 1 patient showed exodeviation for far and vertical deviation for near; 1 patient showed vertical deviation for far and esodeviation for near, and 1 patient had esodeviation for far and exodeviation for near. Mean far exodeviation (FAoD) was of −10,18 ± 11,23 prismatic diopters (PD), mean near exodeviation (NAoD) −17,33 ± 13,30 PD. The angle of deviation (AoD) value was classified as mild (< 8 PD), moderate (9–14 PD) and severe (> 15 PD). At cover test for distance, 15 patients (60%) showed a mild angle of squint, 3 (12%) moderate and 7 (28%) a severe angle for squint. At cover test for near, 7 patients (28%) showed a mild angle of squint, 8 (32%) moderate and 10 (40%) a severe angle for squint.
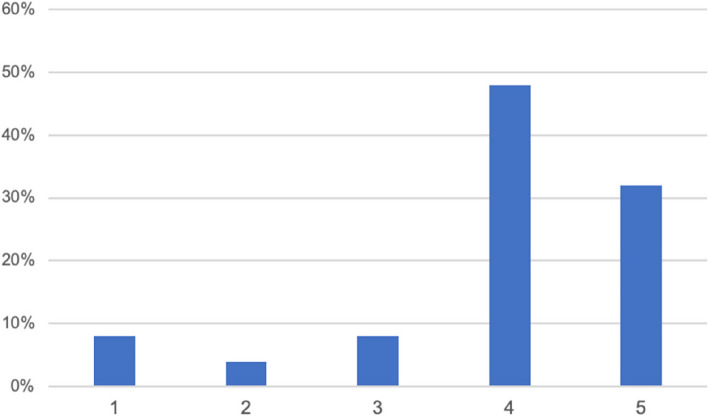
An ocular motility within the normal range (grade1), was observed in 2 of 25 patients (8%), a mild EOM impairment, (grade 2) in 1 of 25 patients (4%), a moderate impairment (grade 3) in 2 of 25 patients (8%), a severe EOM impairment (grade 4) in 12 of 25 patients (48%), and in 8 (32%) patients a complete ophthalmoplegia (grade 5) was observed. These results are reported in Fig. 1.
Fig. 1.
EOM impairment.1: normal motility, 2: mild impairment, 3: moderate impairment; 4: severe impairment; 5: complete ophthalmoplegia
Fusional vergence amplitudes
Convergence amplitudes for far (FCFAs) were within normal range in 5 patients (20%), low in 3 patients (12%), and absent in 15 patients (68%). Convergence amplitudes for near (NCFAs) were within normal range in 3 patients (12%), low in 4 patients (16%), and absent in 18 patients (72%).
Divergence amplitudes for far (FDFAs) were within normal range in 7 patients (28%), low in 3 patients (12%), and absent in 15 patients (60%). Divergence amplitudes for near (NDFAs) were within normal range in 4 patients (16%), low in 3 patients (12%), and absent in 18 patients (72%). General characteristic and ophthalmological features of the study population are shown in Tables 1 and 2.
Table 1.
General characteristics of the study population (n = 25)
| N | % | ||
|---|---|---|---|
| Gender | Male | 15 | 60 |
| Female | 10 | 40 | |
| Phenotype | PEO | 22 | 88 |
| KSS | 3 | 12 | |
| Genotype | SINGLE MTDNA DELETION | 10 | 40 |
| POLG | 7 | 28 | |
| SLC25A4 | 5 | 20 | |
| M.3243A > G | 2 | 8 | |
| TOP3A | 1 | 4 | |
Table 2.
Ophthalmological features of the study population (n = 25)
| N | % | ||
|---|---|---|---|
| Diplopia in casual seeing conditions | Yes | 10 | 40 |
| No | 15 | 60 | |
| Type of strabismus for far | Latent | 8 | 32 |
| Latent/Manifest | 5 | 20 | |
| Manifest | 12 | 48 | |
| Type of strabismus for near | Latent | 4 | 16 |
| Latent/Manifest | 9 | 36 | |
| Manifest | 12 | 48 | |
| FAoD | Mild < 8 | 15 | 60 |
| Moderate 9–15 | 3 | 12 | |
| Severe > 15 | 7 | 28 | |
| NAoD | Mild < 8 | 7 | 28 |
| Moderate 9–15 | 8 | 32 | |
| Severe > 15 | 10 | 40 | |
| EOM impairment | Within the normal range | 2 | 8 |
| 2. mild | 1 | 4 | |
| 3. moderate | 2 | 8 | |
| 4. severe | 12 | 48 | |
| Ophthalmoplegia | 8 | 32 | |
| FCFAs | Normal > 14 | 5 | 20 |
| Low < 14 | 3 | 12 | |
| Absent | 17 | 68 | |
| NCFAs | Normal > 25 | 3 | 12 |
| Low < 25 | 4 | 16 | |
| Absent | 18 | 72 | |
| FDFAs | Normal > 6 | 7 | 28 |
| Low < 6 | 3 | 12 | |
| Absent | 15 | 60 | |
| NDFAs | Normal > 14 | 4 | 16 |
| Low < 14 | 3 | 12 | |
| Absent | 18 | 72 | |
| FBSLs | Diplopia | 5 | 20 |
| Suppression | 8 | 32 | |
| Suppression/Fusion | 3 | 12 | |
| CRN | 6 | 24 | |
| CRA | 3 | 12 | |
| NBSLs | Diplopia | 5 | 20 |
| Suppression | 8 | 32 | |
| Suppression/Fusion | 2 | 8 | |
| Suppression/Diplopia | 3 | 12 | |
| NRC | 6 | 24 | |
| ARC | 1 | 4 | |
| WFDT | Diplopia | 7 | 28 |
| Suppression | 5 | 20 | |
| Suppression/Fusion | 3 | 12 | |
| Suppression/Diplopia | 2 | 8 | |
| NRC | 5 | 20 | |
| ARC | 3 | 12 | |
FAoD (far angle of deviation), NAoD (near angle of deviation), FCFAs (far convergence fusional amplitudes), NCFAs (near convergence fusional amplitudes), FDFAs (far divergence fusional amplitudes), NDFAs (near divergence fusional amplitudes), FBSLs (far Bagolini striated lenses), NBSLs (near Bagolini striated lenses), NRC (normal retinal correspondence), ARC (anomalous retinal correspondence), WFDT (Worth’s four-dot test)
Diplopia evaluation
We evaluated all patients in casual seeing conditions, at BSLs and WFDT.
