Abstract
Individual, strongly electroluminescent Agn molecules (n = 2∼8 atoms) have been electrically written within otherwise nonemissive silver oxide films. Exhibiting characteristic single-molecule behavior, these individual room-temperature molecules exhibit extreme electroluminescence enhancements (>104 vs. bulk and dc excitation on a per molecule basis) when excited with specific ac frequencies. Occurring through field extraction of electrons with subsequent reinjection and radiative recombination, single-molecule electroluminescence is enhanced by a general mechanism that avoids slow bulk material response. Thus, while we detail strong electroluminescence from single, highly fluorescent Agn molecules, this mechanism also yields strong ac-excited electroluminescence from similarly prepared, but otherwise nonemissive, individual Cu nanoclusters.
As device dimensions continue to decrease, developing new low-voltage nanoscale electroluminescent materials is crucial to future optoelectronics (1). While many materials are electroluminescent, most are limited by inefficient charge injection, transport, and subsequent electron–hole recombination to produce photons in the desired spectral region. Recent advances in device fabrication have enabled efficient charge injection and transport to the electroluminescent species (2), but the broad recombination zone, differing speeds of electron and hole transport, and the bulk material response or dielectricity limit excitation rate (3, 4). While also limited by disadvantageous spin statistics (5), slow material response and broad recombination zones limit overall performance. If made electroluminescent, single molecules, with their small size and environment-specific properties, would, themselves, provide very narrow recombination zones without slow bulk dielectric responses. Because material behavior often differs on nanometer dimensions relative to bulk materials because of increased importance of quantum confinement and surface and environmental effects (6), such single-molecule electroluminescent materials are likely to exhibit properties that do not average to yield overall bulk behavior.
While nanomaterials have been created with unique optical properties, single-molecule optical methods rely on laser-induced emission (7–16) and have been relegated to the study of materials without the veil of bulk averaging. While finding utility within electronic devices (17, 18) and even as electrically excited single-photon sources at cryogenic temperatures (19), the useful properties of individual molecules have not, to date, been used to create novel optical or electroluminescent materials with behavior different from that in bulk. Recently, we reported photoactivated fluorescence from individual Agn nanoclusters (n = 2∼8 atoms) (20), which have been observed and calculated to absorb and emit strongly throughout the visible spectrum (21–24). Here we report that these same Agn molecules can be electrically created, thus demonstrating that it is possible to prepare room-temperature electroluminescent single molecules. Generated in situ within silver oxide films, these electrode-spanning individual Agn molecules simultaneously conduct current and emit electroluminescence under dc excitation. Because of the much narrower recombination zone in electrode-spanning single Agn molecules than in crossed nanowires (25) or in cryogenic single-quantum dot devices (19), this single-molecule electroluminescence enables extreme electroluminescence enhancement (>104) at specific ac frequencies relative to dc excitation. By applying these same methods to nonfluorescent Cu samples, we have produced and also strongly enhanced Cu nanocluster electroluminescence from materials that are otherwise nonemissive both in bulk and on the single-molecule level. Thus, harnessing single molecules in electroluminescent devices may provide a general method of circumventing low bulk electroluminescence efficiencies.
Three types of silver oxide samples were used in our studies (20, 26), ranging in thickness from 15 to 80 nm. