ABSTRACT
Introduction:
The COVID-19 pandemic has led to long-term health effects in some patients, known as long COVID. This study aimed to delineate the symptoms of long COVID-19 and determine the presence of neurasthenia in patients one year after COVID-19 infection while excluding other potential causes of fatigue.
Methods:
A cross-sectional study was conducted on 512 RT-PCR-confirmed COVID-19 patients attending a follow-up clinic at least one year after infection. After excluding patients above 60 years, those with pre-existing psychiatric disorders, medical co-morbidities, and current psychiatric diagnoses, 87 patients were included in the final analysis. Patients were evaluated using the Schedule for Clinical Assessment in Neuropsychiatry (SCAN), Fatigue Severity Scale (FSS), and memory scale of PGI-BBD. A semi-structured questionnaire assessed changes in activities of daily living.
Results:
Of the 87 patients, 43 (49.4%) fulfilled the ICD-10 criteria for neurasthenia. Fatigue interfering with daily activities was reported by 41.3% of patients, with a mean FSS score of 6.1 in those with neurasthenia. Other symptoms included muscular aches (35.6%), tension headaches (27.5%), and weakness (31%). Cognitive difficulties, specifically problems with attention and concentration, were observed in 8% of patients. The severity of the initial COVID-19 infection did not correlate with the risk of developing neurasthenia.
Conclusion:
Long COVID symptoms, particularly those resembling neurasthenia, persist in a significant proportion of patients one year after infection. The syndrome of long COVID shows similarities to the ICD-10 diagnosis of neurasthenia, suggesting a potential link between post-COVID symptoms and chronic low-grade inflammation. These findings highlight the need for recognition and management of long-term COVID-19 effects in public health policies.
Keywords: Attention and concentration, cognition, cross-sectional, fatigue, long covid, neurasthenia
Introduction
The COVID-19 (coronavirus disease of 2019) pandemic brought the world to a standstill. While the COVID-19 virus was first reported from China in December 2019,[1] the first RT-PCR-confirmed case was reported from Kashmir in March 2020.[2] It was initially thought to be a viral illness lasting five to fourteen days, however, rapidly accumulating data suggested that it had long-term effects as well.[3] The term long COVID was first introduced in a social media post on Twitter.[4] Taking cognizance of the long-term effects of COVID-19 infection the WHO defined long COVID as the continuation or development of new symptoms three months after the initial SARS COV-2 infection with the symptoms lasting for at least two months with no other explanation. These signs and symptoms include fatigue, headache, sleep difficulties, shortness of breath, dizziness, tinnitus, rashes, gastrointestinal issues, muscle aches and pains.
Long COVID also entails cognitive difficulties such as memory impairment, problems with attention and concentration, working memory, executive function etc.
Fatigue both mental and physical is the cardinal symptom of ICD 10 diagnosis of neurasthenia.[4] The other features are muscular aches and pains, dizziness, tension headaches, irritability; inability to recover through rest, relaxation, and enjoyment; duration exceeding 3 months, and exclusion of organic mental disorders, affective disorders, or panic or generalized anxiety disorder.[5] Mental fatigue presents as a decreased speed of information processing and difficulties in attention and concentration.[6]
Fatigue though a symptom of many disorders continues to be poorly understood. Despite a lot of effort to explain its pathogenic mechanism, current knowledge remains limited. Fatigue can be caused as well as compounded by general medical conditions,[7] old age,[8] psychiatric disorders,[9] inflammatory processes[10] etc.
As is evident from the above discussion that there is a lot of overlap between the clinical symptoms of long COVID and neurasthenia, we, therefore, conducted a cross-sectional study on COVID-19 patients one year after the onset of clinical symptoms to delineate the symptoms of long COVID and determine the presence of neurasthenia in such patients. Additionally, although there is a lot of literature on the presence of fatigue and cognitive difficulties in long-term COVID patients in the short term (time frame of a few months), this is one of the very few studies that has evaluated patients one year after infection with COVID-19. Extensive search on Google Scholar, Medline, and Pubmed reveals that to date there is no published study on long-term covid and neurasthenia.