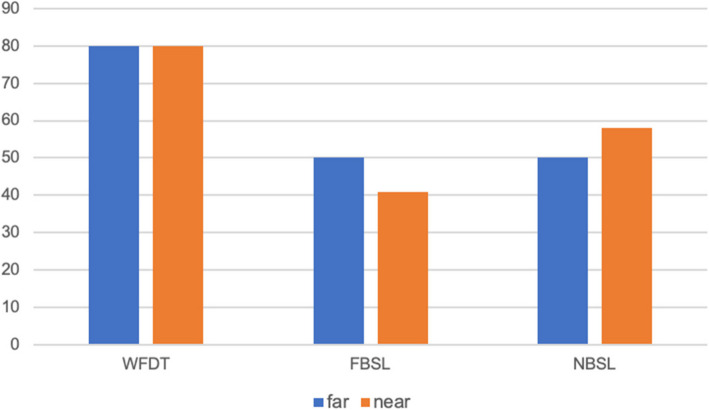
Fifteen patients out 25 (60%) didn’t complain of double vision in casual seeing conditions, while 10 (40%) did. At far cover test 8 (53%) of the 15 patients who did not report diplopia had a manifest strabismus, and 1 (7%) patient had latent/manifest strabismus. At near cover test, 9 (60%) out of 15 patients had a manifest strabismus and 2 (13%) had a latent/manifest strabismus. The results are reported in Fig. 2.
Fig. 2.
Distribution in percentage of strabismus among patients who didn’t complain of diplopia
At BSGs, when evaluated for far, 5 patients showed diplopia (20%), 8 suppression (32%), 3 suppression alternate to fusion (12%), 6 (24%) normal retinal correspondence (NRC) and 3 (12%) anomalous retinal correspondence (ARC)., When evaluated for near, 5 patients had diplopia (20%), 8 suppression (32%), 2 patients suppression alternate to fusion (8%), 3 suppression alternate to diplopia (12%), 6 patients NRC (24%) and 1 ARC (4%).
WFDT showed diplopia in 7 (28%) patients, suppression in 5 (20%) patients, suppression/fusion in 3 patients (12%), suppression/diplopia in 2 (8%) patients, NRC in 5 (20%) patients and ARC in 3 (12%) patients.
Furthermore, the response of patients with manifest or latent/manifest strabismus to the different sensorial tests was assessed.
Percentage of manifest strabismus with suppression mechanism are presented in Fig. 3.
Fig. 3.
Patients in percentage with manifest strabismus with suppression mechanism at different functional tests
Moreover, we investigated how many patients who did not exhibit diplopia during normal vision had latent/manifest and manifest strabismus and evaluated how well these patients responded to sensory tests.
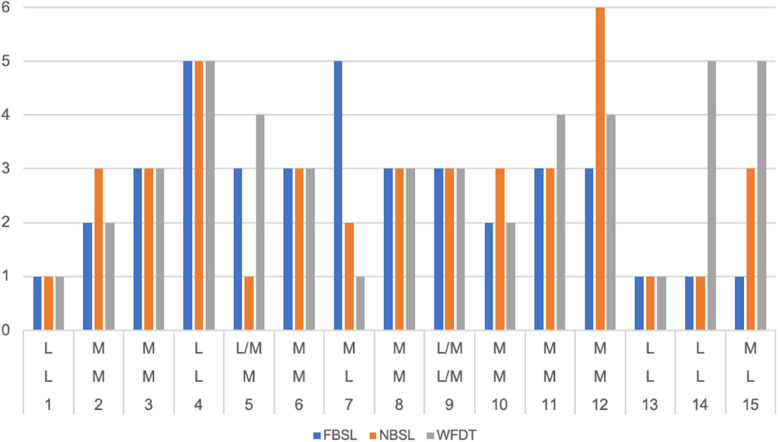
Of the 15 patients who did not complain of diplopia in casual seeing, for distance: 1 (36%) had latent/manifest strabismus, 8(53%) had manifest strabismus, the others were defined as latent. For near, however, 2 (13%) had latent/manifest strabismus and 9 (60%) had manifest strabismus. Only two (13%) patients who did not present diplopia with latent strabismus at far and manifest strabismus at near. The patient with latent/manifest strabismus for distance also had latent/manifest strabismus for near and a suppressive mechanism to both FBSL, NBSL and the WFDT. Of the 8(53%) patients with manifest strabismus for distance, 7 (88%) had manifest strabismus also at near and 1(12%) had latent/manifest strabismus at near. At the FBSL evaluation: 6 (75%) had a suppressive mechanism at distance and 2 (25%) had an ARC, while at near 6 (75%) had a suppressive mechanism, 1 (12.5%) NRC and 1 (12.5%) diplopia/suppression. At WFDT, on the other hand, 3 (37.5%) had a suppression mechanism, 3 (37.5%) had diplopia and 2 (25%) patients an ARC. For near, on the other hand, the two patients with latent/manifest strabismus both showed a suppression mechanism at WFDT and at FBSL and NBSL. Of the 9 patients with manifest strabismus at near, on the other hand, evaluated at FBSL: 5 (55%) had a suppression mechanism, 1 (11%) NRC, 2 (23%) ARC, 1 (11%) fusion/suppression. At the NBSL: 7 (78%) had suppression, 1 (11%) diplopia/suppression and 1(11%) ARC. At WFDT, 3 (33%) showed suppression, 2 (23%) ARC, 2 (23%) diplopia, 1 (11%) NRC and 1 (11%) fusion/suppression. The distribution of the population without diplopia is shown in Fig. 4 and Table 3. On the abscissas, from top to bottom for near strabismus, far strabismus and patients. On the ordinates, numbers from 1 to 6 to define: 1-NRC, 2-ARC; 3-suppression; 4-diplopia; 5-fusion/suppression; 6-diplopia/suppression.
