Abstract
1. Desensitization of acetylcholine (ACh) receptors was studied at the frog neuromuscular junction under voltage clamp.
2. ACh was applied directly to junctional receptors by stimulating the motor nerve with trains of impulses. End-plate currents (e.p.c.s) were used to estimate the total number of channel openings by the junctional ACh receptors, and miniature end-plate currents (m.e.p.c.s) were used to measure changes in post-synaptic sensitivity. Under the conditions of these experiments the changes in m.e.p.c. amplitudes were shown to be post-synaptic in origin and thus provided a measure of desensitization.
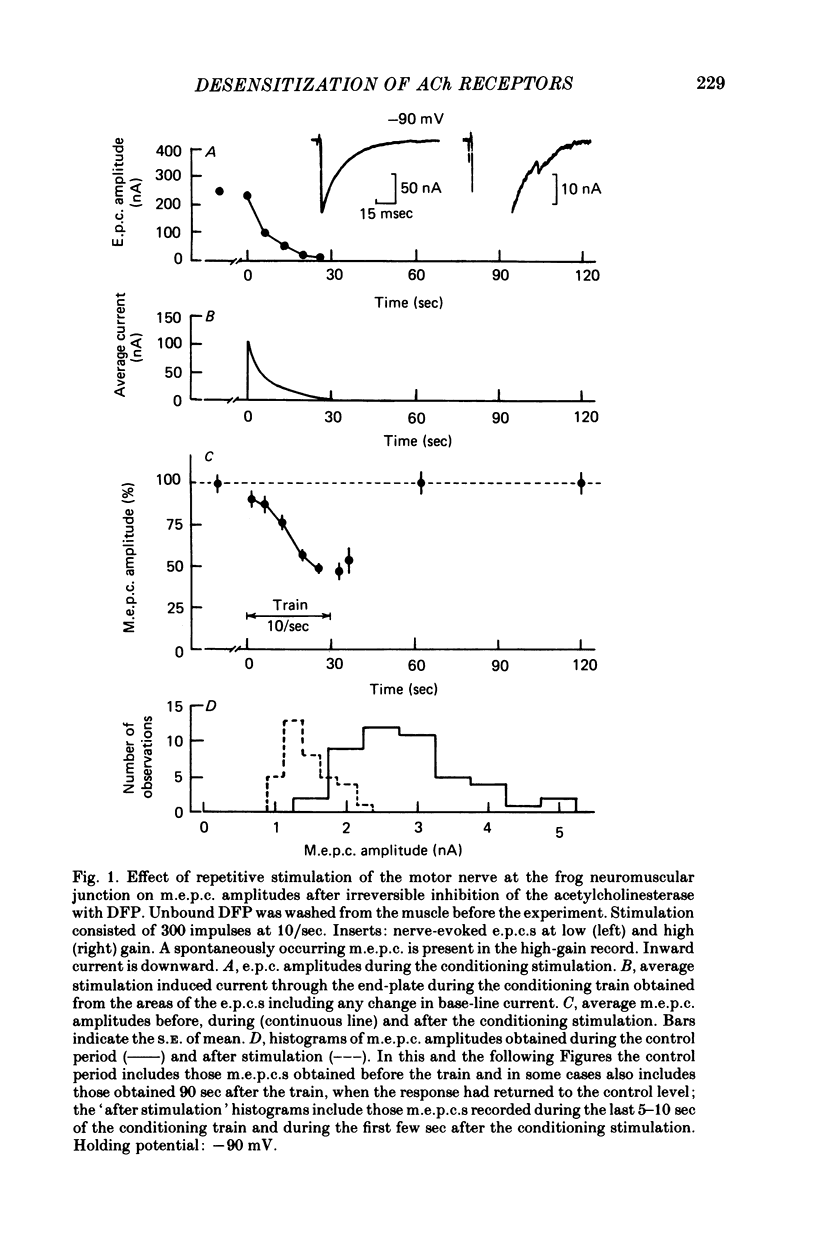
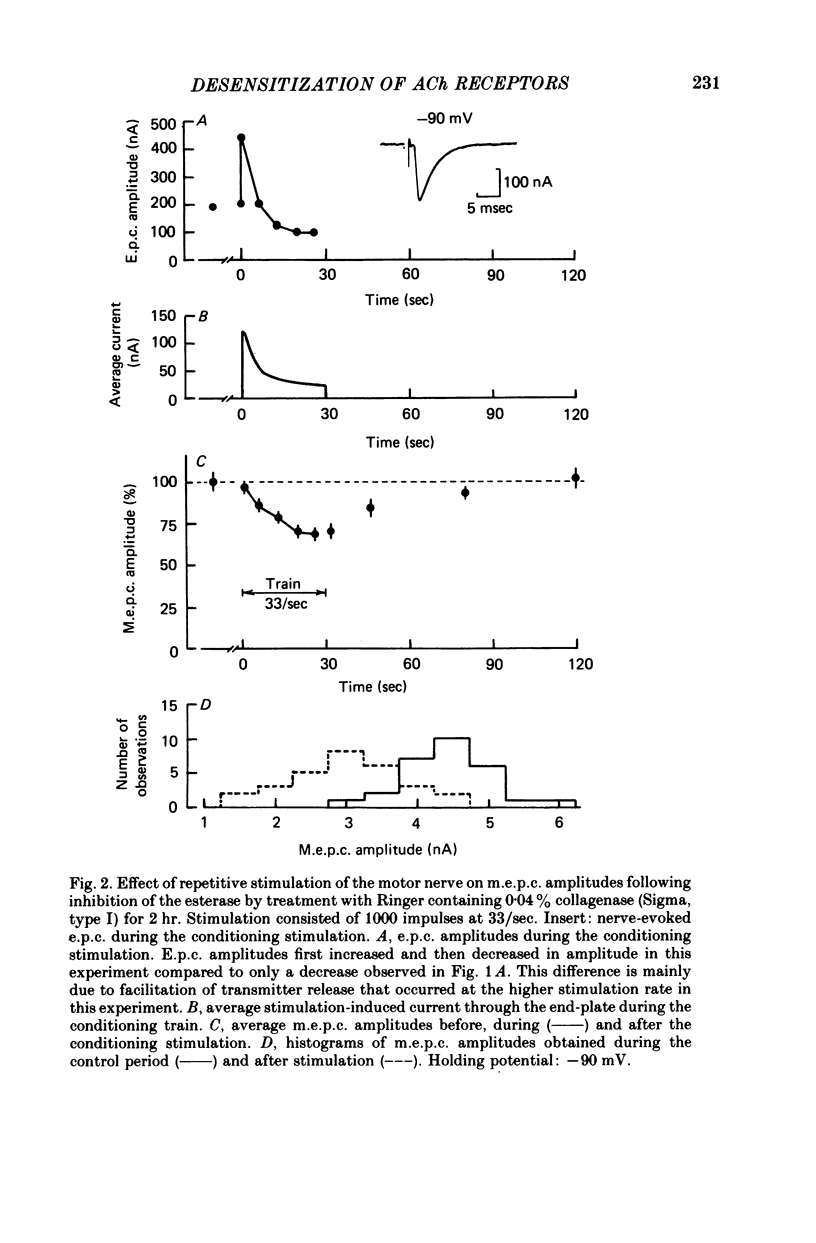
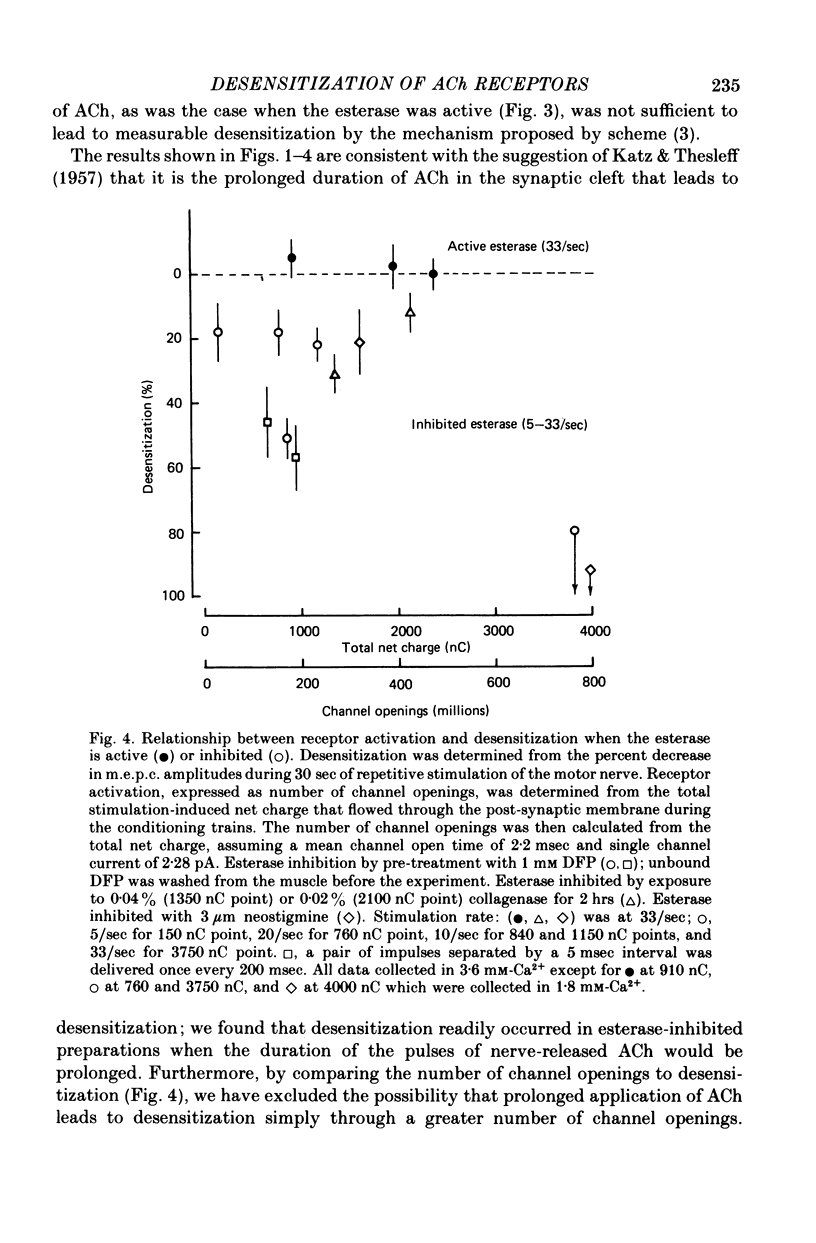
3. When the acetylcholinesterase was inhibited with diisopropylfluorophosphate, neostigmine, or collagenase treatment to prolong the duration of the nerve-released ACh in the synaptic cleft, desensitization developed during repetitive stimulation of 1000 impulses at 5-33 impulses/sec and then recovered after the conditioning trains, with a time constant of about 25 sec.
