Aberrant glycosylation occurs in essentially all types of experimental and human cancers, as has been observed for over 35 years, and many glycosyl epitopes constitute tumor-associated antigens. A long-standing debate is whether aberrant glycosylation is a result or a cause of cancer. Many recent studies indicate that some, if not all, aberrant glycosylation is a result of initial oncogenic transformation, as well as a key event in induction of invasion and metastasis. Glycosylation promoting or inhibiting tumor cell invasion and metastasis is of crucial importance in current cancer research. Nevertheless, this area of study has received little attention from most cell biologists involved in cancer research, mainly because structural and functional concepts of glycosylation in cancer are more difficult to understand than the functional role of certain proteins and their genes in defining cancer cell phenotypes. Glycosylation appears to be considered “in the shade” of more popular topics such as oncogenes and antioncogenes, apoptosis, angiogenesis, growth factor receptors, integrin and caderin function, etc., despite the fact that aberrant glycosylation profoundly affects all of these processes.
The apoptotic signaling triggered by glycosylation presents an immense challenge for future study.
The concept of glycosylation-dependent promotion or inhibition of tumor progression has developed in conjunction with clinicopathological studies. High expression of some glycosyl epitopes promotes invasion and metastasis, leading to shorter 5–10 year survival rates of patients, whereas expression of some other glycosyl epitopes suppresses tumor progression, leading to higher postoperative survival rates (for review see refs. 1 and 38). The former category of epitopes includes β6GlcNAc branching in N-linked structure; sialyl-Tn in O-linked structure; sialyl-Lex, sialyl-Lea, and Ley in either N-linked, O-linked, or lipid-linked structure; GM2, GD3, and sialyl-Gb5 in lipid-linked structure. The latter category includes β4GlcNAc competitive with β6GlcNAc; histo-blood group A and B competitive with sialylated structures including sialyl-Lex and sialyl-Lea; Gb5 competitive with sialyl-Gb5. The expression mechanism of these glycosyl epitopes in terms of status of respective glycosyltransferase genes has been extensively studied (2); however, little is known concerning the mechanisms through which specific glycosyl epitopes induce invasive and metastatic phenotypes of tumor cells.
In this issue of PNAS, Kakugawa et al. (3) report a ganglioside change caused by enhanced expression of ganglioside-specific sialidase “Neu3” in colorectal cancer. Neu3 mRNA level in 32 cases was enhanced 3- to 100-fold compared with nontumor mucosa. Ganglioside sialidase level was also greatly enhanced in these and other cases (total n = 50). Resulting lactosylceramide (Lac-cer) is claimed to inhibit apoptosis, mainly through increased Bcl-2 and decreased caspase expression (Fig. 1, route 1). Although the study is limited to Lac-cer function, a possibility is opened that various desialylated glycosphingolipid (GSL) core structures may affect some yet-unknown process to enhance tumor growth, invasiveness, and metastasis. The results pave the way for new study trends of ganglioside and GSL function in tumor progression.
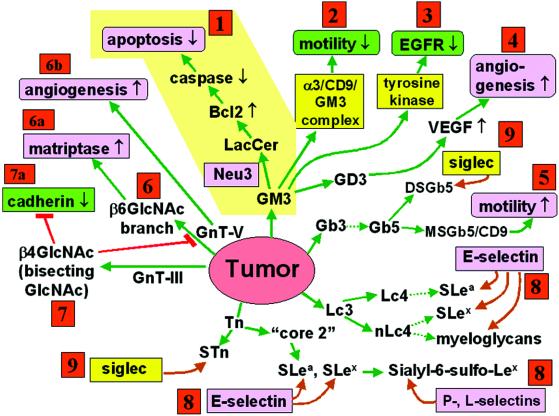
Fig 1.