Fig. 4.
The distribution of the population without diplopia. On the abscissas, from top to bottom are reported latent (L), latent/manifest (L/M) and manifest (M) for near strabismus, far strabismus and patients. On the ordinates numbers from 1 to 6 to define: 1-NRC, 2-ARC; 3-suppression (S); 4-diplopia (D); 5-fusion/suppression (F/S); 6-diplopia/suppression (D/S)
Table 3.
The distribution of the population without diplopia. L: latent; L/M: latent/manifest; M: manifest strabismus; NRC (normal retinal correspondence) ARC (anomalous retinal correspondence); SUP: suppression; F/S: fusion/suppression; D/S: diplopia/suppression; D: diplopia
| Patients | Strabismus Far | Strabismus Near | FBSL | NBSL | WFDT |
|---|---|---|---|---|---|
| 1 | L | L | NRC | NRC | NRC |
| 2 | M | M | ARC | SUP | ARC |
| 3 | M | M | SUP | SUP | SUP |
| 4 | L | L | F/S | F/S | F/S |
| 5 | M | L/M | SUP | NRC | D |
| 6 | M | M | SUP | SUP | SUP |
| 7 | L | M | F/S | ARC | NRC |
| 8 | M | M | SUP | SUP | SUP |
| 9 | L/M | L/M | SUP | SUP | SUP |
| 10 | M | M | ARC | SUP | ARC |
| 11 | M | M | SUP | SUP | D |
| 12 | M | M | SUP | D/S | D |
| 13 | L | L | NRC | NRC | NRC |
| 14 | L | L | NRC | NRC | F/S |
| 15 | L | M | NRC | SUP | F/S |
Ptosis
Ptosis was reported in 22/25 patients (88%). Three (14%) of 22 patients had a monocular eyelid ptosis, whereas 19 cases (86%) had a binocular disease. The mean MRD value was of 1.8 mm (range −2/5 mm) in right eye (RE) and 1.35 mm (range −2/4 mm) in left eye (LE). Mean LF was 8.4 mm (range 2–20 mm) in RE and 7.4 mm (range 2–18 mm) in LE. Only 3 out 25 patients (12%) had a LF higher than 15 mm. Ten out 25 patients (40%) had a previous ptosis surgery, 5 of them received two or more surgeries, 2 cases were planned for a second surgery during the evaluation, and only 3 patients had undergone a single procedure. The surgeries performed were in 7 patients a levator aponeurosis resection/advancement (and in 4 cases was performed a surgical revision), one levator complex duplication with a subsequent levator resection, one frontal sling suspension; one patient has undergone a non-specified surgery.
Analysis of influence of age at onset, evaluation and elapsed time on disease phenotype
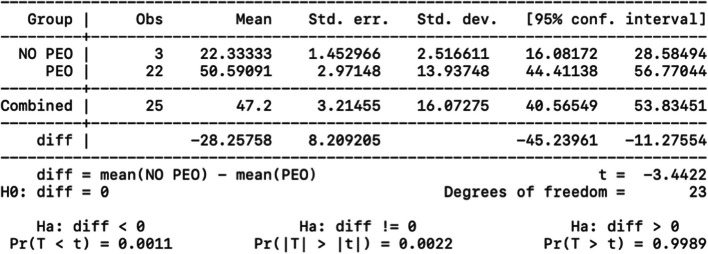
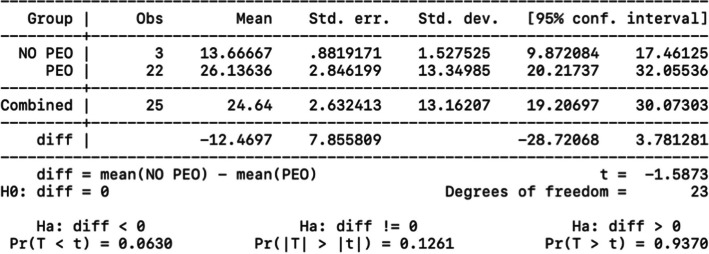
A statistical analysis was performed to correlate disease's onset and type of mitochondrial myopathy. We compared the PEO/PEO plus group and the KSS using a t-test after analyzing the variables for normality with the Shapiro test. As expected, the difference in variance between the averages was statistically significant (p = 0.003); patients develop the PEO phenotype later than the KSS phenotypes. Two-sample t test with equal variances (PEO vs KSS) about disease’s onset is showed in Fig. 5.
