Abstract
The green revolution, characterized by intensive farming practices and synthetic agrochemicals, has been associated with concerns about ecological balance and soil health. This study investigated the impact of organic and conventional farming practices on soil quality and microbial diversity in coffee plantations within the Western Ghats. Soil samples from organic and conventional coffee farms in Ponnampet, Kodagu, Karnataka were collected for physical, chemical, and biological analysis. Organic soils had lower bulk density and particle density, suggesting improved structure and porosity. Organic systems had higher levels of organic carbon, nitrogen, and exchangeable calcium and magnesium. Organic coffee farming exhibited the highest soil quality index value of 0.98, which was higher than that of conventional coffee farming practice (0.87). Organic farming systems demonstrated significantly higher soil microbial respiration rates, reflecting a more active and diverse microbial community. Organic coffee farming systems not only promoted higher microbial biomass but also the higher value of Shannon–Wiener’s index, Simpson’s Diversity Index, Shannon and Simpson evenness index enhanced microbial diversity. These findings underscore the potential of organic coffee farming for sustainable agriculture in the Western Ghats, particularly in terms of enhancing soil health, promoting microbial diversity, and improving long-term soil quality compared to conventional practices.
Keywords: Organic coffee practice, Conventional coffee practice, Soil quality, Soil microbial diversity, Western ghats
Subject terms: Microbiology, Environmental impact
Introduction
Organic farming encompasses an agricultural production system that deliberately refrains from utilizing synthetic fertilizers and pesticides. Organic farming is predicated upon the use of many techniques, including crop rotations, the utilization of animal and green manure, and the application of biological pest control measures1. These strategies are employed to preserve soil productivity, ensure an adequate supply of plant nutrients, and effectively manage and control insects, weeds, and other pests2. It has gained global recognition for its potential to address several pressing challenges in modern agriculture3. Organic farming increases biodiversity in animals, insects, pollinators and other beneficial organisms compared to conventional farming4. Multiple studies indicate enhanced microbial abundance and diversity in organically managed soils compared to conventional practices. Reduced tillage, cover crops, and adding organic fertilizer may lead to a wider range of microorganisms because soil organic carbon provide a carbon heterotrophic microbiota5.
Coffee is a major plantation crop in India, grown in high-altitude southern regions, and contributes significantly to foreign exchange earnings. The industry is vital for the economy, providing jobs and aiding in ecosystem preservation. Understanding the impact of farming methods on coffee cultivation, specifically comparing organic and conventional approaches, is crucial for several reasons related to economic, environmental, and agricultural sustainability. Coffee is a key plantation crop in India, contributing significantly to foreign exchange earnings and thriving in the southern high-altitude regions. The coffee industry is vital for the economy, providing jobs and aiding in ecosystem preservation6. Organic coffee production and consumption have recently gained global importance, accounting for about 4% of global consumption7. This sector emphasizes soil health, biodiversity, and ethical practices.
Over the decades, approaches to evaluating soil quality in the coffee agroecosystems of the Central Western Ghats have advanced from basic chemical testing to more comprehensive assessments that include both physical and biological parameters62. The implementation of the Soil Quality Index (SQI), which uses principal component analysis to select key variables, now allows objective comparisons between organic and conventional management systems. Current assessments place strong emphasis on the diversity of soil microbes and invertebrates, with studies showing that organic practices can boost microbial populations by approximately 34% and contribute to a more diverse soil ecology60. Effective evaluation of sustainability in cultivation systems relies on soil quality indicators that are sensitive to changes, connected to essential ecosystem functions, and are straightforward to measure, interpret, and compare9,10. Recent research highlights that organic coffee farming enhances or maintains soil quality more effectively than conventional systems, notably increasing soil organic carbon by up to 15.6% as well as improving soil structure and water retention60,61. Conversely, conventional methods may lead to a reduction in soil carbon and raise the risks of acidification and compaction. Although some indicators, such as nutrient levels and pH, may show little difference between systems, a broad body of evidence consistently supports organic and regenerative strategies as the best options for preserving long-term soil health and productivity in coffee plantations60,61, .
Microbes play a crucial role in agroecosystems, enhancing crop growth by producing antibiotics and plant hormones, and improving nutrient accessibility, thereby boosting plant quality and yield11. Recently, the soil microbial community’s role in agricultural production has gained interest, as studies show that microbial characteristics can reflect ecosystem processes like crop productivity12decomposition regulation, nutrient cycling13and defence against soil-borne diseases14. Study found a 34% increase in microbial population and higher diversity in organic soils, along with greater soil respiration and FDA activity, indicating more active microbial communities and nutrient cycling. In contrast, conventional systems may show higher urease activity due to fertilizer use6. Organic and shade-grown systems support higher biodiversity, including soil invertebrates, which promotes healthy microbial ecosystems and natural pest control60,61.
The significance of this study lies in its comprehensive evaluation of organic versus conventional coffee farming practices in the Western Ghats of India. The Green Revolution’s intensive agrochemical use has disrupted the ecological balance and soil health, whereas organic farming offers a viable alternative to mitigate these adverse effects15.
While existing research highlights conventional farming’s immediate benefits, such as higher yields and pest control, it often overlooks long-term environmental impacts like soil degradation, biodiversity loss, and heavy metal accumulation. Conversely, research on organic farming underscores its potential to enhance soil health, promote biodiversity, and support sustainable agriculture10. However, there’s a critical gap in empirical evidence comparing these systems’ impacts on soil quality and microbial diversity, especially in biodiversity hotspots like the Western Ghats. This study addresses this gap by providing empirical data on the physical, chemical, and biological properties of soils under organic and conventional coffee farming systems. The findings show that organic farming significantly improves soil structure, organic matter content, and microbial diversity, making it a more sustainable option. These results highlight the importance of adopting organic farming practices in ecologically sensitive areas for long-term soil health and environmental conservation.
This study’s practical applications include developing region-specific organic farming guidelines and establishing soil quality monitoring programs for sustainable coffee cultivation. By comparing the impacts of organic and conventional farming, this research offers valuable insights for promoting sustainable agricultural practices in the Western Ghats and similar regions.
Materials and methods
Overview of field and experimental setup
The study focused on organic and conventional coffee cultivation methods in Ponnampet taluk, Kodagu district, Karnataka, India, which have been practiced for over a decade. The taluk, located 851 m above sea level, experiences a predominantly tropical climate, while the higher hill ranges exhibit montane subtropical conditions, with summer temperatures ranging from 25.5 °C to 28.6 °C and winter temperatures between 12 °C and 15 °C. The annual rainfall varies from 1126 to 2500 mm (Fig. 1). The research site was predominantly planted with Arabica coffee, with plant spacing varying between 0.3 × 0.3 m and 0.4 × 0.4 m, depending on the farmer’s preference. Organic coffee farms were treated with 5–8 tonnes of farmyard manure or compost per acre annually. In contrast, conventional coffee farming systems adhered to a standard fertilizer application of 40:30:40 (N: P2O5:K2O kg ha− 1 year), with nitrogen applied in three separate instances. Weed management in conventional farms typically involved one herbicide application and two manual weeding sessions, while organic farms relied on three rounds of manual weeding using a bush cutter. Organic systems addressed pest and disease issues using neem-based formulations for sucking pests, lime swabbing, pheromone traps, Broca traps for white stem borers, and a 0.5% Bordeaux spray for rust control. Apart from these, both systems employed similar crop management practices.