The symptoms of long COVID have been proposed to be caused by a variety of causes including psychiatric disorders,[9] medical co-morbidities,[7] organ damage[10] advancing age[8], and inflammation.[10] We excluded all proposed causes in our sample apart from inflammation.
Material and Methods
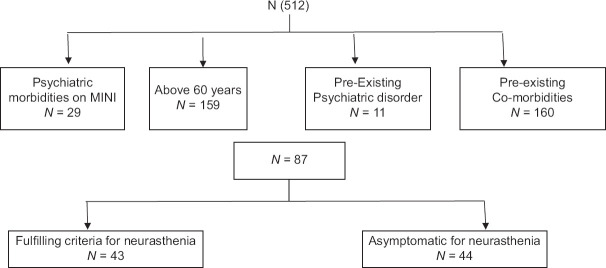
512. consecutive RT-PCR (reverse transcriptase polymerase chain reaction) confirmed patients attending a COVID-19 follow-up clinic at least 1 year after infection were evaluated using Schedule for Clinical Assessment in Neuropsychiatry (SCAN) [Figure 1]. Detailed socio-demographic profiles, medical history, and current medical records were obtained from all patients. All patients above 60 years of age (n = 159), those with pre-existing psychiatric disorders (n = 11), those with medical co-morbidities (n = 160) [Table 1] and those with any psychiatric diagnosis on SCAN (29) were excluded [Table 2]. The reason for such exclusion was that fatigue is a common accompaniment of all these conditions and we wanted to exclude all other potential causes of fatigue in our cohort. The remaining patients with a history of COVID-19 infection were included in the study. Whenever fatigue was the chief complaint, the fatigue severity scale was used to quantify fatigue, and where patients reported cognitive symptoms the memory scale of PGI-BBD was used. Patients were also asked whether exertion made their fatigue worse. The patient’s activities of daily living (ADL) were also enquired into and a comparison between the patient’s pre- and post-COVID level of functioning (social, occupational or educational, and personal) was made by using a semi-structured questionnaire. In addition information was obtained about severity of COVID-19 infection. Infection was defined as moderate-severe if the patient required hospitalization or supplementary oxygen.
Figure 1.
Chart depicting patient selection for study
Table 1.
Comorbidity and post-covid symptoms (n=512)
| Comorbidity | Post-covid symptoms | |||
|---|---|---|---|---|
|
| ||||
| Yes | No | Total | ||
| Hypertension | Frequency | 21 | 26 | 47 |
| % Age | 11.4 | 8.2 | 9.4 | |
| % Total | 4.2 | 5.2 | 9.4 | |
| Type II diabetes mellitus | Frequency | 17 | 30 | 47 |
| % Age | 9.2 | 9.5 | 9.4 | |
| % Total | 3.4 | 6.0 | 9.4 | |
| Chronic obstructive pulmonary disease | Frequency | 4 | 3 | 7 |
| % Age | 2.2 | 0.9 | 1.4 | |
| % Total | 0.8 | 0.6 | 1.4 | |
| Hypothyroidism | Frequency | 13 | 23 | 36 |
| % Age | 7.1 | 7.3 | 7.2 | |
| % Total | 2.6 | 4.6 | 7.2 | |
| Others | Frequency | 8 | 15 | 23 |
| % Age | 4.3 | 4.7 | 4.6 | |
| % Total | 1.6 | 3.0 | 4.6 | |
| None | Frequency | 121 | 219 | 340 |
| % age | 65.8 | 69.3 | 68.0 | |
| % Total | 24.2 | 43.8 | 68.0 | |
| Total | Frequency | 184 | 316 | 500 |
| % Age | 100 | 100 | 100 | |
| % Total | 36.8 | 63.2 | 100 | |
Table 2.