Fig. 5.
Two-sample t-test with equal variances (PEO vs KSS) about disease’s onset
The age at evaluation was also analyzed. The difference in variance between the means was statistically significant (p = 0.001): individuals with PEO come at the evaluation later, as showed in Fig. 6.
Fig. 6.
Two-sample t-test with equal variances (PEO vs KSS) about disease’s evaluation
To assess if the diplopia was onset disease-dependent, the same test was also conducted considering as variable the time of disease, i,e,. the difference between the age at evaluation and the age of onset, representing a not age-related time.
The results were not statistically significant (p = 0.06) (Fig. 7). Therefore, time-of-disease influences the age of onset of the disease, but not the presence of diplopia.
Fig. 7.
Two-sample t-test with equal variances (PEO vs KSS) about time of disease
Moreover, according to Hosmer and Lemeshow's multivariable model construction, the variables identified as prospective predictors of diplopia at regression logistic (onset p = 0.018, time p = 0.165, angle of squint for near p = 0.172, ocular motility p = 0.199) did not provide statistically significant results (Table 4), indicating the presence of confounding factors.
Table 4.
Hosmer and Lemeshow's multivariable model construction
| Diplopia | Coef | St.Err | T-value | P-value | [95% Conf | Interval] | Sig |
|---|---|---|---|---|---|---|---|
| Onset | 1.166 | .156 | 1.15 | .252 | .897 | 1.515 | |
| Time | .893 | .088 | −1.14 | .253 | .736 | 1.084 | |
| Angle dev at near | 2.043 | 4.334 | 0.34 | .736 | .032 | 130.608 | |
| Ocular motility | 2.04 | 2.707 | 0.54 | .591 | .152 | 27.47 | |
| Constant | .799 | 5.707 | −0.03 | .975 | 0 | 957,798.85 | |
| Mean dependent var | 0.400 | SD dependent var | 0.500 | ||||
| Pseudo r-squared | 0.668 | Number of obs | 25 | ||||
| Chi-square | 22.477 | Prob > chi2 | 0.001 | ||||
| Akaike crit. (AIC) | 25.174 | Bayesian crit. (BIC) | 33.706 | ||||
*** p < .01, ** p < .05, * p < .1
Discussion
The first case of PEO, attributing the clinical manifestations to disorders of the ocular nerves, was reported about 100 years before description of the first mitochondrial disorder with the introduction in the medical field of this new group of diseases [26] and the later report of the first pathogenic mtDNA variants [27, 28], that ushered MDs in the nowadays molecular era. Myopathic etiology of PEO was documented in the mid-1950s [29–32]. In 1958, Kearns and Sayre reported a clinically distinct form of PEO, KSS, characterized by multisystemic involvement [12]. PEO phenotypes are characterized by a remarkable molecular complexity for the association with pathogenic variants of both mitochondrial and nuclear DNA [1, 33].
Although ocular myopathy was among the first clinical manifestations associated with MDs, to date there have been few ophthalmological studies in small cohorts of patients investigating the clinical consequences of ocular muscle involvement associated with primary mitochondrial dysfunction [16, 34–36].
Compared with other skeletal muscles, extraocular muscles have distinct properties that make them selectively vulnerable to muscular disorders. Progressive external ophthalmoplegia and ptosis represent the most striking oculomotor clinical manifestations of mitochondrial myopathy. In contrast to myasthenic signs, the eyelids and EOMs impairment is permanent and progressively worsening.
Even after maximal bilateral surgery markedly enhances ocular alignment and may alleviate diplopia symptoms; but, due to the degenerative nature of the condition, strabismus frequently recurs [37].
Over a 33-months study period, this multidisciplinary prospective study analyzed 25 patients with a MDs diagnosis with particular regard to oculomotor impairment and sensorimotor evaluation.
Contrary to the findings of Wai Man et al., our investigation revealed that the patients had favorable visual acuity of around 0.09 logMAR. The absence of visual field assessment constitutes a significant drawback of the study [38].
Divergent strabismus, according to Kim et al., was the most prevalent type of ocular deviation with a severe ocular motility impairment or a complete ophthalmoplegia in 80% of the patients [39].
Ptosis is the most common ocular finding with EOM involvement in MDs. It can also occur as first symptom [40, 49].
In our study we found that only 5/21 patients (24%) showed a normal LF, higher than 12 mm. The ptosis was symmetrical, with a MRD difference between the two eyes below or equal to 2 mm in 21 of 23 patients (91%), and an asymmetric presentation in only 2 of 23 patients (9%). These results are consistent with literature where MD are often bilateral and symmetric, although an asymmetric presentation has been reported [41]. Moreover, Ptosis in MDs is characterized by a low levator muscle function (LF), usually below 8–10 mm, one of the factors causing the surgery challenging [42–44].
Unlike the eyelid ptosis ophthalmoplegia is usually deemed asymptomatic but only a few non-prospective studies have analyzed ocular motility disorders.
Despite the lack of double vision is considered a clinical characteristic of mitochondrial diseases and it often allows the differential diagnosis from other neurological conditions including ocular myasthenia or myasthenia gravis [43–48] in the early stages, we found that 40% of our patients complained of constant or inconstant diplopia in casual seeing condition and showed manifest strabismus both at far (53%) and at near (60%), and only 8% of patients had normal ocular motility.