Fig. 1.
Annual temperature, humidity, and rainfall of Ponnampet Taluk, Kodagu district for the year 2022–2023.
Soil sample collection and analysis
This study analyzed soil from 50 farming systems, equally divided between organic and conventional methods. Under each farming system,, a minimum of 8 to 10 spots were chosen in a sampling area (location) and samples from these spots were consolidated to get one composite soil sample for each location. Fifty such composite samples were collected from conventional and fifty from organic coffee farming systems. Soil samples were collected to a depth range of 0 to 0.6 m, adhering to standard protocols with the aid of a motorized auger. Each representative soil sample was split into two subsets. The first subset was air-dried, sieved through a 2 mm mesh, and stored in a moisture-free setting for subsequent analysis. The second subset was refrigerated and used to assess the soil’s biological attributes.
Standard procedures were employed to ascertain the physicochemical and biological attributes of the soil samples. The International Pipette method was used to determine the composition of sand, silt, and clay in the samples16. The Keen Raczkowski cup method16 was utilized to measure the pore space and maximum water-holding capacity (MWHC). Soil pH and electrical conductivity (EC) were gauged using a pH meter and a conductivity meter, respectively, in a 1:2 soil-water ratio. The soil organic carbon was determined using K2Cr2O7 as an oxidizing agent (1 N) and back titrating with 0.5 N FAS method17. The available N was estimated by the alkaline KMnO4 method where organic matter present in the soil is oxidized with a hot alkaline KMnO4 solution in the presence of NaOH. The ammonia (NH3) that evolved during oxidation was distilled and trapped in a boric acid mixed indicator solution. The amount of ammonia trapped was estimated by titrating with standard acid18available P by extraction with Bray’s extractant (i.e., 0.025 M HCl and 0.03 M NH4F) and was determined colourimetrically by the ascorbic acid method. The intensity of the blue colour was read at 660 nm using a spectrophotometer19 and available K by extraction with 1 N ammonium acetate (pH 7.0) and fed directly to a flame photometer20. Soil-available calcium, magnesium, sulphur, iron, zinc, copper and manganese were determined using inductively coupled plasma optical emission spectrometry (ICP-OES). Dehydrogenase activity was estimated based on the principle that 2,3,5-triphenyl tetrazolium chloride (TTC) which is used as an electron acceptor, is reduced to triphenyl formazan (TPF), which imparts colour21. The quantity of TPF in terms of the colour intensity formed was measured using a spectrophotometer at 485 nm wavelength. Acid phosphatase activity (AcidPhos) was measured using the p-nitrophenyl phosphate technique22. It is based on the incubation of soil samples mixed with a buffer solution of p-nitrophenylphosphate at 37 °C for 1 h. The released p-nitrophenol is stained and measured spectrophotometrically at 400 nm. Soil respiration was measured as the CO2 evolved from the moist soil23. Urease activity and fluorescein diacetate (FDA) were assayed by the standard method24.
The soil carbon stock was estimated using the parameters namely the soil thickness, bulk density and carbon content using the below-mentioned formula.
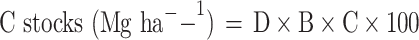 |
Where D, B, and C represent the soil thickness (cm), BD (g cm[– [3), and C content (%) respectively4.
Soil Microbiological enumeration and microbial diversity indices
The samples were diluted 10− 4 in 90 mL Ringer’s solution. The diluted samples were plated on Nutrient Agar for bacteria, Martin’s Rose Bengal Agar for fungi, Ken Knights and Munaier’s Agar for actinomycetes, and Buffered Yeast Agar for yeast in 1 mL aliquots Optimal temperature for triplicate plates was 25℃±1℃. To count functional/physiological microorganisms, standard microbiological methods were used. PSM, Azotobacter, and fluorescent pseudomonad functional groups in soil samples were quantified by Pikovskaya Agar, Waksman Number 77, and King’s B. Microbial colonies were counted and reported as CFUs g− 1 after 3 days for bacteria and yeast, 5 days for fungi, and 7 days for actinomycetes25. Culturable microbial diversity indexes for organic and conventional agriculture were calculated using standard methods.
Diversity indices
Various diversity indices were employed to assess the richness and dominance of microorganisms in conventional and organic coffee systems. Standard methods were used to determine the microbial diversity indices for the different treatments26.
The Shannon–Wiener’s index (H′) was chosen as an index of species relative abundance or richness using the following equation.
 |
Where, H′: Shannon-Weiner index of diversity.
pi: The proportion of each species in the sample.
pi = ni/N Where ni: Number of individuals of species.
N: Total number of species present.
The Simpson’s index (D) was chosen as an index of dominance using the following equation.
 |
Where, D: Simpson Index, n: total number of organisms of a particular species.
N: Total number of species present.
The Shannon evenness (E) or species equitability index was calculated using the following equation.
 |
Where: H′ =Shannon- Weiner index, S = Total species number, Hmax = In(S) Maximum diversity possible.
The Simpson evenness (E) was calculated using the following equation.
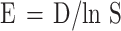 |
where, E = equitability/evenness index, D = Simpson’s index, S = number of species in a population.
Soil quality index (SQI)
Soil quality assessment involves a three-step process that guided the development of the current tool27 (Fig. 2). This includes selecting the minimum data set (MDS) and integrating the indicator scores into the soil quality index (SQI). Data were streamlined into the MDS using univariate statistical analysis and the correlation matrix of indicators, helping identify variables with significant treatment effects. The indicators selected for the Soil Quality Index were chosen based on their sensitivity to changes in land use and management practices, as well as their relevance to key soil functions such as nutrient cycling, microbial activity, and structural integrity. These indicators were identified using a combination of statistical significance (p < 0.05), principal component analysis (PCA), and correlation matrix filtering. This approach aligns with established methodologies in soil quality assessment, ensuring that the minimum data set (MDS) reflects the most informative and non-redundant variables for evaluating soil health across farming systems27. The data were processed using SPSS software, and PCA was applied to each significant indicator to explore relationships among them by statistically grouping them into PC factors using the varimax rotation procedure. PCs with eigenvalues greater than one9 and explaining at least 5% of data variation were selected for additional analysis to identify indicators. For each PC, the indicator with the highest factor loading (positive or negative) was selected for scoring. If multiple factors were retained within one PC, multivariate correlation was employed to reduce data redundancy. Highly correlated variables (> 0.60) were deemed redundant, and only one was included in the MDS28. Remaining variables were removed, but if highly weighted variables were not correlated, each was considered crucial and included in the MDS.