Glycosylation defining malignancy (invasive and metastatic phenotype of tumors). Tumor cell malignancy is defined by several key phenotypes: apoptosis (route 1), motility (routes 2 and 5), EGF receptor tyrosine kinase (route 3), angiogenesis (routes 4 and 6b), matriptase (matrix-destroying enzyme) activity (route 6a), self-adhesion (through cadherin) (route 7a), adhesion to ECM (through integrin), adhesion to ECs and platelets (through E- or P-selectin) (route 8), adhesion to blood cells and other parenchymatous cells (through siglecs) (route 9). Each phenotype is up- or down-regulated (↑, ↓) by different status of glycosylation as explained in the text and indicated in the figure. Phenotypes with ↑ or ↓ and green color inhibit tumor invasiveness. Those with ↑ or ↓ and pink color promote invasiveness. Glycosyl epitopes capable of binding to specific ligands (pink color without arrow) promote invasiveness. Ligands with yellow color have variable or unclear effect on invasiveness. Note that a given phenotype is produced by different glycosylations, and a given glycosylation produces different phenotypes. Phenotypic changes have cooperative effects on malignancy. For example, GM3 inhibits motility through α3/CD9 complex and also inhibits EGF receptor tyrosine kinase (routes 2 and 3). Reduction of GM3 inhibits apoptosis (route 1), but promotes motility and proliferation (negative route 2 and 3 effect). Essentially all glycosylation pathways catalyzed by multiple glycosyltransferases and their genes are well established (for review see 2). However, the mechanism by which each type of glycosylation affects the various phenotypes remains to be studied. Structures of GSLs are abbreviated according to International Union of Pure and Applied Chemistry–International Union of Biochemistry nomenclature recommendations. S, sialyl; MS, monosialyl; DS, disialyl.
The linkage between Lac-cer expression and inhibition of apoptosis, described by Kakugawa et al. (3) is novel; however, a key point to be clarified is how Lac-cer induces signaling to enhance Bcl-2. Apoptosis was previously found to be associated with Ley expressed in various types of cancer (4), and with Lex in colonic adenocarcinoma HT29 cells caused by enhanced α1 → 3FT (5). Continuous expression of GM3 and N-glycosylation in Chinese hamster ovary mutant ldlD, associated with CD9 and CD82, induces apoptosis (6). Thus, the apoptotic signaling triggered by glycosylation presents an immense challenge for future study.
Kakugawa et al. (3) regard decrease of GM3 and increase of Lac-cer as the major target of Neu3. However, they paid little attention to the cell biological function of GM3, which is regarded as a target of enhanced Neu3 expression. GM3 inhibits cell motility/invasiveness when complexed with CD9 or CD82 (6), which are antimetastatic membrane proteins collectively termed “tetraspanin” (7, 8). In various colorectal (9) and bladder cancer (10) cell lines, GM3 and CD9 are coexpressed, and inhibit Matrigel- or laminin-5-dependent cell motility through α3 integrin/CD9/GM3 complex in glycolipid-enriched microdomain (11) (Fig. 1, route 2). However, a different ganglioside monosialo-Gb5, complexed with CD9 in breast cancer MCF7 cells, strongly enhances motility/invasiveness (39) (Fig. 1, route 5).
Neu3 may enhance malignancy through reduction of the motility-inhibitory effect of GM3/CD9. Similar considerations may apply to the inhibitory effect of GM3 on epidermal growth factor (EGF) receptor tyrosine kinase (e.g., ref. 12) (Fig. 1, route 3). There have been many studies on effect of gangliosides on various growth factor receptor tyrosine kinases (for review see ref. 13). Tyrosine kinases in growth factor receptors, particularly EGF, are a current target of anticancer therapy (14); therefore, effects of gangliosides and N-glycosylation on EGF receptor (15) are of central importance.
GD3 may promote tumor cell motility and growth, possibly through angiogenesis and vascular endothelial growth factor (VEGF) production, as evidenced by antisense approach using GD3 synthase gene (16) (Fig. 1, route 4). GM3 in mouse ependymoblastoma tumor cell line EPEN inhibits tumor growth and angiogenesis, whereas induction of GM2, GM1, and GD1a synthesis through β4GalNAc-T transfection leads to higher vascularization and enhanced VEGF production (17).