However, it is even more interesting to point out that the large percentage (60–73%) of patients with no complaints of diplopia in casual seeing condition had a manifest or latent/manifest strabismus at FAoD and NAoD. In addition, the near and distance fusional capabilities in convergence and in divergence (CFAs and DFAs) were absent in 68 and 72% of the whole sample respectively.
At sensory tests (BSLs and WFDT) most patients with manifest strabismus showed suppression of one eye or alternate diplopia and suppression.
It is unclear how the capacity to suppress one eye and eliminate the second image, characteristic of children and limited by the plastic age, might develop in these adult patients. Richardson et al. [50] proposed a reactivation of the plasticity of the visual system thanks to the steadily progressive nature of the disease [30, 51–53]. Drachman and Kisilevsky attribute the infrequency of the clinical manifestation to the different causes: symmetric limitation of motility, ptotic eyelid, suppression [47, 54]. Wallace et al. hypothesized that the mechanism was dependent on the patient's ability to ignore the second image [55]. Anyway, it seems not to be in accordance with the fundaments of binocular vision, for which the plastic age of visual system should end at 8–10 years of age [56]. Von Noorden outlined the possibility that patients who develop manifest deviation as adult can ignore the second fastidious image, in particular if deviation is wide and with the second image tending to pose at the periphery of visual field. The alleged mechanism is likely to be at the psychological level and different from the suppression mechanism, that represents an active process of inhibition of visual afference [56].
According to Heighton, the nuclear gene most associated with DNA variation was POLG [57].
In addition, our data is in line with the previous knowledge that, compared to the other phenotypes, PEO manifests at a later age than KSS and that the presence of diplopia does not correlate with disease duration [58, 59]. As demonstrated by Zhao et al., it is essential to consider the genetic profile of patients with PEO, since, as highlighted by the author, the length and locations of mtDNA deletions may influence onset age and clinical phenotypes. [60] For this reason, even If no mtDNA deletions are identified, whole mtDNA sequencing should be performed to detect possible other mtDNA pathogenic variants. Moreover, if no mtDNA variant is detected, nuclear genes that may cause PEO should be investigated. [61]
Moreover, through multivariate analysis, we have demonstrated that no predictive factors for diplopia can be identified. These findings are completely new to the scientific literature and contribute significantly to our understanding of this disease.
Although limited by the small sample and the bias that only patients who complained of eye motility problems at the neurological examination were enrolled, to the best of our knowledge this is the first prospective multidisciplinary study analyzing the eyelid and ocular motility impairment in MDs patients, and evaluating how the double vision and sensorial modifications impact in these patients.
Conclusions
Our findings on ophthalmoplegia, ptosis, and strabismus are consistent with literature, although a high number of patients with no diplopia in casual seeing state had evident or latent/manifest strabismus at FAoD and NAoD. In addition, 68% and 72% of the sample lacked near and distance fusional capacities in convergence and divergence (CFAs and DFAs). Most apparent strabismus patients had one-eye suppression or alternate diplopia and suppression at sensory tests (BSLs and WFDT). It is unknown how these adult patients acquired the ability to suppress one eye and remove the second image.
We also found that diplopia is not related to disease duration but to disease onset, which manifests later in the PEO phenotype than in KSS. Multivariate study showed that no variable can predict diplopia.
This study confirms the need for a multi-specialistic approach for a correct deep phenotyping of patients with primary mitochondrial disease, useful for identifying the peculiar clinical characteristics and needs of patients affected by this neurogenetic disorder, but also for suggesting important insights into possible disease mechanisms.
Acknowledgements
G.P. and S.S. are members of the European Reference Network for Neuromuscular Diseases (Project ID No. 870177).
Abbreviations
- ARC
Anomalous retinal correspondence
- BCVA
Best-corrected visual acuity
- CPEO
Chronic progressive external ophthalmoplegia
- FAoD
Far angle of deviation
- FAs
Fusional amplitudes
- FBSLs
Far Bagolini striated lenses
- FCFAs
Far convergence fusional amplitudes
- FDFAs
Far divergence fusional amplitudes
- KSS
Kearns-Sayre Syndrome
- LE
Left eye
- LF
Levator function
- MD
Mitochondrial disease
- MRD
Margin-reflex distance
- NAoD
Near angle of deviation
- NBSLs
Near Bagolini striated lenses
- NCFAs
Near convergence fusional amplitudes
- NDFAs
Near divergence fusional amplitudes
- NRC
Normal retinal correspondence
- PEO
Progressive external ophthalmoplegia
- PMM
Primary mitochondrial myopathies
- RE
Right eye
- RPE
Retinal pigmentary epithelium
- WFDT
Worth’s four-dot test
Authors’ contributions
Conceptualization, G.S., G.P. and F.G.; methodology, G.P., G.S. and V.C.; validation, G.S. and G.P.; formal analysis, M.C.S.; investigation D.B., V.C. and C.F.; writing—original draft preparation, F.G and D.B.; writing—review and editing, F.G and. D.B.; visualization, S.S.; supervision, S.S.; project administration, G.S. and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
The authors did not receive support from any organization for the submitted work.
Data availability
The data that support the findings of this study are available from the corresponding author, D.B., upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the Fondazione Policlinico Universitario A. Gemelli-IRCCS, Rome.
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gustavo Savino and Federico Giannuzzi contributed equally to this work.