Fig. 2.

Conceptual model for computing soil quality index57.
Every MDS indicator observation was normalized for SQI computation. The normalized indicator value is the “indicator score” (S). Each indicator in the linear scoring method is categorized as “more is better”, “less is better”, or “optimum is better”. For “more is better,” divide each observation by the highest observed value, resulting in a score of 1 for the highest and a score of < 1 for the rest. To score “less is better,” divide the lowest observed value by each observation, resulting in a score of 1 for the lowest value indicator and a score of < 1 for others Up to the threshold level, indicator observations are scored as “more is better” for “optimum is better” and then as “less is better.”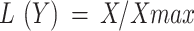 ‟More is better” approach (1)
‟More is better” approach (1)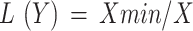 ‟Less is better” approach (2).
‟Less is better” approach (2).
Where,
L(Y) is the linear score varying from 0 to 1.
X is the soil indicator value.
Xmax is the maximum value of each soil indicator.
Xmin is the minimum value of each soil indicator.
Once a score is assigned to each indicator, weight is computed for them by using the PCA results. Each PC explained a certain amount of variation (%) to the total variation (%). This percentage of variation divided by the total per cent variation gave the weighted factor (W) for each selected indicator from the PCA29. The SQI is computed by integrating the score and weight factor of each indicator. This can be explained by the following equation:
Where, Si = Score for subscripted variable.
Wi = Weighing factor derived from the PCA.
Data analysis
A descriptive analysis (range, average, and standard deviation) described the data. SPSS version 29.0.1.0 was used to analyse soil properties using a suitable independent t-test approach to examine the significance of the overall differences between farming systems. Differences in farming systems were statistically significant (P 0.05). Pearson correlation was used to determine variable relationships. The SPSS 29.0 software also performed the MDS through PCA for SQI selection.
Results
Soil physical characteristics
The soil texture in organic and conventional farming systems was sandy clay loam, and there were no significant differences in the sand, silt, and clay levels (Table 1). The conventional farming system soils had the highest average sand, silt, and clay content (65.08%, 10.72%, and 24.23%), while the organic soils had the lowest. The mean value of BD (1.36 g cm− 3) and MWHC (51.57%) were substantially greater in conventional and organic farming systems, respectively (Table 1).
Table 1.
Influence of conventional and organic coffee farming practices on soil physical and chemical properties of soil.
| Parameters | Conventional coffee farming system | Organic coffee farming system | SE ± m | C.D (p = 0.05) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Mean | SD | Min. | Max. | Mean | SD | |||
| Sand (%) | 60.60 | 82.10 | 65.08 | 4.29 | 56.44 | 81.69 | 64.28 | 6.37 | 0.21 | NS |
| Silt (%) | 16.02 | 37.52 | 10.72 | 4.29 | 3.18 | 28.43 | 10.51 | 6.27 | 0.065 | NS |
| Clay (%) | 20.43 | 41.93 | 24.23 | 4.28 | 15.74 | 40.99 | 23.98 | 5.32 | 0.072 | NS |
| BD (g cm–3) | 1.09 | 1.52 | 1.36 | 0.09 | 1.03 | 2.61 | 1.28 | 0.21 | 0.02 | 0.06 |
| PD (g cm–3) | 2.40 | 2.90 | 2.68 | 0.13 | 2.43 | 4.19 | 2.75 | 0.30 | 0.032 | NS |
| MWHC (%) | 35.36 | 76.30 | 41.29 | 8.37 | 24.14 | 60.06 | 51.57 | 7.59 | 0.94 | 2.09 |
| Porosity (%) | 35.88 | 64.09 | 54.90 | 6.13 | 37.95 | 72.53 | 50.73 | 5.68 | 6.28 | NS |
| pH (1:2 soil water ratio) | 4.01 | 638.00 | 5.48 | 0.60 | 3.69 | 6.62 | 5.17 | 0.67 | 0.10 | 0.265 |
| EC (dS m− 1) | 104.86 | 31.62 | 104.86 | 94.67 | 35.90 | 290.6 | 97.5 | 51.82 | 1.25 | 3.68 |
| OC (g kg− 1) | 10.95 | 18.60 | 15.51 | 1.77 | 13.85 | 18.35 | 16.10 | 1.79 | 0.23 | 0.66 |
| N (kg ha− 1) | 211.99 | 496.12 | 377.27 | 53.46 | 218.89 | 489.22 | 388.78 | 50.86 | 5.17 | 14.72 |
| P2O5 (kg ha− 1) | 75.02 | 1253.55 | 358.57 | 214.12 | 132.09 | 1585.0 | 574.13 | 273.8 | 35.02 | 99.89 |
| K2O (kg ha− 1) | 64.68 | 867.91 | 196.49 | 143.18 | 95.65 | 935.15 | 380.84 | 252.05 | 18.08 | 51.56 |
| Exch. Ca [cmol (P) kg− 1] | 1.90 | 11.95 | 5.69 | 2.11 | 2.30 | 15.05 | 6.97 | 2.80 | 0.27 | 0.78 |
| Exch. Mg [cmol (P) kg− 1] | 1.25 | 9.25 | 3.64 | 1.82 | 0.65 | 11.25 | 3.74 | 2.18 | 0.21 | NS |
| Available S (mg kg− 1) | 4.75 | 29.05 | 29.15 | 3.99 | 14.50 | 74.00 | 41.98 | 1.55 | 4.31 | 12.95 |
| Fe (mg kg− 1) | 1.11 | 19.87 | 6.86 | 4.08 | 2.43 | 45.41 | 11.32 | 8.87 | 0.87 | 2.48 |
| Mn (mg kg− 1) | 0.58 | 6.27 | 2.24 | 1.24 | 0.44 | 3.68 | 1.61 | 0.82 | 0.13 | 0.37 |
| Cu (mg kg− 1) | 0.16 | 6.38 | 0.95 | 1.19 | 0.47 | 3.02 | 0.89 | 0.52 | 0.12 | NS |
| Zn (mg kg− 1) | 0.39 | 7.08 | 1.07 | 1.13 | 0.30 | 4.93 | 0.91 | 0.77 | 0.69 | NS |
| Cr (mg kg− 1) | 0.0005 | 0.0075 | 0.003 | 0.0018 | 0.0005 | 0.005 | 0.002 | 0.0011 | 0.0003 | 0.001 |
| Ni (mg kg− 1) | 0.02 | 2.54 | 0.282 | 0.39 | 0.18 | 1.74 | 0.346 | 0.29 | 0.02 | 0.057 |
| Pb (mg kg− 1) | 0.07 | 0.44 | 0.205 | 0.08 | 0.03 | 0.37 | 0.141 | 0.08 | 0.008 | 0.023 |
| Cd (mg kg− 1) | 0.001 | 0.03 | 0.005 | 0.0049 | 0.001 | 0.01 | 0.004 | 0.0025 | 0.0003 | 0.001 |
NS – Non-significant, SD – Standard deviation, BD – bulk density, PD – Particle density, MWHC – Maximum Water Holding Capacity, SE ± m - Standard error of mean, C.D (p = 0.05) – Critical difference.