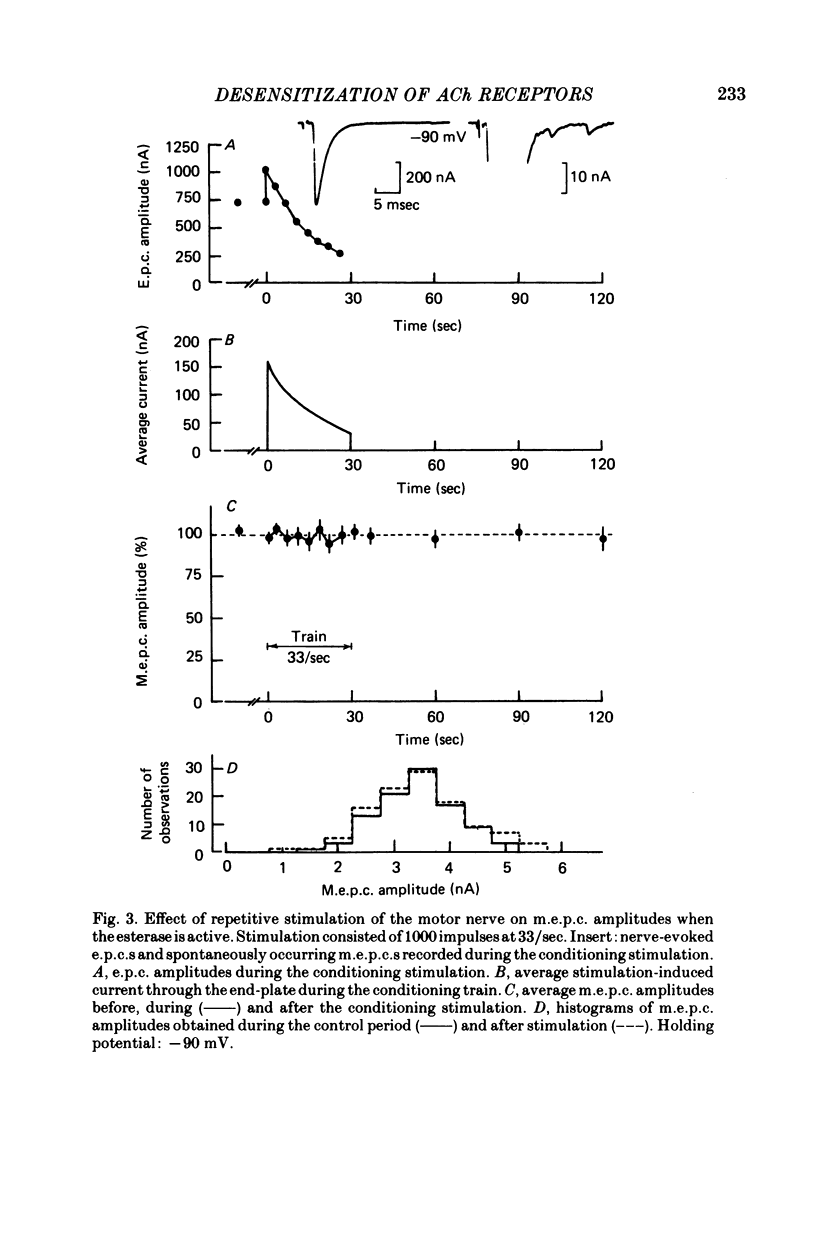
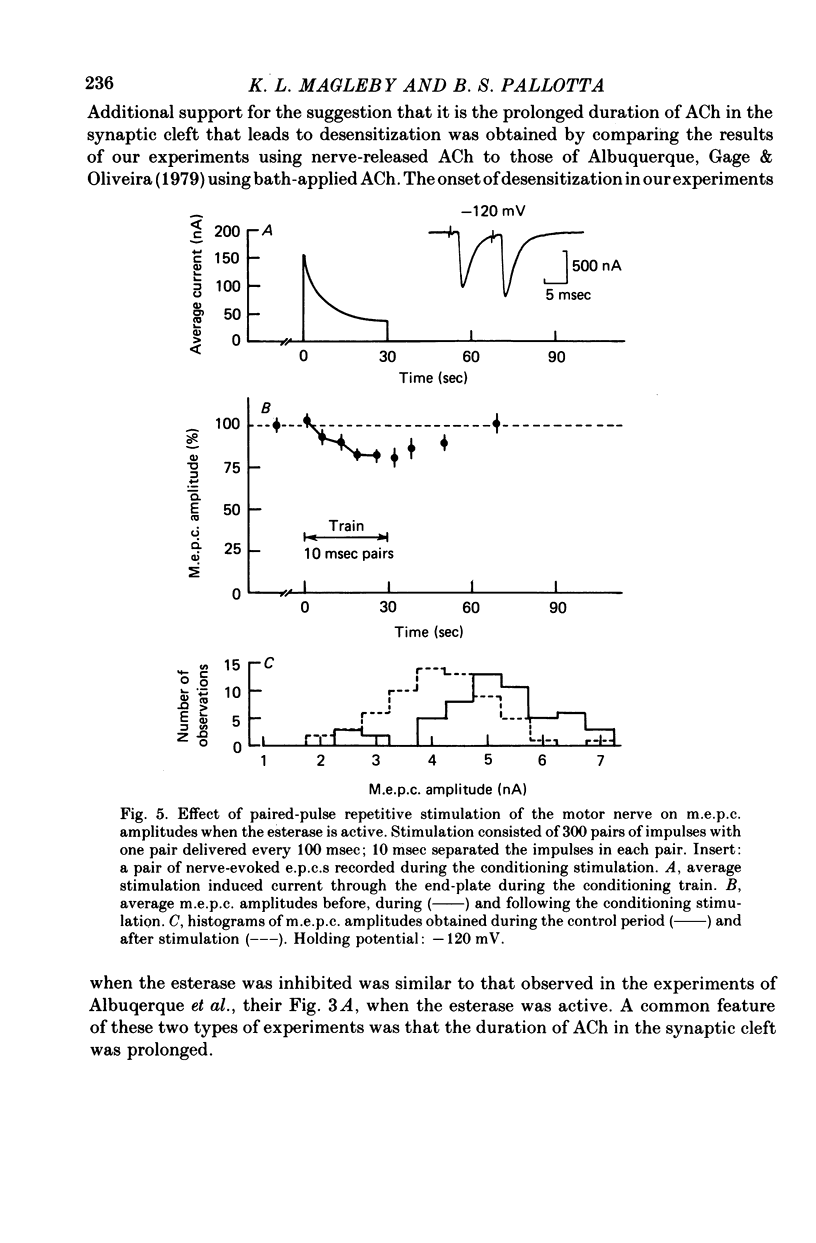
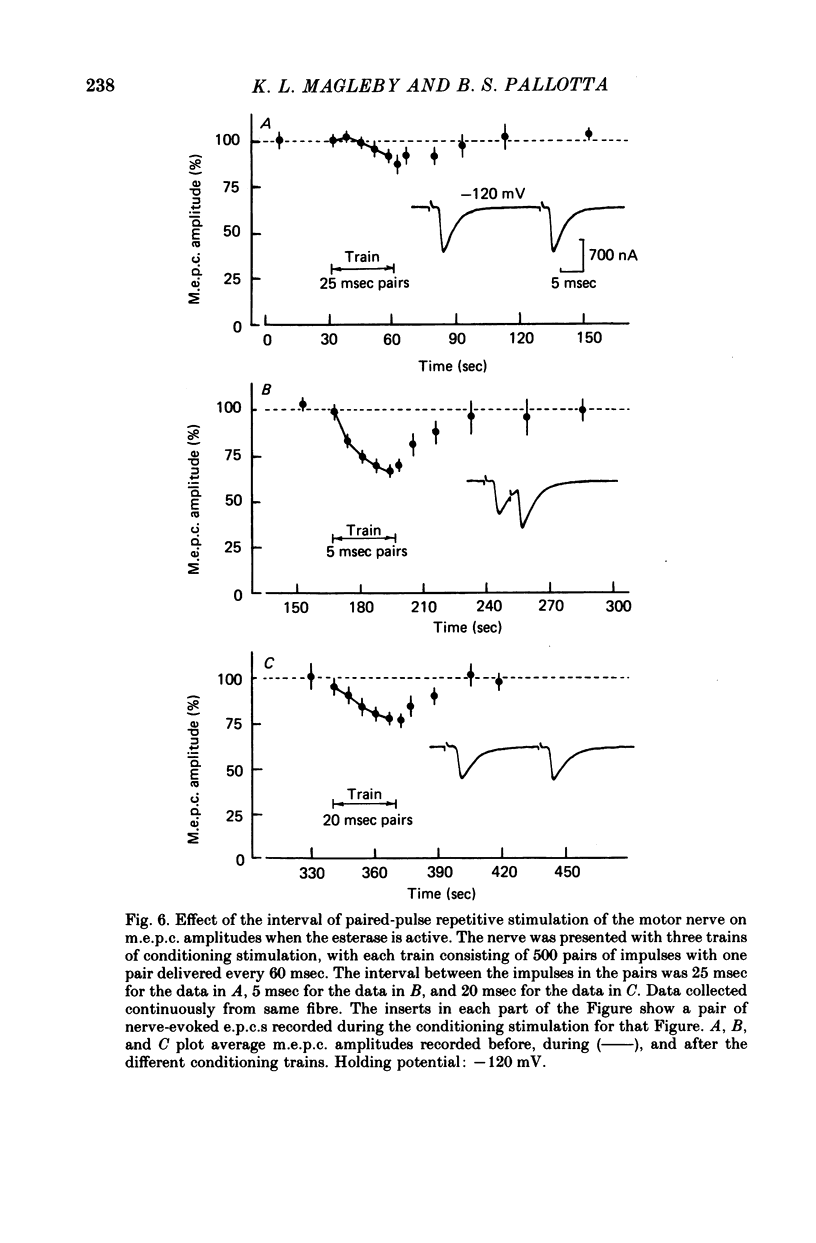
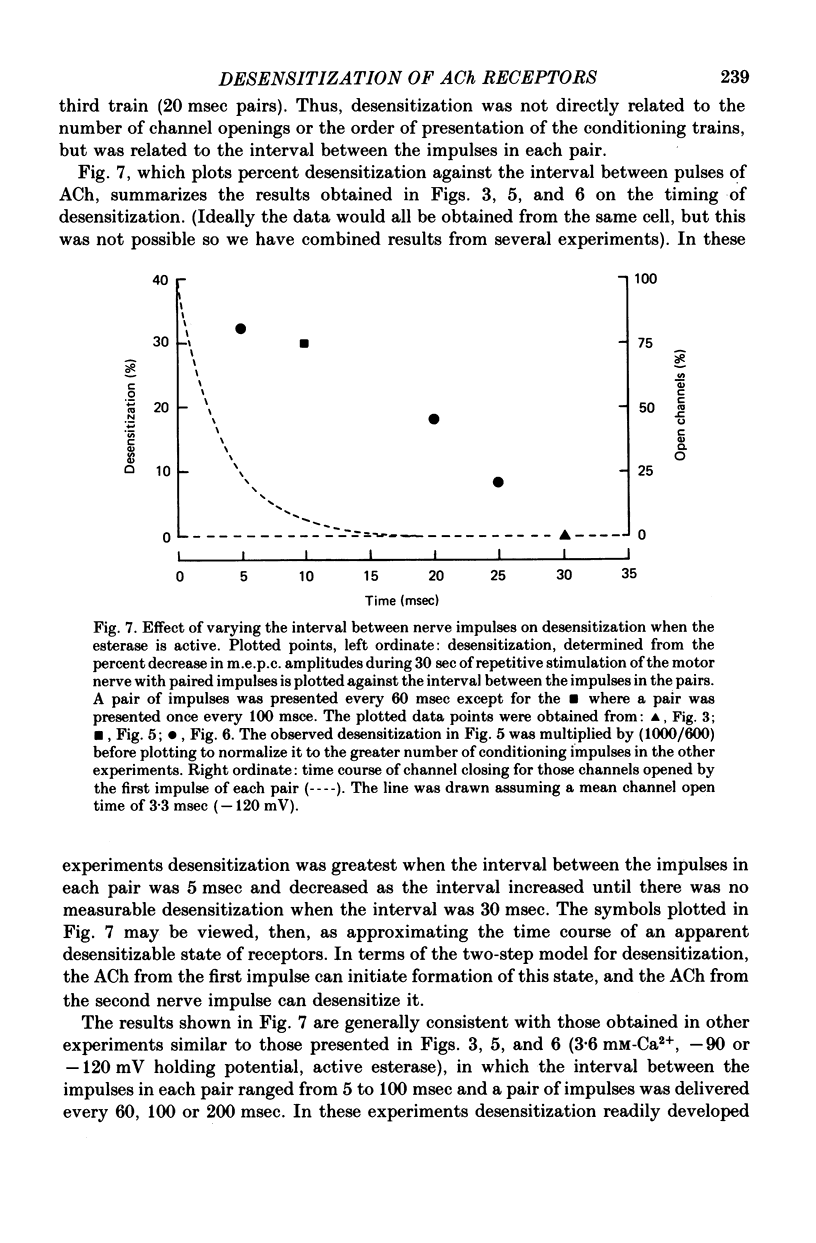
4. When the acetylcholinesterase was active so that the duration of ACh in the synaptic cleft resulting from each nerve impulse was brief (< 300 μsec), desensitization developed in response to 300-500 pairs of nerve stimuli if the interval between the impulses of each pair was 25 msec or less. When the interval was 30 msec or greater, however, measurable desensitization did not occur, even if the total number of channel openings was many times greater than in the experiments with shorter intervals or inhibited esterase where desensitization readily occurred.