References
- 1.Ng YS, Bindoff LA, Gorman GS, Klopstock T, Kornblum C, Mancuso M, et al. Mitochondrial disease in adults: recent advances and future promise. Lancet Neurol. 2021;20(7):573–84. 10.1016/s1474-4422(21)00098-3. [DOI] [PubMed] [Google Scholar]
- 2.Skladal D, Halliday J, Thorburn DR. Minimum birth prevalence of mitochondrial respiratory chain disorders in children. Brain. 2003;126(Pt 8):1905–12. 10.1093/brain/awg170. [DOI] [PubMed] [Google Scholar]
- 3.Gorman GS, Chinnery PF, DiMauro S, Hirano M, Koga Y, McFarland R, et al. Mitochondrial diseases Nat Rev Dis Primers. 2016;20(2):16080. 10.1038/nrdp.2016.80. [DOI] [PubMed] [Google Scholar]
- 4.Musumeci O, Barca E, Lamperti C, Servidei S, Comi GP, Moggio M, et al. Lipomatosis Incidence and Characteristics in an Italian Cohort of Mitochondrial Patients. Front Neurol. 2019;10:160. 10.3389/fneur.2019.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montano V, Orsucci D, Carelli V, La Morgia C, Valentino ML, Lamperti C, et al. Adult-onset mitochondrial movement disorders: a national picture from the Italian Network. J Neurol. 2022;269(3):1413–21. 10.1007/s00415-021-10697-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grier J, Hirano M, Karaa A, Shepard E, Thompson JLP. Diagnostic odyssey of patients with mitochondrial disease: Results of a survey. Neurol Genet. 2018;4(2): e230. 10.1212/nxg.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deschauer M, Müller T, Dreha S, et al. Familiäre mitochondriale chronisch progressive externe Ophthalmoplegie Fünf Familien mit unterschiedlicher Genetik. Nervenarzt. 2001;72:122–9. 10.1007/s001150050724. [DOI] [PubMed] [Google Scholar]
- 8.Mancuso M, McFarland R, Klopstock T, Hirano M. International Workshop:: Outcome measures and clinical trial readiness in primary mitochondrial myopathies in children and adults. Consensus recommendations. 16–18 November 2016, Rome, Italy. Neuromuscul Disord. 2017 Dec;27(12):1126–37. 10.1016/j.nmd.2017.08.006 [DOI] [PMC free article] [PubMed]
- 9.Montano V, Gruosso F, Carelli V, Comi GP, Filosto M, Lamperti C, et al. Primary mitochondrial myopathy: Clinical features and outcome measures in 118 cases from Italy. Neurol Genet. 2020;6(6): e519. 10.1212/nxg.0000000000000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Barcelos IP, Emmanuele V, Hirano M. Advances in primary mitochondrial myopathies. Curr Opin Neurol. 2019;32(5):715–21. 10.1097/wco.0000000000000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emmanuele V, Ganesh J, Vladutiu G, Haas R, Kerr D, Saneto RP, et al. Time to harmonize mitochondrial syndrome nomenclature and classification: A consensus from the North American Mitochondrial Disease Consortium (NAMDC). Mol Genet Metab. 2022;136(2):125–31. 10.1016/j.ymgme.2022.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kearns TP, Sayre GP. Retinitis pigmentosa, external ophthalmophegia, and complete heart block: unusual syndrome with histologic study in one of two cases. AMA Arch Ophthalmol. 1958;60(2):280–9. [PubMed] [Google Scholar]
- 13.Berenberg RA, Pellock JM, DiMauro S, Schotland DL, Bonilla E, Eastwood A, et al. Lumping or splitting? “Ophthalmoplegia-plus” or Kearns-Sayre syndrome? Ann Neurol. 1977;1(1):37–54. 10.1002/ana.410010104. [DOI] [PubMed] [Google Scholar]
- 14.Rowland L, Hays A, DiMauro S, De Vivo D, Behrens M. Diverse clinical disorders associated with morphological abnormalities of mitochondria. Mitochondrial pathology in muscle diseases. 1983:141–58.
- 15.Rowland LP, Blake DM, Hirano M, Di Mauro S, Schon EA, Hays AP, et al. Clinical syndromes associated with ragged red fibers. Rev Neurol (Paris). 1991;147(6–7):467–73. [PubMed] [Google Scholar]
- 16.McClelland C, Manousakis G, Lee MS. Progressive External Ophthalmoplegia. Curr Neurol Neurosci Rep. 2016;16(6):53. 10.1007/s11910-016-0652-7. [DOI] [PubMed] [Google Scholar]
- 17.Schaefer AM, et al. Mitochondrial disease in adults: a scale to monitor progression and treatment. Neurology. 2006;66:1932–4. 10.1212/01.wnl.0000219759.72195.41. [DOI] [PubMed] [Google Scholar]
- 18.Ruttum M, Von Noorden GK. The Bagolini striated lens test for cyclotropia. Doc Ophthalmol. 1984;58:131–9. 10.1007/BF00140911. [DOI] [PubMed] [Google Scholar]
- 19.Bak, Eunoo*; Yang, Hee Kyung*; Hwang, Jeong-Min. Validity of the Worth 4 Dot Test in Patients with Red-Green Color Vision Defect. Optometry and Vision Science 94(5):p 626–629, May 2017. | 10.1097/OPX.0000000000001058 [DOI] [PubMed]