Scan diagnosis in the studied population (n=512)
| Scan diagnosis | Frequency | Percentage (%) |
|---|---|---|
| PTSD | 1 | 0.2 |
| MDD | 16 | 3.2 |
| BPAD | 1 | 0.2 |
| Panic disorder | 9 | 1.8 |
| OCD | 2 | 0.4 |
| Total | 29 | 5.8 |
Instrument
The SCAN is a semi-structured clinical interview which requires a trained clinician to administer. It is based on the present state examination (PSE). It has good reliability and validity. In this study the SCAN was employed which aligns with ICD-10. It includes all common disorders of mental health including neurocognitive disorders.
PGI-BBD: (Post graduate battery of brain dysfunction) is a sophisticated collection of various tests that are used to quantify cognitive dysfunction, impairment, decline or deficits in clinical settings. The PGI battery of brain dysfunction measures well-known cognitive functions of the brain behavior such as intelligence (both performance and verbal), memory, perceptual acuity, and transference from one hemisphere to another. While the test is supposed to be used as a whole, it can also be used in parts based on special Circumstances. Each of these tests has separate norms, thus making it easy for them to be used autonomously. We used only the memory subscale of the PGI BBD as was mandated by the scope of our study design. The memory scale contains 10 sub-tests: remote memory, recent memory, mental balance, attention, delayed recall, immediate recall, retention for similar pairs, retention for dissimilar pairs, visual retention, and recognition psychometric properties.
FSS:(Fatigue Severity Scale) The FSS is a nine-item questionnaire primarily focusing on the motor aspects of fatigue, the main emphasis being the assessment of the severity of fatigue symptoms and its impact on individual daily functioning. Each item of the questionnaire is scored on a seven-point Likert scale ranging from 1(completely disagree) to 7 (completely agree). The mean score of the nine items is used as the FSS score. As recommended, we used a cut-off score of 4 in our study.
Semi structured questionaire for ADL
Questions were asked regarding patients’ ability to move up and down a flight of stairs, ability to change clothes and bathe, ability to perform household chores (cleaning, meal preparation, shopping, managing transportation), and patients were asked to make a comparison with their pre-covid level of functioning.
Statistical analysis
The recorded data was compiled and analyzed using SPSS. Continuous variables were expressed as mean +/− SD and categorical variables were summarized as frequencies and percentages. For inferential statistics, Pearson’s Chi-Square was used, 2-sided P values were reported, and the P value of 0.05 was considered as statistically significant.
Results
43 patients in our cohort fulfilled the criteria for neurasthenia, which constitutes 49.4% of the cohort. These patients also had impairment in their daily functioning.
The sociodemographic profile is presented in [Table 3]. The difference in age, gender, education, occupation, marital status, and residence (rural versus urban) between those with and without neurasthenia was not statistically significant.
Table 3.
Sociodemographic profile of included population (n=87)
| Variable | Severity of covid-19 infection | Total | |
|---|---|---|---|
|
| |||
| Mild | Moderate–severe | ||
| Age | |||
| <40 | 19 | 18 | 37 |
| 40–60 | 24 | 26 | 50 |
| Gender | |||
| Females | 18 | 29 | 47 |
| Males | 25 | 15 | 40 |
| Marital Status | |||
| Married | 38 | 40 | 78 |
| Unmarried | 5 | 4 | 9 |
| Education | |||
| Uneducated | 4 | 8 | 12 |
| School | 17 | 20 | 37 |
| College | 22 | 16 | 38 |
| Occupation | |||
| Unemployed | 3 | 5 | 8 |
| Employed | 40 | 39 | 79 |
| Address | |||
| Rural | 25 | 17 | 42 |
| Urban | 18 | 27 | 45 |
| Severity Of Covid-19 | |||
| Home Quarantine | 37 | 31 | 68 |
| Hospitalized | 6 | 13 | 19 |
The severity of COVID-19 infection did not correlate with the risk of developing neurasthenia.