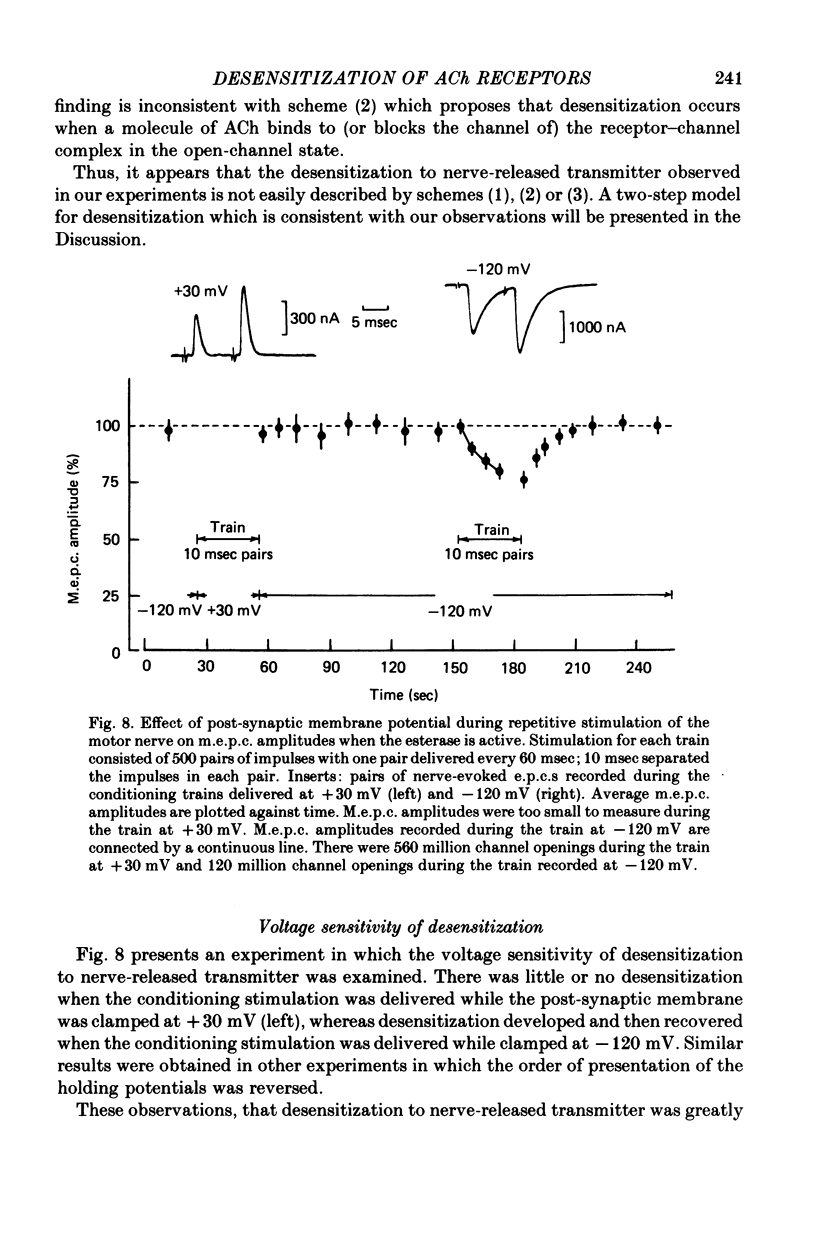
5. The desensitization observed to pairs of impulses was enhanced by chlorpromazine and decreased when the post-synaptic membrane was depolarized, properties similar to those described previously for desensitization to bath and ionophoretic application of ACh.
6. These results indicate that desensitization to nerve-released transmitter is not a simple consequence of receptor activation, is not due to blockade of the open receptor channels by ACh, and does not result from ACh binding directly to desensitized receptors with a resulting shift in the receptor population towards the desensitized state.
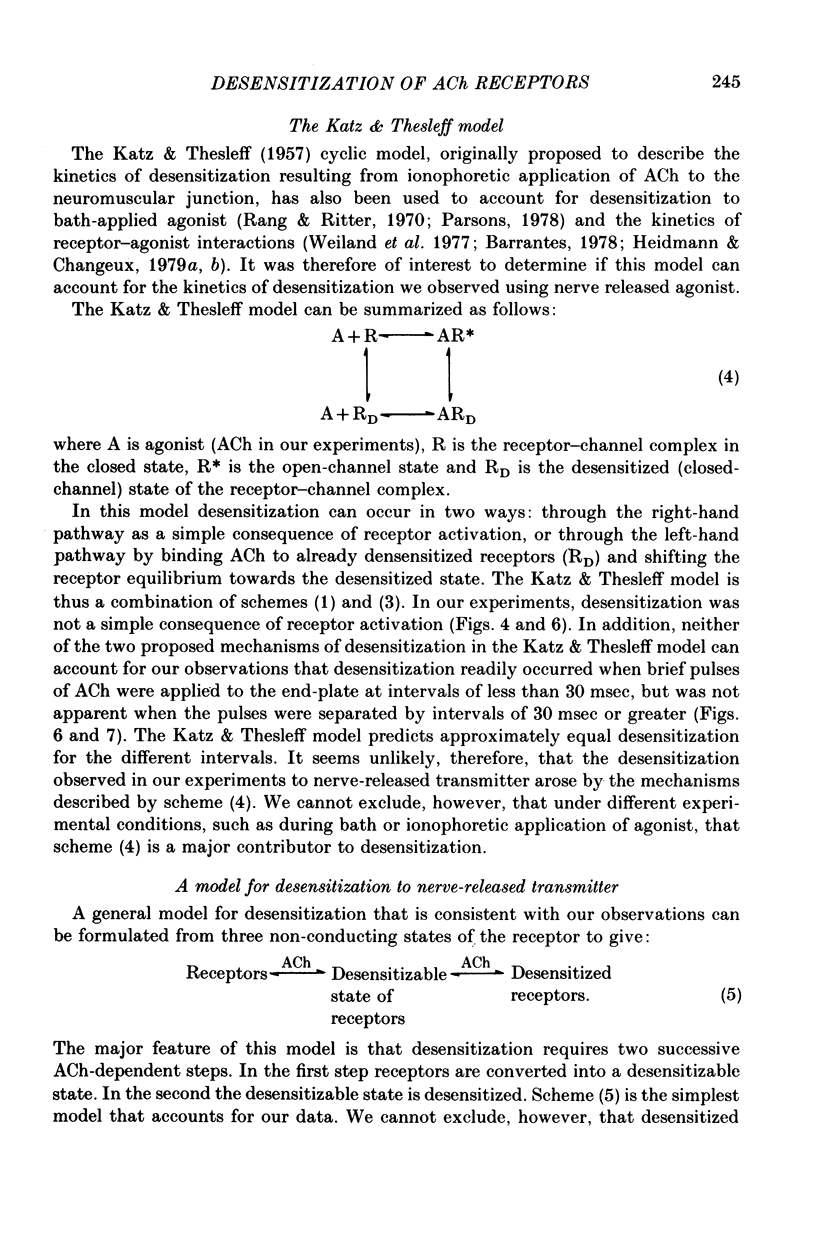
7. We suggest that the desensitization observed to nerve-released transmitter is a two-step process with both steps initiated by ACh. In the first step ACh converts some receptors into a desensitizable state which has an apparent lifetime of less than 30 msec; in the second step ACh desensitizes the desensitizable state.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Dwyer T. M., Hille B. The permeability of endplate channels to monovalent and divalent metal cations. J Gen Physiol. 1980 May;75(5):493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. A study of desensitization using voltage clamp. Pflugers Arch. 1975 Oct 28;360(2):135–144. doi: 10.1007/BF00580536. [DOI] [PubMed] [Google Scholar]
- Adams P. R., Sakmann B. A comparison of current-voltage relations for full and partial agonists. J Physiol. 1978 Oct;283:621–644. doi: 10.1113/jphysiol.1978.sp012523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T., Karczmar A. G. Effects of anticholinesterases and of sodium fluoride on neuromyal desensitization. Neuropharmacology. 1980 Apr;19(4):393–403. doi: 10.1016/0028-3908(80)90192-6. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Gage P. W., Oliveira A. C. Differential effect of perhydrohistrionicotoxin on 'intrinsic' and 'extrinsic' end-plate responses. J Physiol. 1979 Dec;297(0):423–442. doi: 10.1113/jphysiol.1979.sp013049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes F. J. Agonist-mediated changes of the acetylcholine receptor in its membrane environment. J Mol Biol. 1978 Sep 5;124(1):1–26. doi: 10.1016/0022-2836(78)90144-4. [DOI] [PubMed] [Google Scholar]
- Betz W., Sakmann B. "Disjunction" of frog neuromuscular synapses by treatment with proteolytic enzymes. Nat New Biol. 1971 Jul 21;232(29):94–95. doi: 10.1038/newbio232094a0. [DOI] [PubMed] [Google Scholar]
- Betz W., Sakmann B. Effects of proteolytic enzymes on function and structure of frog neuromuscular junctions. J Physiol. 1973 May;230(3):673–688. doi: 10.1113/jphysiol.1973.sp010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregestovksi P. D., Bukharaeva E. A., Iljin V. I. Voltage clamp analysis of acetylcholine receptor desensitization in isolated mollusc neurones. J Physiol. 1979 Dec;297(0):581–595. doi: 10.1113/jphysiol.1979.sp013058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Steinbach J. H., Stevens C. F. An analysis of the dose-response relationship at voltage-clamped frog neuromuscular junctions. J Physiol. 1978 Aug;281:421–444. doi: 10.1113/jphysiol.1978.sp012431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Peper K., Sterz R. Determination of dose-response curves by quantitative ionophoresis at the frog neuromuscular junction. J Physiol. 1978 Aug;281:395–419. doi: 10.1113/jphysiol.1978.sp012430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Walther C., Peper K. Junctional and extrajunctional acetylcholine receptors in normal and denervated frog muscle fibres. Noise analysis experiments with different agonists. Pflugers Arch. 1976 Oct 15;366(1):1–9. doi: 10.1007/BF02486555. [DOI] [PubMed] [Google Scholar]