Signficant values are in bold.
Soil chemical characteristics
Both farming systems had moderately acidic soils, with conventional farming showing a significantly higher pH (5.48) than organic farming (5.17). Electrical conductivity (EC) was also significantly higher in conventional farming (0.21 dS m− 1) compared to organic farming (0.14 dS m− 1) (Table 1). Organic farming showed a slightly higher mean carbon stock (121.15 Mg ha– 1) with less data variation, whereas conventional farming had a lower mean carbon stock and a higher standard deviation (25.25 Mg ha– 1), indicating greater variability among conventional sites. (Fig. 3). Organic farming systems had significantly higher levels of organic carbon (15.51 g kg− 1), available nitrogen (388.78 kg ha-1, phosphorus (574.17 kg ha− 1), and potassium (380.85 kg ha− 1). Additionally, organic farming exhibited significantly higher exchangeable calcium (6.97 cmol (P+) kg− 1), available sulphur (42.15 mg kg− 1), and numerically higher magnesium (3.74 cmol (P+) kg− 1) and micronutrients (Fe, Mn, and B). Conventional farming, conversely, showed higher heavy metal content (Cr, Ni, Pb, and Cd).
Fig. 3.

Mean C stocks of the organic and conventional coffee farming practices.
Soil biological attributes
Soil respiration was found to be much greater in the organic farming system, specifically measuring 45.51 CO2 mg 50 g− 1 compared to the conventional farming system (39.30 CO2 mg 50 g− 1) (Table 2). The average values of the MBC, MBN, urease, dehydrogenase, acid phosphatase and FDA were also found significantly higher in organic farming systems (843.67, 217.74 µg g − 1, 130.39 µg NH4 + -N g− 1 h− 1), 500.24 µg TPF g− 1 24 h− 1, 29.35 µg PNP g− 1 h− 1, and 997.65 µg g− 1, respectively) compared to conventional farming systems (Table 2).
Table 2.
Influence of conventional and organic coffee farming practices on soil biological properties and microbial enumeration in soil.
| Parameters | Conventional coffee farming system | Organic coffee farming system | SE ± m | C.D (p = 0.05) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | Min | Max | Mean | SD | |||
| Soil respiration (mg 50 g-1) | 24.47 | 38.88 | 39.3 | 3.87 | 11.36 | 33.56 | 45.51 | 4.81 | 1.93 | 5.89 |
| SMBC (µg g -1) | 287.30 | 827.73 | 476.75 | 119.04 | 657.46 | 1043.20 | 843.67 | 104.27 | 14.64 | 41.74 |
| SMBN (µg g -1) | 110.70 | 175.8 | 142.17 | 17.57 | 131.25 | 378.03 | 217.74 | 54.37 | 5.46 | 15.48 |
|
UREASE (µg NH4 + N g-1 h-1) |
64.64 | 139.49 | 112.86 | 14.74 | 89.92 | 167.59 | 130.39 | 16.08 | 1.60 | 4.58 |
|
DHA (µg TPF g-1 24 h-1) |
248.30 | 720.89 | 413.84 | 103.50 | 389.86 | 618.57 | 500.24 | 61.85 | 11.25 | 32.08 |
|
APA (µg PNP g-1 h-1) |
14.57 | 42.29 | 24.28 | 6.07 | 22.87 | 36.29 | 29.35 | 3.63 | 0.6 | 1.88 |
| FDA (µg g-1) | 396.58 | 1116.71 | 649.49 | 162.11 | 24.47 | 38.88 | 997.65 | 3.87 | 19.01 | 54.20 |
| Soil microbial population (CFU*104) | ||||||||||
| Bacteria | 69.11 | 64.89 | 69.11 | 2.32 | 92.49 | 82.31 | 92.49 | 6.18 | 0.59 | 1.73 |
| Fungi | 4.60 | 4.20 | 4.60 | 0.22 | 7.49 | 5.21 | 7.49 | 0.77 | 0.09 | 0.25 |
| Actinomycetes | 12.46 | 11.39 | 12.46 | 0.59 | 18.45 | 15.25 | 18.45 | 1.61 | 0.16 | 0.46 |
| Yeast | 15.43 | 14.1 | 15.43 | 0.73 | 38.17 | 25.34 | 38.17 | 4.06 | 0.50 | 1.49 |
| PSM | 2.12 | 1.94 | 2.12 | 0.10 | 4.77 | 3.25 | 4.77 | 0.50 | 0.08 | 0.18 |
| Azotobacter | 1.75 | 1.60 | 1.75 | 0.08 | 2.48 | 2.15 | 2.48 | 0.21 | 0.02 | 0.06 |
| P. fluorescens | 14.92 | 13.64 | 14.92 | 0.71 | 23.84 | 13.25 | 23.84 | 2.93 | 0.356 | 1.03 |
NS – Non-significant, SD – Standard deviation, SMBC – Soil Microbial Biomass Carbon, SMBN - Soil Microbial Biomass Nitrogen, DHA – Dehydrogenase activity, APA – Acid phosphatase activity, FDA - Fluorescein Diacetate Hydrolysis, SE ± m - Standard error of mean, C.D (p = 0.05) – Critical difference.
Soil Microbiological enumeration and microbial diversity indices
Organic coffee farm soils had significantly higher average levels of bacteria, fungi, actinomycetes, and yeast populations (92.49, 7.49, 18.45, and 38.17 CFU×104) compared to conventional coffee farming soils (69.11, 4.60, 12.46, and 15.43 CFU×104) (Table 2). The phosphorus-solubilizing microorganisms, azotobacter, and pseudomonas florescence populations showed a similar pattern. The Shannon-Wiener Index measures species diversity, with higher values indicating greater diversity. For example, an H index of 0 indicates the presence of only one species. Organic farming soils have shown the highest H index at 2.42, surpassing that of conventional farming soils at 2.32 (Fig. 4). In parallel, the Simpson’s Diversity Index, which spans from 0 to 1, measures biodiversity in a specific area, with organic farming soils achieving the peak D index of 0.35 (Fig. 4). Moreover, the reciprocal of the Simpson index (1/D) accounts for species abundance and distribution uniformity, with organic farming soils registering the maximum Shannon evenness index of 0.58 (Fig. 4). The Simpson’s evenness index, derived by dividing the inverse Simpson index by the species count, also recorded higher figures in organic coffee soils at 0.61, in contrast to conventional farming.
Fig. 4.
Box and whisker plots of the Microbial diversity indices as influenced by the organic and conventional coffee practices.
Principal component analysis (PCA)
Principal component analysis scree plots show soil quality evaluation factors. Figure 5 shows that only the two PCs with eigenvalues over one are considered in this analysis. PC 1 had a 14.07 eigenvalue and 82.76% variance. Bulk, MWHC, pH, OC, nitrogen, phosphorus, calcium, magnesium, soil respiration, urease, dehydrogenase MBC and MBN are soil quality parameters. Table 4 shows these parameters had the highest factor loading. PC 2 had an eigenvalue of 2.66, indicating a 15.64% fluctuation. Electrical conductivity, acid phosphatase, and FDA significantly affected this variation (Tables 3 and 4). Thus, these variables were chosen as the minimum data set for the Soil Quality Index for organic soils across plantation depths and ages.
Fig. 4.
Box and whisker plots of the Microbial diversity indices as influenced by the organic and conventional coffee practices.
Table 3.
Eigenvalues from principal component analysis (PCA) of soil quality parameters.
| Principal component | Initial eigenvalues | Weightage factor | ||
|---|---|---|---|---|
| Total | % Variance | Cumulative % | ||
| 1 | 14.07 | 82.76 | 82.76 | 0.84 |
| 2 | 2.66 | 15.64 | 98.41 | 0.16 |
| Total | 1.00 | |||
Singnificant values are in bold.
Table 4.
Principal component analysis of soil quality parameters.
| Component matrix | ||
|---|---|---|
| Parameters | Components | |
| PC1 | PC2 | |
| BD | − 0.981 | 0.118 |
| MWHC | 0.93 | − 0.256 |
| Soil pH | 0.865 | − 0.501 |
| EC | − 0.612 | 0.784 |
| OC | 0.999 | 0.011 |
| Available N | 0.922 | 0.307 |
| Available P2O5 | 0.978 | − 0.199 |
| Available K2O | 0.782 | − 0.569 |
| Exch. Ca | 0.993 | 0.005 |
| Exch. Mg | 0.997 | − 0.059 |
| SR | 0.993 | 0.059 |
| Urease | 0.969 | 0.244 |
| DHA | 0.967 | 0.251 |
| AcP | 0.683 | 0.731 |
| MBN | 0.998 | 0.058 |
| MBC | 0.996 | 0.088 |
| FDA | 0.637 | 0.763 |
| Highest factor (HF) | 0.999 | 0.784 |
| 10% of HF | 0.8991 | 0.7056 |
PC: principal component.
Boldface eigenvalues correspond to the PCs examined for the index. Boldface factor loadings are considered highly weighed; Bold-underlined factors correspond to the indicators included in the index.
Selection of minimum data set (MDS)
For each principal component in the MDS, indicators with weighted loading values within 10% of the highest weighted loading were chosen29. Each variable is a determinant in a principal component analysis with many variables28. Variables were kept in the MDS if their correlation coefficient (r < 0.60). The principal component loading matrix shows that the first principal component used in MDS selection was associated with 13 high-weighted variables within 10% of the highest factor loading (Table 4). Among the 13 variables, “less is better” for bulk density and EC; “optimum is better” for soil pH, water holding capacity, available N, P2O5, K2O, urease activity and soil respiration; and “more is better” for organic carbon, MBN, MBC, DHA, AcP, and FDA activity. These 13 variables had a statistically significant correlation coefficient. As shown in Table 4, the final MDS only included organic carbon in PC 1, the weighted variable EC, acid phosphatase, and FDA were included in MDS in PC2 due to their low correlation Table 4. The more is better approach is used for organic carbon, acid phosphatase, and FDA indicators and less is better approach is used for EC to score the soil quality index. The weighted factor for variables selected under a certain PC was obtained by dividing the amount of variance explained by that PC by the total percentage of variance explained by all PCs with eigenvectors greater than 1, as shown in Table 3. PC 1, with a weight of 0.84, likely explains the majority of the dataset’s variability, while PC 2, with a weight of 0.16, contributes to a smaller portion of the variance.
Soil quality index
The development of the soil quality index involved the conversion of soil properties into numerical scores (Table 5). The soil quality index in organic and conventional coffee plantations was determined using the following key indicators: organic carbon, electrical conductivity, acid phosphatase and Fluorescein diacetate hydrolysis. Organic coffee farming showed a higher Soil Quality Index (0.98) compared to conventional farming (0.87) (Table 6).
Table 5.
Score, weight and soil quality index (SQI) values of selected minimum data set variables under organic and conventional coffee farming system.
| Parameters | OC | EC | AcP | FDA | SQI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | W | T | S | W | T | S | W | T | S | W | T | ||
| Organic | 1.00 | 0.84 | 0.84 | 0.92 | 0.05 | 0.046 | 1.00 | 0.05 | 0.05 | 1.00 | 0.05 | 0.05 | 0.98 |
| Conventional | 0.90 | 0.84 | 0.75 | 1.00 | 0.05 | 0.05 | 0.82 | 0.05 | 0.041 | 0.65 | 0.05 | 0.03 | 0.87 |
W- Weightage factor, S- Score value, T-Total, OC - Organic Carbon, EC - Electrical Conductivity, AcP- Acid Phosphatase, FDA- Fluorescein Diacetate Hydrolysis, SQI-Soil Quality Index.
Signficant values are in bold.
Table 6.
Percent contribution of the soil quality indicators to the soil quality index under different coffee farming practices.
| Parameters | OC | EC | AcP | FDA |
|---|---|---|---|---|
| Organic | 85.71 | 4.69 | 5.10 | 5.10 |
| Conventional | 86.21 | 5.75 | 4.71 | 3.45 |
OC Organic Carbon, EC Electrical Conductivity, AcP Acid Phosphatase, FDA Fluorescein Diacetate Hydrolysis, SQI Soil Quality Index.
Discussion
Soil physical properties
The soil texture was found to be non-significant among both the coffee farming practices due to the inherent soil properties of the study area, which are less influenced by farming practices and more by parent material and topography consistent with previous studies indicating that short- to medium-term management has limited impact on soil texture58. Traditional agricultural systems use extensive tillage, which breaks down soil aggregates, leading to increased erosion and the loss of finer particles such as silt and clay. Conventional farming uses more synthetic fertilizers, which can seep into the soil and increase clay content30. Reduced tillage and organic matter additions in organic farming help maintain soil structure and reduce erosion31,32. Organic farming reduced soil bulk density and increased water-holding capacity and porosity33,34. Organic matter increases soil structure, and water retention, and reduces leaching, runoff, and evapotranspiration, increasing water availability35.