- 20.Kim HY. Statistical notes for clinical researchers: Chi-squared test and Fisher’s exact test. Restor Dent Endod. 2017;42(2):152–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Surjanovic N, Loughin TM. Improving the Hosmer-Lemeshow goodness-of-fit test in large models with replicated Bernoulli trials. J Appl Stat. 2023;51(7):1399–411. 10.1080/02664763.2023.2272223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Y, Du J, Wang W, et al. Novel biallelic mutations in POLG gene: large deletion and missense variant associated with PEO. Neurol Sci. 2021;42:4271–80. 10.1007/s10072-021-05380-2. [DOI] [PubMed] [Google Scholar]
- 23.Park KP, Kim HS, Kim ES, Park YE, Lee CH, Kim DS. SLC25A4 and C10ORF2 Mutations in Autosomal Dominant Progressive External Ophthalmoplegia. J Clin Neurol. 2011 Mar;7(1):25 30. 10.3988/jcn.2011.7.1.25 [DOI] [PMC free article] [PubMed]
- 24.Mancuso, M., Orsucci, D., Angelini, C. et al. The m.3243A>G mitochondrial DNA mutation and related phenotypes. A matter of gender?. J Neurol 261, 504–510 (2014). 10.1007/s00415-013-7225-3 [DOI] [PubMed]
- 25.Erdinc, D., Rodríguez-Luis, A., Fassad, M. R., Mackenzie, S., Watson, C. M., Valenzuela, S., Xie, X., Menger, K. E., Sergeant, K., Craig, K., Hopton, S., Falkous, G., Genomics England Research Consortium, Poulton, J., Garcia-Moreno, H., Giunti, P., de Moura Aschoff, C. A., Morales Saute, J. A., Kirby, A. J., Toro, C., … Nicholls, T. J. (2023). Pathological variants in TOP3A cause distinct disorders of mitochondrial and nuclear genome stability. EMBO molecular medicine, 15(5), e16775. 10.15252/emmm.202216775 [DOI] [PMC free article] [PubMed]
- 26.Luft R, Ikkos D, Palmieri G, Ernster L, Afzelius B. A case of severe hypermetabolism of nonthyroid origin with a defect in the maintenance of mitochondrial respiratory control: a correlated clinical, biochemical, and morphological study. J Clin Invest. 1962;41(9):1776–804. 10.1172/jci104637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holt IJ, Harding AE, Morgan-Hughes JA. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988;331(6158):717–9. 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- 28.Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, et al. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988;242(4884):1427–30. 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- 29.Sandifer PH. Chronic progressive ophthalmoplegia of myopathic origin. J Neurol Neurosurg Psychiatry. 1946;9(3):81–3. 10.1136/jnnp.9.3.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiloh LG, Nevin S. Progressive dystrophy of the external ocular muscles (ocular myopathy). Brain. 1951;74(2):115–43. 10.1093/brain/74.2.115. [DOI] [PubMed] [Google Scholar]
- 31.Beckett RS, Netsky MG. Familial ocular myopathy and external ophthalmoplegia. AMA Arch Neurol Psychiatry. 1953;69(1):64–72. 10.1001/archneurpsyc.1953.02320250070007. [DOI] [PubMed] [Google Scholar]
- 32.Schwarz GA, Liu CN. Chronic progressive external ophthalmoplegia. AMA Arch Neurol Psychiatry. 1954;71(1):31–53. 10.1001/archneurpsyc.1954.02320370033003. [DOI] [PubMed] [Google Scholar]
- 33.Orsucci D, Angelini C, Bertini E, Carelli V, Comi GP, Federico A, et al. Revisiting mitochondrial ocular myopathies: a study from the Italian Network. J Neurol. 2017;264(8):1777–84. 10.1007/s00415-017-8567-z. [DOI] [PubMed] [Google Scholar]
- 34.Chen BS, Harvey JP, Gilhooley MJ, et al. Mitochondria and the eye—manifestations of mitochondrial diseases and their management. Eye. 2023;37:2416–25. 10.1038/s41433-023-02523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritchie AE, Griffiths PG, Chinnery PF, et al Eye movement recordings to investigate a supranuclear component in chronic progressive external ophthalmoplegia: a cross-sectional study British Journal of Ophthalmology 2010;94:1165–1168. [DOI] [PMC free article] [PubMed]
- 36.Zhu CC, Traboulsi EI, Parikh S. Ophthalmological findings in 74 patients with mitochondrial disease. Ophthalmic Genet. 2017 Jan-Feb;38(1):67–69. 10.3109/13816810.2015.1130153. Epub 2016 Mar 30. PMID: 27029465. [DOI] [PubMed]
- 37.Tinley C, Dawson E, Lee J. The Management of Strabismus in Patients with Chronic Progressive External Ophthalmoplegia. Strabismus. 2010;18(2):41–7. 10.3109/09273971003758388. [DOI] [PubMed] [Google Scholar]
- 38.Yu Wai Man, C., Smith, T., Chinnery, P. et al. Assessment of visual function in chronic progressive external ophthalmoplegia. Eye 20, 564–568 (2006). 10.1038/sj.eye.6701924 [DOI] [PubMed]
- 39.Kim JY, Yang HK, Kim N, Kim MJ, Cho SI, Seong M-W, Park SS, Hwang J-M. Strabismus in chronic progressive external ophthalmoplegia. Acta Ophthalmol. 2021;99:e274–80. 10.1111/aos.14558. [DOI] [PubMed] [Google Scholar]
- 40.Richardson C, Smith T, Schaefer A, Turnbull D, Griffiths P. Ocular motility findings in chronic progressive external ophthalmoplegia. Eye (Lond). 2005;19(3):258–63. 10.1038/sj.eye.6701488. [DOI] [PubMed] [Google Scholar]