Perhaps the most widely occurring glycosylation change inducing malignancy is enhanced β6GlcNAc side chain branching of N-linked structure, caused by enhanced activity of GnT-V (18) (Fig. 1, route 6), and counteracting β4GlcNAc (bisecting GlcNAc) synthesized by GnT-III (Fig. 1, route 7). The level of both epitopes is determined by the balance between GnT-V and GnT-III (19). This topic was elaborated by cloning of genes for these two enzymes. Up-regulation of GnT-V gene, promoted by oncogene family Ets (20, 21), leads to enhanced expression of β6GlcNAc branching, whereas GnT-III gene reduces such branching. Enhanced GnT-III gene inhibits β6GlcNAc branching, leading to suppression of metastasis, in B16 melanoma (22). In this process, one of the targets appears to be E-cadherin, in which enhanced β4GlcNAc reduces β6GlcNAc branching, leading to enhanced cadherin-dependent cell-to-cell adhesion and consequent suppression of metastasis (23) (Fig. 1, route 7a). Thus, GnT-V displays prometastatic effect, whereas GnT-III is antimetastatic. The prometastatic effect of GnT-V is ascribable to stabilization of active matriptase by addition of β6GlcNAc side chain (24) (Fig. 1, route 6a). Surprisingly, the secreted form of GnT-V per se is angiogenic, which also promotes metastasis (25) (Fig. 1, route 6b).
Another series of in-depth studies of prometastatic glycosylation deals with SLex and SLea, both of which have been identified as tumor-associated antigens and as E-selectin epitopes, and may promote adhesion of tumor cells to endothelial cells. Clinically, expression of SLex (26) and SLea (27) is inversely correlated with postoperative survival rate of patients. Experimentally, human lung adenocarcinoma cell lines showing higher metastatic potential in nude mice express long-chain SLex defined by mAb FH6 (28). The importance of SLex carried by long-chain polyLacNAc in inducing metastasis was demonstrated in Lex-expressing B16/F1 melanoma cells transfected with α1 → 3 FucT-III gene (29), and in a few colorectal cancer cell lines reactive with FH6 and showing preferential adhesion to hepatocytes (30). On the other hand, SLex defined by IgG3 mAb SNH4, which reacts with SLex regardless of carrier chain, is expressed highly “just ahead of the invasive front” of colorectal cancer (31). Thus, the same SLex epitope carried by different carbohydrate chains produces different adhesive and/or invasive properties of tumor cells.
Most human cancer cell lines do not bind to P-selectin unless P-selectin glycoprotein ligand-1 (PSGL-1) gene is transfected (32). Sialyl-6-sulfo-Lex, identified as l-selectin ligand (33), also binds to E- and P-selectin (34). Therefore, cancer cells expressing sialyl-6-sulfo-Lex, without PSGL-1, may display P-selectin-dependent adhesion (Fig. 1, route 8).
Many types of disialogangliosides and sialylated O- or N-linked glycans are targets of a variety of siglecs with different specificities (35, 36) (Fig. 1, route 9). The tumor biological significance of siglec-dependent binding of tumor cells to target cells remains to be elucidated.
Finally, I will emphasize one unique property of GSLs directly involved in cell adhesion through GSL-to-GSL interaction between counterpart microdomains organized with signal transducers. Many tumor-associated GSL antigens belong to this category, and form “glycosynapses”. Tumor progression is associated with overexpression of defined types of GSLs involved in GSL-dependent adhesion of tumor cells to target cells, which activates signal transducers to enhance motility and invasiveness (37).
As suggested by Kakugawa et al. (3), Neu3 inhibitors could be useful in development of antitumor drugs. Similarly, inhibitors of various other processes described above, such as GnT-V, selectin epitopes, selectin per se, and specific GSLs, could be realistic targets for development of antimetastatic reagents in the future.
See companion article on page 10718.
References
- 1.Hakomori S. (1996) Cancer Res. 56, 5309-5318. [PubMed] [Google Scholar]
- 2.Taniguchi N., Honke, K. & Fukuda, M., (2002) Handbook of Glycosyltransferases and their Related Genes (Springer, Tokyo).
- 3.Kakugawa Y., Wada, T., Yamaguchi, K., Yamanami, H., Ouchi, K., Sato, I. & Miyagi, T. (2002) Proc. Natl. Acad. Sci. USA 99, 10718-10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiraishi K., Suzuki, K., Hakomori, S. & Adachi, M. (1993) Glycobiology 3, 381-390. [DOI] [PubMed] [Google Scholar]
- 5.Akamatsu S., Yazawa, S., Zenita, K., Matsumoto, H., Tachikawa, T. & Kannagi, R. (1996) Glycoconj. J. 13, 1021-1029. [DOI] [PubMed] [Google Scholar]
- 6.Ono M., Handa, K., Withers, D. A. & Hakomori, S. (1999) Cancer Res. 59, 2335-2339. [PubMed] [Google Scholar]
- 7.Cajot J.-F., Sordat, I., Silvestre, T. & Sordat, B. (1997) Cancer Res. 57, 2593-2597. [PubMed] [Google Scholar]
- 8.Adachi M., Taki, T., Ieki, Y., Huang, C., Higashiyama, M. & Miyake, M. (1996) Cancer Res. 56, 1751-1755. [PubMed] [Google Scholar]
- 9.Ono M., Handa, K., Sonnino, S., Withers, D. A., Nagai, H. & Hakomori, S. (2001) Biochemistry 40, 6414-6421. [DOI] [PubMed] [Google Scholar]
- 10.Satoh M., Ito, A., Nojiri, H., Handa, K., Numahata, K., Ohyama, C., Saito, S., Hoshi, S. & Hakomori, S. (2001) Int. J. Oncol. 19, 723-731. [PubMed] [Google Scholar]
- 11.Kawakami, Y., Kawakami, K., Steelant, W. F. A., Ono, M., Baek, R. C., Handa, K., Withers, D. A. & Hakomori, S. (2002) J. Biol. Chem., in press. [DOI] [PubMed]
- 12.Bremer E. G., Schlessinger, J. & Hakomori, S. (1986) J. Biol. Chem. 261, 2434-2440. [PubMed] [Google Scholar]
- 13.Yates A. J. & Rampersaud, A. (1998) in Sphingolipids as Signaling Modulators in the Nervous System, eds. Ledeen, R. W., Hakomori, S., Yates, A. J., Schneider, J. S. & Yu, R. K. (Ann. N.Y. Acad. Sci., New York), Vol. 845, pp. 57–71. [Google Scholar]
- 14.Steinberg D. (2002) The Scientist 16, 29. [Google Scholar]
- 15.Wang X.-Q., Sun, P., O'Gorman, M., Tai, T. & Paller, A. S. (2001) Glycobiology 11, 515-522. [DOI] [PubMed] [Google Scholar]
- 16.Zeng G., Gao, L., Birkle, S. & Yu, R. K. (2000) Cancer Res. 60, 6670-6676. [PubMed] [Google Scholar]
- 17.Manfredi M. G., Lim, S., Claffey, K. P. & Seyfried, T. N. (1999) Cancer Res. 59, 5392-5397. [PubMed] [Google Scholar]
- 18.Pierce M., Buckhaults, P., Chen, L. & Fregien, N. (1997) Glycoconj. J. 14, 623-630. [DOI] [PubMed] [Google Scholar]
- 19.Schachter H. (1986) Biochem. Cell Biol. 64, 163-181. [DOI] [PubMed] [Google Scholar]
- 20.Kang R., Saito, H., Ihara, Y., Miyoshi, E., Koyama, N., Sheng, Y. & Taniguchi, N. (1996) J. Biol. Chem. 271, 26706-26712. [DOI] [PubMed] [Google Scholar]