All patients reported some degree of fatigue, however, fatigue interfering with activities of daily living and compromising the functioning of the patient as measured by the fatigue severity scale was reported by 41.3% (36) patients. Muscular aches and pains were reported by 35.6% (31) patients, dizziness was reported by 5.7% (5) patients, tension headaches were reported by 27.5% (24) patients, and weakness by 31% (27) patients. The mean score on the fatigue severity scale of patients having neurasthenia was 6.1.
Forgetfulness with inability to concentrate was reported by 8% (7) patients. On the memory scale of PGI-BBD, these 7 patients had no dysfunction on the subscale of remote memory, recent memory, mental balance, delayed recall, immediate recall, verbal retention for similar pairs, verbal retention for dissimilar pairs, visual retention, and recognition. However, all seven patients had a dysfunction rating score of 2 on tests of attention and concentration on the PGI-BBD [Table 4].
Table 4.
Results on the PGI-BBD (n=7)
| Sub-test | I | II | III | IV | V | VI | VII | VIII | IX | X |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean raw scores, (median raw score) | 6, (6) | 5, (5) | 5.4, (6) | 6.5, (7) | 8.7, (9) | 10.2, (10) | 5, (5) | 12, (12) | 10.3, (10) | 10, (10) |
| Dysfunction rating (n=7) | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
I=remote memory, II=recent memory, III=mental balance, IV=Attention and concentration, V=delayed recall, VI=immediate recall, VII=verbal retention for similar pairs, VIII=verbal retention for dissimilar pairs, IX=visual retention, and X=recognition
Discussion
Neurasthenia has been historically conceptualized as a disorder resulting at times of social change with increased psychosocial stress. Neurasthenia-like symptom complexes have been described by various terms over time such as chronic fatigue syndrome, post-viral fatigue syndrome, etc., with a focus on immune dysregulation as the pathophysiology and onset associated with antecedent viral infections.[11] CFS is thought to be precipitated by a viral insult, major life event, or environmental toxins. Additionally, post-exertional malaise is thought of as an important diagnostic construct in chronic fatigue syndrome whereby exertion brings about increased fatigue and a decrease in exercising capacity.[12] However, despite much research such associations remain unproven, and such distinctions are arbitrary. There remains a lot of overlap between the clinical characteristics of neurasthenia and chronic fatigue syndrome with fatigue as the cardinal symptom.
Stress whether psychosocial or biological is thought to activate inflammatory processes.[9]
Neurasthenia as such can be thought of as a marker of inflammation caused by medium and long term effects of infection with COVID 19 or the associated psychosocial changes. There is a need to look into the effects of such inflammation on various other organ systems such as the brain, heart, etc., and the effects of such inflammatory processes on long-term morbidity and mortality.
Treatment in the form of adequate nutrition, rest, and daily graded exercise may be used for recovery.[13] Additional treatments that need further study are antidepressants that have been postulated to have anti-inflammatory properties[12] and there is some data on their benefit in long COVID symptoms.
In our study, 49.4% of the sample fulfilled ICD-10 criteria for neurasthenia. There was significant impairment in the daily functioning of individuals. Additionally, there was no significant post-exertional malaise in our study sample.
The prevalence of neurasthenia from a clinical sample in India has been reported to be 5%.[14] In a nationally representative sample in the U.S., among the total sample, the adjusted prevalence rates of lifetime and 12-month neurasthenia with exclusionary criteria were 2.22 and 1.19%.[15]
Since ours was also a clinical sample this study suggests an increased prevalence post-infection with COVID-19.
41% of our sample reported fatigue while 8% of patients reported some degree of cognitive impairment characterized by problems in attention and concentration.