- 41.Wabbels B, Ali N, Kunz WS, Roggenkämper P, Kornblum C. Chronic progressive external ophthalmoplegia and Kearns-Sayre syndrome : interdisciplinary diagnosis and therapy. Ophthalmologe. 2008;105(6):550–6. 10.1007/s00347-007-1643-5. [DOI] [PubMed] [Google Scholar]
- 42.Lane CM, Collin JR. Treatment of ptosis in chronic progressive external ophthalmoplegia. Br J Ophthalmol. 1987;71(4):290–4. 10.1136/bjo.71.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shorr N, Christenbury JD, Goldberg RA. Management of ptosis in chronic progressive external ophthalmoplegia. Ophthalmic Plast Reconstr Surg. 1987;3(3):141–5. 10.1097/00002341-198703030-00005. [DOI] [PubMed] [Google Scholar]
- 44.Ahn J, Kim NJ, Choung HK, Hwang SW, Sung M, Lee MJ, et al. Frontalis sling operation using silicone rod for the correction of ptosis in chronic progressive external ophthalmoplegia. Br J Ophthalmol. 2008;92(12):1685–8. 10.1136/bjo.2008.144816. [DOI] [PubMed] [Google Scholar]
- 45.Schoser BG, Pongratz D. Extraocular mitochondrial myopathies and their differential diagnoses. Strabismus. 2006;14(2):107–13. 10.1080/09273970600701218. [DOI] [PubMed] [Google Scholar]
- 46.Stanworth A. OCULAR MYOPATHIES. Trans Ophthalmol Soc U K. 1962;1963(83):515–30. [PubMed] [Google Scholar]
- 47.Drachman DA. Ophthalmoplegia plus. The neurodegenerative disorders associated with progressive external ophthalmoplegia. Arch Neurol. 1968 Jun;18(6):654–74. 10.1001/archneur.1968.00470360076008 [DOI] [PubMed]
- 48.Koerner F, Schlote W. Chronic progressive external ophthalmoplegia: association with retinal pigmentary changes and evidence in favor of ocular myopathy. Arch Ophthalmol. 1972;88(2):155–66. 10.1001/archopht.1972.01000030157005. [DOI] [PubMed] [Google Scholar]
- 49.Danta G, Hilton RC, Lynch PG. Chronic progressive external ophthalmoplegia. Brain. 1975;98(3):473–92. 10.1093/brain/98.3.473. [DOI] [PubMed] [Google Scholar]
- 50.Richardson DK, Gray JE, Gortmaker SL, Goldmann DA, Pursley DM, McCormick MC. Declining severity adjusted mortality: evidence of improving neonatal intensive care. Pediatrics. 1998;102(4 Pt 1):893–9. 10.1542/peds.102.4.893. [DOI] [PubMed] [Google Scholar]
- 51.Hansen AC, Logan JH. Chronic progressive external ophthalmoplegia. A review and case report. J Natl Med Assoc. 1966 Nov;58(6):436–41. [PMC free article] [PubMed]
- 52.Malbrán ES. Myopathic ophthalmoplegia externa. Int Ophthalmol Clin. 1966 Fall;6(3):711–22. 10.1097/00004397-196609000-00020 [DOI] [PubMed]
- 53.Bau V, Zierz S. Update on chronic progressive external ophthalmoplegia. Strabismus. 2005;13(3):133–42. 10.1080/09273970500216432. [DOI] [PubMed] [Google Scholar]
- 54.Kisilevsky E, Freund P, Margolin E. Mitochondrial disorders and the eye. Survey of Ophthalmology. 2020 2020/05/01/;65(3):294–311. 10.1016/j.survophthal.2019.11.001 [DOI] [PubMed]
- 55.Wallace DK, Sprunger DT, Helveston EM, Ellis FD. Surgical management of strabismus associated with chronic progressive external ophthalmoplegia. Ophthalmology. 1997;104(4):695–700. 10.1016/s0161-6420(97)30250-4. [DOI] [PubMed] [Google Scholar]
- 56.von Noorden GKCE. Binocular Vision and Motility: Theory and Management of Strabismus. 6th ed. St Louis: Mosby; 2002. [Google Scholar]
- 57.Julia N. Heighton, Lauren I. Brady, Bekim Sadikovic, Dennis E. Bulman, Mark A. Tarnopolsky, Genotypes of chronic progressive external ophthalmoplegia in a large adult-onset cohort, Mitochondrion, Volume 49, 2019, Pages 227–231, ISSN 1567–7249, 10.1016/j.mito.2019.09.002. [DOI] [PubMed]
- 58.Orsucci D, CaldarazzoIenco E, Rossi A, Siciliano G, Mancuso M. Mitochondrial Syndromes Revisited. J. Clin Med. 2021;10(6):1249. 10.3390/jcm10061249.PMID:33802970;PMCID:PMC8002645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beecher G, Gavrilova RH, Mandrekar J, Naddaf E. Mitochondrial myopathies diagnosed in adulthood: clinico-genetic spectrum and long-term outcomes. Brain Commun. 2024 Feb 14;6(2):fcae041. 10.1093/braincomms/fcae041. PMID: 38434220; PMCID: PMC10906953. [DOI] [PMC free article] [PubMed]
- 60.Zhao Y, Hou Y, Zhao X, Liufu T, Yu M, Zhang W, Xie Z, Zhang VW, Yuan Y, Wang Z. The clinical, myopathological, and genetic analysis of 155 Chinese mitochondrial ophthalmoplegia patients with mitochondrial DNA single large deletions. Mol Genet Genomic Med. 2024;12: e2328. 10.1002/mgg3.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodríguez-López C, García-Cárdaba LM, Blázquez A, et al Clinical, pathological and genetic spectrum in 89 cases of mitochondrial progressive external ophthalmoplegia Journal of Medical Genetics 2020;57:643–646. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, D.B., upon reasonable request.