Soil chemical properties
The lower soil pH observed in organic farming systems can perhaps be attributed to the generation of weak organic acids during the decomposition process of organic leftovers that are incorporated into the soil33. The decomposition of organic materials, such as leaves, and plant residues, releases organic acids into the soil, which can lower the pH36. The use of organic fertilizers, particularly those high in ammonium, can contribute to soil acidification as ammonium is converted to nitrate, releasing hydrogen ions (H+)37,38. However, higher EC values were recorded in conventional coffee farming due to the continuous use of synthetic fertilizers and pesticides than in organic farming methods. Practices such as deep ploughing and frequent tillage can disrupt soil structure and reduce the retention of salts and nutrients, contributing to lower EC39. Higher organic carbon, major nutrients in organic coffee farming the persistent application of organic manures increased organic carbon and vital nutrients40. Organic farming involves adding compost, manure, and crop residues, which decompose and contribute to higher SOC levels41. Higher microbial activity in organic farming systems breaks down organic matter, increasing SOC and nutrient availability42. Similarly, exchangeable bases and available S content in organic coffee systems are due to the addition of manure, compost, and leaf litter from shade-grown trees, which releases exchangeable bases. Improved soil structure with organic matter helps retain calcium and magnesium. The regular inorganic fertilizer uses depleted exchangeable Ca and Mg43. Significantly higher micronutrients (Fe, Mn, Zn, Cu, and B) were found in organic farming and heavy metal content (Cr, Ni, Pb and Cd) built up in conventional coffee farming systems due to the continuous addition of different sources of organic matter as well as leaf litter, which may have added micronutrients upon decomposition, whereas in conventional farming systems, long-term continuous use of inorganic fertilizers leads to depletion of micronutrients and also build up heavy metals in the soil. Conventional farming, showed higher heavy metal content (Cr, Ni, Pb, and Cd), likely due to the prolonged use of chemical fertilizers, phosphate-based inputs, and certain pesticides that contain or contribute to heavy metal accumulation59. The study found that conventional crops had the highest metal concentrations in soil, likely due to field practices. Pesticides, including rodenticides, fungicides, insecticides, herbicides, and nematicides, contain heavy metals as active ingredients44. A previous study on Peruvian coffee pesticides showed that none of the 601 pesticides analyzed were present in the organic system, while glyphosate was found in some conventional coffee bean samples45. Additionally, the composition of fertilizer formulations also influences the presence of specific metals in the soil. Consequently, the application of fertilizers has led to persistent increases in Cd, Ni, and Pb levels in the soil46.
Soil biological attributes
Soil respiration, MBC, MBN, urease, dehydrogenase, acid phosphatase and FDA were also found significantly higher in organic farming systems (p < 0.05). Organic matter acts as a nutrient reservoir for soil microbes, which are instrumental in soil respiration. Soils rich in organic matter promote a more diverse and abundant microbial ecosystem47. This increased microbial activity further amplifies soil respiration. Soil microbial biomass, which influences nutrient cycling in the soil, is significantly affected by various production practices, including organic and conventional methods4849. The carbon derived from microbial biomass was the most critical soil microbiological characteristic in differentiating the various management methods50. The presence of microbial biomass nitrogen in organic coffee plots may be linked to the application of nitrogen-rich organic fertilizers51. The presence of increased FDA activity in organic coffee production suggests a greater level of microbiological activity in comparison to the conventional type of coffee cultivation6. Conventional farming practices, which involve chemical fertilizers and pesticides, could potentially disrupt the soil ecosystem and lead to lower FDA activity52.
Soil Microbiological enumeration and microbial diversity indices
Organic coffee farming increases these culturable (bacteria, fungi, actinomycetes, and yeast populations) and beneficial (PSB, azotobacter, and pseudomonas fluorescence) microbial populations by adding soil organic matter to the topsoil, mulching with fruit and coffee residues, and adding leaves, which nourish the rhizosphere and benefit the population52.
Higher Shannon-Wiener Index (H) was found in the soils of organic farming systems compared to conventional farming systems due to the imposition of limitations on the utilization of chemical fertilizers and pesticides within organic fields, together with the implementation of organic fertilizers which in turn increase organic carbon, microbial biomass carbon, and microbial biomass nitrogen can be identified as significant elements that might contribute to the augmentation of microbial diversity53Moreover, the greater diversity of microbial communities in organic soils converts carbon from organic matter into biomass more efficiently, requiring less energy and optimizing resource utilization54.
Soil quality index
The soil quality index is a collection of indicators that yield quantitative data regarding a soil’s capacity to carry out one or more tasks. Organic coffee farming showed a higher Soil Quality Index compared to conventional farming, possibly due to the positive impact of organic management systems, including organic fertilization and the addition of plant residues47 (Table 5)64. found that while tree-based systems improved SQI compared to conventional agriculture in semi-arid India, values rarely exceeded 0.8, and reaching SQI > 0.8 generally requires long-term organic management and minimal disturbance, as supported by recent reviews. The primary cause of differences is synthetic fertilizers and pesticides in conventional farming, which reduce soil biodiversity and negatively affect carbon fixation, nutrient cycling, and disease suppressiveness while organic systems incorporate more organic matter through composting, mulching, and cover cropping, serving as food for soil microorganisms and increasing microbial richness compared to conventional fertilization, enhancing microbial diversity that improve soil structure and water retention which in turn affects the soil quality.
Several soil quality indicators contribute to the soil quality index with soil organic carbon being a key indicator due to its role in nutrient cycling, soil aggregation, and as a nutrient source for microorganisms27. In arid and semi-arid regions, soil salinity is a reliable soil quality indicator as it affects plant growth and soil microbes. The enzyme FDA, produced by proteases, lipases, and esterases, is a reliable measure of soil microbial activity55. Phosphatase activity, closely associated with soil organic matter, organic phosphorus, inorganic phosphorus, and nitrogen availability, is a reliable soil quality predictor56.
Conclusion
Organic coffee farming improves soil fertility, structure, and microbial diversity, leading to enhanced soil health and potentially higher coffee quality. Studies have shown that organic practices, by increasing soil organic matter and biological activity, can positively influence bean composition, resulting in better aroma, flavor, and antioxidant content. The = organic farming systems had significantly higher soil quality indicators, including enhanced soil respiration, microbial biomass, and enzyme activities compared to conventional systems. Organic farming also showed higher levels of essential nutrients (Ca, Mg, S) and micronutrients, while conventional farming had concerning heavy metal accumulation. These findings provide valuable evidence from the Western Ghats, highlighting the positive impact of organic farming on soil health and microbial diversity. Future research should explore soil parameter relationships, long-term ecosystem services, and non-culturable microorganisms, and develop region-specific organic farming guidelines and soil quality monitoring programs for sustainable coffee cultivation. Organic farming can reduce dependence on external inputs, increase production, and protect the environment. These findings suggest that organic farming may support sustainable coffee production while improving soil quality and microbial diversity.