Systematic reviews indicate a pooled prevalence of post-COVID-19 fatigue to vary among 45%,[16] 52%,[17] and 64%.[18]
In one meta-analysis the prevalence of fatigue was 40%.[19] In another meta-analysis the prevalence of fatigue was around 64%[20] while the prevalence of cognitive symptoms was around 20% in one study.[21]
Age greater than 50 years has been associated with an increased risk of developing fatigue in various studies,[22] we did not find such an association in our study, partly because of the small sample size and majorly because we excluded people aged above 60 in our study. Similarly, the female gender has been associated with an increased risk of developing fatigue,[22] however no such association was found in our study. The severity of the disease was not associated with the development of long COVID symptoms. Disease severity also had an inconsistent impact on fatigue, with most studies finding no association with severe acute disease or fatigue prevalence in severity categories.[23]
Long COVID symptoms have been thought to result from various processes including inflammatory processes, organ damage, and psychiatric illnesses.[7,9,10]
Cognitive symptoms of long COVID include the full gamut of symptoms including impairment in language, executive function, visuospatial functions, attention and concentration, memory impairment, etc., Post-COVID-19 dementia has also been reported especially in the elderly population. We used the memory subscale of the PGI-BBD in our study, the memory parameters were mostly unimpaired in our population. This could in part be due to the young cohort in our study. In 8% of the sample significant problems with attention and concentration were found which is akin to the brain fog or mental fatigue of neurasthenia. Several other studies have also reported problems with attention and concentration post-COVID-19 infection.
Pandemics are known to induce high levels of stress and precipitate mental health problems in the populations affected as has previously been experienced with other pandemics such as the 2003 Severe Acute Respiratory Syndrome (SARS), the 2009 H1N1 pandemic, etc., Quarantine, isolation, and other such necessary measures that need to be imposed to prevent the spread of infection also contribute towards the burden of mental health issues, such as post-traumatic stress disorder, major depressive disorder,[24] etc., Other factors that contribute include psychosocial conditions prevailing around the pandemic such as loneliness, isolation, life-threatening situations in the form of ICU admissions hospitalization death of loved ones, etc. A lot of research has already been generated which points towards an increase in the burden of mental health morbidity owing to the COVID-19 pandemic.[25] An increase in such disorders such as anxiety disorders, depressive disorders, etc.,[24] has been reported.
Additionally, there is a commonality of symptoms between neurasthenia and depression as well as anxiety disorders, in fact, in the past many patients of neurasthenia were subsequently diagnosed as having other mental health disorders; however, we excluded depression and anxiety disorders in our cohort. Also, these are different disorders in ICD-10 diagnostic systems with low mood and loss of interest as the sine quo non in depressive disorders and fear and worry as the sine quo non in all anxiety disorders whereas neurasthenia has fatigue as the cardinal symptom. We used the SCAN to exclude all psychiatric disorders. Hence the symptoms cannot be attributed to any psychiatric co-morbidity.
Infection with COVID-19 can lead to long-term pulmonary complications such as fibrotic lung disease, post-COVID lung fibrosis or post-ARDS fibrosis, bronchiectasis, pulmonary vascular disease, and chronic cough. These complications may in turn cause shortness of breath and fatigue, resembling the long COVID syndrome. Chronic cardiac problems such as myocarditis, pericarditis, myocardial infarction, cardiac failure, etc., can also cause long COVID symptoms.[26] Hence the long COVID syndrome is also thought to result from organ damage; however, all patients included in the study were thoroughly examined and detailed medical examination and investigation of study subjects was negative.
Patients above 60 years of age were excluded from the study as old age is known to be associated with complaints of fatigue with associated difficulties with performing activities of daily living.
Fatigue can be caused by a variety of medical illnesses. As such we excluded patients with hypertension, diabetes, dyslipidemia, and asthma from our study.
As we excluded the effects of medical illness, organ damage owing to Covid, psychiatric co-morbidity, and old age as potential causes of fatigue in our study subjects, it points towards the presence of neurasthenia as an independent entity as a long-term complication of COVID-19 infection and it is worthwhile to consider low-grade chronic inflammation as a cause of post-COVID symptoms and neurasthenia.
In summation, long-term COVID exerts significant physical and cognitive effects even one-year post-infection, and these effects may be mediated via inflammation. Long COVID is a reality that needs to be endorsed in public health policy not only validating the pain of sufferers but also devising effective treatments. The syndrome of long COVID is very similar to the ICD-10 diagnosis of neurasthenia.
Limitations
Diagnosis of long COVID is challenging, as long COVID symptoms (fatigue, headache, brain fog, musculoskeletal pain, shortness of breath (SoB), chest pain) are common in the general population. Additionally, without pre-illness participant baseline data, the impact of COVID-19 on pre-existing symptoms cannot be determined.
Conclusion
COVID-19 epidemic as an acute life threatening viral infection evoked unrivalled public health response but long COVID despite having evidence in its support is still ill defined, less recognized and scarcely treated condition. Neurasthenia as described in ICD-10 seems to be closely associated with long COVID and needs further investigation focusing on similarities and differences and common pathophysiological links.
Conflicts of interest
There are no conflicts of interest.
Funding Statement
Nil.
References
- 1.Spiteri G, Fielding J, Diercke M, Campese C, Enouf V, Gaymard A, et al. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Euro Surveill. 2020;25:2000178. doi: 10.2807/1560-7917.ES.2020.25.9.2000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan SMS, Qurieshi MA, Haq I, Majid S, Ahmad J, Ayub T, et al. Seroprevalence of SARS-CoV-2-specific IgG antibodies in Kashmir, India, 7 months after the first reported local COVID-19 case: Results of a population-based seroprevalence survey from October to November 2020. BMJ Open. 2021;11:e053791. doi: 10.1136/bmjopen-2021-053791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castanares-Zapatero D, Chalon P, Kohn L, Dauvrin M, Detollenaere J, Maertens de Noordhout C, et al. Pathophysiology and mechanism of long COVID: A comprehensive review. Ann Med. 2022;54:1473–87. doi: 10.1080/07853890.2022.2076901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awoyemi T, Ebili U, Olusanya A, Ogunniyi KE, Adejumo AV. Twitter sentiment analysis of long COVID syndrome. Cureus. 2022;14:e25901. doi: 10.7759/cureus.25901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang C, Zhao H, Espín E, Tebbutt SJ. Association of SARS-CoV-2 infection and persistence with long COVID. Lancet Respir Med. 2023;11:504–6. doi: 10.1016/S2213-2600(23)00142-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faber LG, Maurits NM, Lorist MM. Mental fatigue affects visual selective attention. PLoS One. 2012;7:e48073. doi: 10.1371/journal.pone.0048073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fritschi C, Quinn L. Fatigue in patients with diabetes: A review. J Psychosom Res. 2010;69:33–41. doi: 10.1016/j.jpsychores.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu DS, Lee DT, Man NW. Fatigue among older people: A review of the research literature. Int J Nurs Stud. 2010;47:216–28. doi: 10.1016/j.ijnurstu.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Harvey SB, Wessely S, Kuh D, Hotopf M. The relationship between fatigue and psychiatric disorders: Evidence for the concept of neurasthenia. J Psychosom Res. 2009;66:445–54. doi: 10.1016/j.jpsychores.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianka K, Tina S, Julie L. Role of inflammation in human fatigue: Relevance of multidimensional assessments and potential neuronal mechanisms. Front Immunol. 2017;8:21. doi: 10.3389/fimmu.2017.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhola P, Chaturvedi SK. Neurasthenia: Tracing the journey of a protean malady. Int Rev Psychiatry. 2020;32:491–9. doi: 10.1080/09540261.2020.1758638. [DOI] [PubMed] [Google Scholar]