- 21.Buckhaults P., Chen, L., Freigen, N. & Pierce, M. (1997) J. Biol. Chem. 271, 19575-19581. [DOI] [PubMed] [Google Scholar]
- 22.Yoshimura M., Nishikawa, A., Ihara, Y., Taniguchi, S. & Taniguchi, N. (1995) Proc. Natl. Acad. Sci. USA 92, 8754-8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshimura M., Ihara, Y., Matsuzawa, Y. & Taniguchi, N. (1996) J. Biol. Chem. 271, 13811-13815. [DOI] [PubMed] [Google Scholar]
- 24.Ihara S., Miyoshi, E., Ko, J. H., Murata, K., Nakahara, S., Honke, K., Dickson, R. B., Lin, C.-Y. & Taniguchi, N. (2002) J. Biol. Chem. 277, 16960-16967. [DOI] [PubMed] [Google Scholar]
- 25.Saito T., Miyoshi, E., Sasai, K., Nakano, N., Eguchi, Y., Honke, K. & Taniguchi, N. (2002) J. Biol. Chem. 277, 17002-17008. [DOI] [PubMed] [Google Scholar]
- 26.Nakamori S., Kameyama, M., Imaoka, S., Furukawa, H., Ishikawa, O., Sasaki, Y., Kabuto, T., Iwanaga, T., Matsushita, Y. & Irimura, T. (1993) Cancer Res. 53, 3632-3637. [PubMed] [Google Scholar]
- 27.Kannagi R. (1997) Glycoconj. J. 14, 577-584. [DOI] [PubMed] [Google Scholar]
- 28.Inufusa H., Kojima, N., Yasutomi, M. & Hakomori, S. (1991) Clin. Exp. Metastasis 9, 245-257. [DOI] [PubMed] [Google Scholar]
- 29.Ohyama C., Tsuboi, S. & Fukuda, M. (1999) EMBO J. 18, 1516-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ota M., Takamura, N. & Irimura, T. (2000) Cancer Res. 60, 5261-5268. [PubMed] [Google Scholar]
- 31.Ono M., Sakamoto, M., Ino, Y., Moriya, Y., Sugihara, K., Muto, T. & Hirohashi, S. (1996) Cancer 78, 1179-1186. [DOI] [PubMed] [Google Scholar]
- 32.Handa K., White, T., Ito, K., Fang, H., Wang, S. & Hakomori, S. (1995) Int. J. Oncol. 6, 773-781. [DOI] [PubMed] [Google Scholar]
- 33.Mitsuoka C., Sawada-Kasugai, M., Ando-Furui, K., Izawa, M., Nakanishi, H., Nakamura, S., Ishida, H., Kiso, M. & Kannagi, R. (1998) J. Biol. Chem. 273, 11225-11233. [DOI] [PubMed] [Google Scholar]
- 34.Ohmori K., Kanda, K., Mitsuoka, C., Kanamori, A., Kurata-Miura, K., Sasaki, K., Nishi, T., Tamatani, T. & Kannagi, R. (2000) Biochem. Biophys. Res. Commun. 278, 90-96. [DOI] [PubMed] [Google Scholar]
- 35.Brinkman-Van der Linden E. C. & Varki, A. (2000) J. Biol. Chem. 275, 8625-8632. [DOI] [PubMed] [Google Scholar]
- 36.Crocker P. R. & Varki, A. (2001) Immunology 103, 137-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hakomori S. (2002) Proc. Natl. Acad. Sci. USA 99, 225-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muramatsu T. (1993) Glycobiology 3, 294-296. [DOI] [PubMed] [Google Scholar]
- 39.Steelant, W. F., Kawakami, Y., Ito, A., Handa, K., Bruyneel, E. A., Mareel, M. & Hakomori, S. (2002) FEBS Lett., in press. [DOI] [PubMed]