Author contributions
A. A.V. M. - Design of the work, Drafting the article, Data analysis, K.M.R. - Design of the work, revision of the article, Final approval of article; G.K.- Drafting the article, Revision of the article; B.N.- Revision of the article, Data analysis; U.K.S.N. - Data analysis and interpretation; M.M.H - Design of the experimental setup; R.H.B - Data collection; V.P - Data analysis and interpretation; S.B.N. - Data collection; H.T.S - Design of the work.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lokesh, M. N. Organic farming for sustainable agriculture in india: an overview and policy initiatives. Int. J. Creative Res. Thoughts. 11, 499–512 (2023). [Google Scholar]
- 2.Reganold, J. P. Comparison of soil properties as influenced by organic and conventional farming systems. Am. J. Altern. Agric.3, 144–155 (1988). [Google Scholar]
- 3.Tripathi, S., Srivastava, P., Devi, R. S. & Bhadouria, R. Influence of synthetic fertilizers and pesticides on soil health and soil microbiology. In agrochemicals detection, treatment and remediation. (eds Butterworth-Heinemann) 25–54 (2020).
- 4.Wang, W. et al. Consistent responses of the microbial community structure to organic farming along the middle and lower reaches of the Yangtze river. Sci. Rep.6, 35046 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lupwayi, N. Z. et al. Pyrosequencing reveals profiles of soil bacterial communities after 12 years of conservation management on irrigated crop rotations. Appl. Soil. Eco. 121, 65–73 (2017). [Google Scholar]
- 6.Velmourougane, K. Impact of organic and conventional systems of coffee farming on soil properties and culturable microbial diversity. Scientifica21, 1–10 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bijoor, H. Organic coffee: the politically correct coffee. Indian Coffee. 62, 6–8 (1998). [Google Scholar]
- 8.Santos, J. B. D. et al. Soil macrofauna in organic and conventional coffee plantations in Brazil. Biota. Neotrop.18, 101–113 (2018). [Google Scholar]
- 9.Brejda, J. I. et al. Identification of regional soil quality factors and indicators of central and Southern high plains. Soil. Sci. Soc. Am. J.64, 2115–2124 (2000). [Google Scholar]
- 10.Krishnasamy, S. & Thomas, V. Ayngaran Foundation-Organic farming: A sustainable and healthy way of agriculture. Available SSRN 4443835 (2023).
- 11.Bacon, C. W., Palencia, E. R. & Hinton, D. M. Abiotic and biotic plant stress-tolerant and beneficial secondary metabolites produced by endophytic Bacillus species. In Plant Microbes Symbiosis: Applied Facets (Springer, 2015). [Google Scholar]
- 12.Ding, L. et al. Increased microbial functional diversity under long-term organic and integrated fertilization in a paddy soil. Appl. Microb. Biotechnol.102, 1969–1982 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Hartmann, M. et al. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J.9, 1177–1194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao, J., Liang, Y. & Huang, D. Organic farming improves soil microbial abundance and diversity under greenhouse condition: A case study in Shanghai (Eastern China). Sustain10, 3825 (2018). [Google Scholar]
- 15.Kumari, P. et al. Organic and natural farming to boost soil immunity. In Advancements in Microbial Biotechnology for Soil Health 249–293 (Springer Nature, (2024).
- 16.Piper, C. S. Soil and plant analysis. Hans Pub. Bombay, Asian Ed. 368–374 (1966).
- 17.Walkley, A. & Black, C. A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid Titration method. Soil. Sci.37, 29–38 (1934). [Google Scholar]
- 18.Subbaiah, B. V. & Asija, G. L. A rapid procedure for the Estimation of available nitrogen in soils. Curr. Sci.25, 259–260 (1956). [Google Scholar]
- 19.Bray, R. H. & Kurtz, L. T. Determination of total, organic, and available forms of phosphorus in soils. Soil. Sci.59, 39–46 (1945). [Google Scholar]
- 20.Jackson, M. L. Soil chemical analysis. Prentice Hall India Private Ltd.498 (1973).
- 21.Casida, L. E., Klein, D. A. & Santoro, T. Soil dehydrogenase activity. Soil. Sci.98, 371–376 (1964). [Google Scholar]
- 22.Tabatabai, M. A. & Bremner, J. M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil. Bio Biochem.1, 301–307 (1969). [Google Scholar]
- 23.Anderson, J. P. E., Page, A. L., Miller, R. H. & Keeney, D. R. Soil respiration. In (ed Page, A. L.) Methods of Soil Analysis, Part 2 (2nd ed., 831–871). Madison, WI: ASA and SSSA (1982). [Google Scholar]
- 24.Tabatabai, M. A. & Bremner, J. M. Assay of urease activity in soils. Soil. Bio Biochem.4, 479–487 (1972). [Google Scholar]
- 25.Ben-David, A. & Davidson, C. E. Estimation method for serial Dilution experiments. J. Microbiol. Methods. 107, 214–221 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Hill, T. C. J., Walsh, K. A., Harris, J. A. & Moffett, B. F. Using ecological diversity measures with bacterial communities. FEMS Microbio. Eco.. 43, 1–11 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Andrews, S. S., Karlen, D. L. & Cambardella, C. A. The soil management assessment framework: a quantitative soil quality evaluation method. Soil. Sci. Soci Am. J.68, 1945–1962 (2004). [Google Scholar]
- 28.Legaz, B. V. et al. Soil quality, properties, and functions in life cycle assessment: an evaluation of models. J. Clean. Prod.140, 502–515 (2017). [Google Scholar]
- 29.Bhardwaja, A. K., Jasrotia, P., Hamiltona, S. K. & Robertsona, G. P. Ecological management of intensively cropped agro-ecosystems improves soil quality with sustained productivity. Agri Ecol. Envi. 140, 419–429. 10.1016/j.agree.2011.01.005 (2011). [Google Scholar]
- 30.Blanchet, G., Gavazov, K., Bragazza, L. & Sinaj, S. Responses of soil properties and crop yields to different inorganic and organic amendments in a Swiss conventional farming system. Agric. Ecosyst. Environ.230, 116–126 (2016). [Google Scholar]
- 31.Kobierski, M., Lemanowicz, J., Wojewodzki, P. & Kondratowicz-Maciejewska, K. The effect of organic and conventional farming systems with different tillage on soil properties and enzymatic activity. Agron10, 1809 (2020). [Google Scholar]
- 32.Shah, A. N. et al. Soil compaction effects on soil health and crop productivity: an overview. Environ. Sci. Pollut Res.24, 10056–10067 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Sihi, D. et al. Evaluation of soil health in organic vs. conventional farming of basmati rice in North India. J. Pl Nutr. Soil. Sci.180, 389–406 (2017). [Google Scholar]