- 12.Cortes Rivera M, Mastronardi C, Silva-Aldana CT, Arcos-Burgos M, Lidbury BA. Myalgic encephalomyelitis/chronic fatigue syndrome: A comprehensive review. Diagnostics (Basel) 2019;9:91. doi: 10.3390/diagnostics9030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larun L, Brurberg KG, Odgaard-Jensen J, Price JR. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev. 2016;12:CD003200. doi: 10.1002/14651858.CD003200.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paralikar V, Sarmukaddam S, Agashe M, Weiss MG. Diagnostic concordance of neurasthenia spectrum disorders in Pune, India. Soc Psychiat Epidemiol. 2007;42:561–72. doi: 10.1007/s00127-007-0196-x. [DOI] [PubMed] [Google Scholar]
- 15.Molina KM, Chen CN, Alegría M, Li H. Prevalence of neurasthenia, comorbidity, and association with impairment among a nationally representative sample of US adults. Soc Psychiatry Psychiatr Epidemiol. 2012;47:1733–44. doi: 10.1007/s00127-012-0489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoshijima H, Mihara T, Seki H, Hyuga S, Kuratani N, Shiga T. Incidence of long-term post-acute sequelae of SARS-CoV-2 infection related to pain and other symptoms: A living systematic review and meta-analysis. PLoS One. 2023;18:e0250909. doi: 10.1371/journal.pone.0250909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cares-Marambio K, Montenegro-Jiménez Y, Torres-Castro R, Vera-Uribe R, Torralba Y, Alsina-Restoy X, et al. Prevalence of potential respiratory symptoms in survivors of hospital admission after coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Chron Respir Dis. 2021;18:14799731211002240. doi: 10.1177/14799731211002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malik P, Patel K, Pinto C, Jaiswal R, Tirupathi R, Pillai S, et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)—A systematic review and meta-analysis. J Med Virol. 2021;94:253–62. doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poole-Wright K, Guennouni I, Sterry O, Evans RA, Gaughran F, Chalder T. Fatigue outcomes following COVID-19: A systematic review and meta-analysis. BMJ Open. 2023;13:e063969. doi: 10.1136/bmjopen-2022-063969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malik P, Patel K, Pinto C, Jaiswal R, Tirupathi R, Pillai S, et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (hrqol)-A systematic review and meta-analysis. J Med Virol. 2022;94:253–62. doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceban F, Ling S, Lui LMW, Lee Y, Gill H, Teopiz KM, et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin ES, Gold LS, Hough CL, Katz PP, Bunnell AE, Wysham KD, et al. Patient-reported functional outcomes 30 days after hospitalization for COVID-19. PM R. 2022;14:173–82. doi: 10.1002/pmrj.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-de-las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Palacios-Ceña M, Rodríguez-Jiménez J, de-la-Llave-Rincón AI, et al. Fatigue and dyspnoea as main persistent post-COVID-19 symptoms in previously hospitalized patients: Related functional limitations and disability. Respiration. 2022;101:132–41. doi: 10.1159/000518854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srivastava K, Chaudhry S, Sowmya AV, Prakash J. Mental health aspects of pandemics with special reference to COVID-19. Ind Psychiatry J. 2020;29:1–8. doi: 10.4103/ipj.ipj_64_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang TJ, Rabheru K, Peisah C, Reichman W, Ikeda M. Loneliness and social isolation during the COVID-19 pandemic. Int Psychogeriatr. 2020;32:1217–20. doi: 10.1017/S1041610220000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh SJ, Baldwin MM, Daynes E, Evans RA, Greening NJ, Jenkins RG, et al. Respiratory sequelae of COVID-19: Pulmonary and extrapulmonary origins, and approaches to clinical care and rehabilitation. Lancet Respir Med. 2023;11:709–25. doi: 10.1016/S2213-2600(23)00159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]