- FATT P. The electromotive action of acetylcholine at the motor end-plate. J Physiol. 1950 Oct 16;111(3-4):408–422. doi: 10.1113/jphysiol.1950.sp004492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiekers J. F., Spannbauer P. M., Scubon-Mulieri B., Parsons R. L. Voltage dependence of desensitization. Influence of calcium and activation kinetics. J Gen Physiol. 1980 May;75(5):511–529. doi: 10.1085/jgp.75.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Action potentials without contraction in frog skeletal muscle fibers with disrupted transverse tubules. Science. 1967 Dec 29;158(3809):1702–1703. doi: 10.1126/science.158.3809.1702. [DOI] [PubMed] [Google Scholar]
- Hall Z. W., Kelly R. B. Enzymatic detachment of endplate acetylcholinesterase from muscle. Nat New Biol. 1971 Jul 14;232(28):62–63. doi: 10.1038/newbio232062a0. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Fast kinetic studies on the allosteric interactions between acetylcholine receptor and local anesthetic binding sites. Eur J Biochem. 1979 Feb 15;94(1):281–296. doi: 10.1111/j.1432-1033.1979.tb12894.x. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Fast kinetic studies on the interaction of a fluorescent agonist with the membrane-bound acetylcholine receptor from Torpedo marmorata. Eur J Biochem. 1979 Feb 15;94(1):255–279. doi: 10.1111/j.1432-1033.1979.tb12893.x. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The nature of the prolonged endplate depolarization in anti-esterase treated muscle. Proc R Soc Lond B Biol Sci. 1975 Dec 31;192(1106):27–38. doi: 10.1098/rspb.1975.0149. [DOI] [PubMed] [Google Scholar]
- Kordas M. The effect of membrane polarization on the time course of the end-plate current in frog sartorius muscle. J Physiol. 1969 Oct;204(2):493–502. doi: 10.1113/jphysiol.1969.sp008926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Albuquerque E. X., Daly J., Barnard E. A. A study of the irreversible cholinesterase inhibitor, diisopropylfluorophosphate, on time course of end-plate currents in frog sartorius muscle. J Pharmacol Exp Ther. 1974 May;189(2):499–512. [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocil F. Dependence of acetylcholine desensitization on the membrane potential of frog muscle fibre and on the ionic changes in the medium. J Physiol. 1970 Oct;210(3):507–518. doi: 10.1113/jphysiol.1970.sp009223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocit F. The effect of temperature on desensitization kinetics at the post-synaptic membrane of the frog muscle fibre. J Physiol. 1975 Jul;249(2):285–300. doi: 10.1113/jphysiol.1975.sp011016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Terrar D. A. Factors affecting the time course of decay of end-plate currents: a possible cooperative action of acetylcholine on receptors at the frog neuromuscular junction. J Physiol. 1975 Jan;244(2):467–495. doi: 10.1113/jphysiol.1975.sp010808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Weinstock M. M. Nickel and calcium ions modify the characteristics of the acetylcholine receptor-channel complex at the frog neuromuscular junction. J Physiol. 1980 Feb;299:203–218. doi: 10.1113/jphysiol.1980.sp013120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey A. A. Further studies of the effect of calcium on the time course of action of carbamylcholine at the neuromuscular junction. J Gen Physiol. 1970 Sep;56(3):407–419. doi: 10.1085/jgp.56.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey A. A. The effect of calcium on the desensitization of membrane receptors at the neuromuscular junction. J Gen Physiol. 