- 34.Gopinath, K. A. et al. Impact of organic and integrated production systems on yield and seed quality of rainfed crops and on oil properties. Front. Nutr.10, 112–127 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seitz, S. et al. Conservation tillage and organic farming reduce soil erosion. Agron. Sustainable Develop. 39, 1–10 (2019). [Google Scholar]
- 36.Fatima, B. et al. Accompanying effects of sewage sludge and pine needle Biochar with selected organic additives on the soil and plant variables. Waste Manage.153, 197–208 (2022). [DOI] [PubMed] [Google Scholar]
- 37.Raza, S. et al. Inorganic carbon losses by soil acidification jeopardize global efforts on carbon sequestration and climate change mitigation. J. Clean. Prod.315, 128036 (2021). [Google Scholar]
- 38.Alkharabsheh, H. M. et al. Biochar and its broad impacts in soil quality and fertility, nutrient leaching and crop productivity: A review. Agron1, 993 (2021). [Google Scholar]
- 39.Singh, M., Yadav, D. B., Yadav, S., Mor, V. & Poonia, B. S. Potential of conservation agricultural practices on soil quality, carbon sequestration, salinity management and productivity of rainfed areas: conservation agricultural practices on soil quality. J. Soil. Salinity Water Qual.16, 329–344 (2024). [Google Scholar]
- 40.Jurburg, S. D., Shek, K. L. & McGuire, K. Soil microbial composition varies in response to coffee agroecosystem management. FEMS Microbio Eco. 96, 164 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Aulakh, C. S., Sharma, S., Thakur, M. & Kaur, P. A review of the influences of organic farming on soil quality, crop productivity and produce quality. J. Pl Nut. 45, 1884–1905 (2022). [Google Scholar]
- 42.Chowdhury, S. et al. Role of cultural and nutrient management practices in carbon sequestration in agricultural soil. Adv. Agron.166, 131–196 (2021). [Google Scholar]
- 43.Muche, M., Kokeb, A. & Molla, E. Assessing the physicochemical properties of soil under different land use types. J. Environ. Anal. Toxicol.5, 1–10 (2015). [Google Scholar]
- 44.Guadalupe, G. A., García, L., Chavez, S. G. & Doménech, E. Ecological and health risk assessment of metals in organic and conventional Peruvian coffee from a probabilistic approach. Agronomy14, 2817 (2024a). [Google Scholar]
- 45.Guadalupe, G. A., García, L., Quispe-Sánchez, L. & Doménech, E. The fault tree analysis (FTA) to support management decisions on pesticide control in the reception of Peruvian parchment coffee. Food Control. 166, 110729 (2024b). [Google Scholar]
- 46.Rashid, A. et al. Heavy metal contamination in agricultural soil: environmental pollutants affecting crop health. Agronomy13, 1521 (2023). [Google Scholar]
- 47.Gajda, A. M., Czyż, E. A., Furtak, K. & Jończyk, K. Effects of crop production practices on soil characteristics and metabolic diversity of microbial communities under winter wheat. Soil. Res.57, 124–131 (2019). [Google Scholar]
- 48.Araujo, A. S. F. et al. V. B. Microbiological process in agroforestry systems. A review. Agron. Sustain. Develop. 32, 215–226 (2012). [Google Scholar]
- 49.Bhunia, S., Bhowmik, A., Mallick, R. & Mukherjee, J. Agronomic efficiency of animal-derived organic fertilizers and their effects on biology and fertility of soil: A review. Agron11, 823 (2021). [Google Scholar]
- 50.Paudel, D. et al. Elucidating the effects of organic vs. conventional cropping practice and rhizobia inoculation on rhizosphere microbial diversity and yield of peanut. Environ. Microbiome. 18, 60 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh, K. P., Suman, A., Singh, P. N. & Srivastava, T. K. Improving quality of sugarcane-growing soils by organic amendments under subtropical Climatic conditions of India. Bio Fert Soils. 44, 367–376 (2007). [Google Scholar]
- 52.Lori, M. et al. Organic management enhances soil quality and drives microbial community diversity in cocoa production systems. Sci. Total Environ.34, 155–165 (2022). [DOI] [PubMed] [Google Scholar]
- 53.Chaudhary, V., Rehman, A., Mishra, A., Chauhan, P. S. & Nautiyal, C. S. Changes in bacterial community structure of agricultural land due to long-term organic and chemical amendments. Microb. Eco. 64, 450–460 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Roberts, F. S. Measurement of biodiversity: richness and evenness. In (eds Kaper, H. & Roberts, F.) Mathematics of Planet Earth. Mathematics of Planet Earth, vol 5. Springer, Cham. 10.1007/978-3-030-22044-0_8 (2019). [Google Scholar]
- 55.Green, V. S., Stott, D. E. & Diack, M. Assay for fluorescein diacetate hydrolytic activity: optimization for soil samples. Soil. Bio Biochem.38, 693–701 (2006). [Google Scholar]
- 56.Liu, H. et al. Strategic tillage on a grey vertosol after fifteen years of no-till management had no short-term impact on soil properties and agronomic productivity. Geoderma267, 146–155 (2016). [Google Scholar]
- 57.Pradeep, K. et al. Soil quality assessment under different land use systems of Gandhi Krishi vigyana kendra, Bengaluru. Pharma Innov. J.12, 1517–1526 (2023). [Google Scholar]
- 58.Bronick, C. J. & Lal, R. Soil structure and management: a review. Geoderma124, 3–22 (2005). [Google Scholar]
- 59.Sharma, R. K., Agrawal, M. & Marshall, F. M. Heavy metal contamination of soil and vegetables in suburban areas of varanasi, India. Ecotoxicology Environ. Safety, 66, 258–266 . [DOI] [PubMed]
- 60.Le, Q. V. et al. A study of regenerative farming practices and sustainable coffee of ethnic minorities farmers in the central highlands of Vietnam. Front. Sustain. Food Syst.5, 712–733 (2021). [Google Scholar]
- 61.Jones, K. et al. Organic management in coffee: a systematic review of the environmentconomic and social benefits and trade-offs for farmers. Agroecol. Sustain. Food Syst. 1–35 62.Poncet, V., et al. Which diversification trajectories make coffee farming more sustainable? Curr. Opin. Environ. Sustain. 68, 101–432 (2024). (2025).
- 62.Poncet, V. et al. Which diversification trajectories make coffee farming more sustainable? Curr. Opin. Environ. Sustain.68, 101–432 (2024). [Google Scholar]
- 63.Maniraho, E., Nkezabera, J. A., Mupenzi, C. & Nishimwe, G. Soil Quality Indicators Under Conventional and Organic Coffee Farming Systems in Rwandapp.305–311 (International Journal of Plant and Soil Science, 2022).
- 64.Uthappa, A. R. et al. Comparative analysis of soil quality indexing techniques for various tree based land use systems in semi-arid India. Frontiers in Forests and Global Change, 6, p.1322660. (2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.