1966 May;49(5):963–976. doi: 10.1085/jgp.49.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Transmitter induced calcium entry across the post-synaptic membrane at frog end-plates measured using arsenazo III. J Physiol. 1980 Mar;300:197–212. doi: 10.1113/jphysiol.1980.sp013158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastuk W. L., Parsons R. L. Factors in the inactivation of postjunctional membrane receptors of frog skeletal muscle. J Gen Physiol. 1970 Aug;56(2):218–249. doi: 10.1085/jgp.56.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Noise analysis of drug induced voltage clamp currents in denervated frog muscle fibres. J Physiol. 1976 Jul;258(3):705–729. doi: 10.1113/jphysiol.1976.sp011442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTSUKA M., ENDO M., NONOMURA Y. Presynaptic nature of neuromuscular depression. Jpn J Physiol. 1962 Dec 15;12:573–584. doi: 10.2170/jjphysiol.12.573. [DOI] [PubMed] [Google Scholar]
- Pallotta B. S., Webb G. D. The effects of external Ca++ and Mg++ on the voltage sensitivity of desensitization in Electrophorus electroplaques. J Gen Physiol. 1980 Jun;75(6):693–708. doi: 10.1085/jgp.75.6.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast U., Schimerlik M. I., Raftery M. A. Ligand-induced changes in membrane-bound acetylcholine receptor observed by ethidium fluorescence. 2. Stopped-flow studies with agonists and antagonists. Biochemistry. 1979 May 15;18(10):1891–1901. doi: 10.1021/bi00577a007. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritter J. M. On the mechanism of desensitization at cholinergic receptors. Mol Pharmacol. 1970 Jul;6(4):357–382. [PubMed] [Google Scholar]
- Rosenberry T. L. Quantitative simulation of endplate currents at neuromuscular junctions based on the reaction of acetylcholine with acetylcholine receptor and acetylcholinesterase. Biophys J. 1979 May;26(2):263–289. doi: 10.1016/S0006-3495(79)85249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzzier F., Scuka M. Effect of repetitive stimulation on the frog neuromuscular transmission. Pflugers Arch. 1979 Nov;382(2):127–132. doi: 10.1007/BF00584213. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Scubon-Mulieri B., Parsons R. L. Desensitization and recovery at the frog neuromuscular junction. J Gen Physiol. 1977 Apr;69(4):431–447. doi: 10.1085/jgp.69.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI N. Effects of calcium on the conductance change of the end-plate membrane during the action of transmitter. J Physiol. 1963 Jun;167:141–155. doi: 10.1113/jphysiol.1963.sp007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THESLEFT S. The mode of neuromuscular block caused by acetylcholine, nicotine, decamethonium and succinylcholine. Acta Physiol Scand. 1955 Oct 27;34(2-3):218–231. doi: 10.1111/j.1748-1716.1955.tb01242.x. [DOI] [PubMed] [Google Scholar]
- Terrar D. A. Influence of SKF-525A congeners, strophanthidin and tissue-culture media on desensitization in frog skeletal muscle. Br J Pharmacol. 1974 Jun;51(2):259–268. doi: 10.1111/j.1476-5381.1974.tb09656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathey J. C., Nass M. M., Lester H. A. Numerical reconstruction of the quantal event at nicotinic synapses. Biophys J. 1979 Jul;27(1):145–164. doi: 10.1016/S0006-3495(79)85208-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland G., Georgia B., Lappi S., Chignell C. F., Taylor P. Kinetics of agonist-mediated transitions in state of the cholinergic receptor. J Biol Chem. 1977 Nov 10;252(21):7648–7656. [PubMed] [Google Scholar]



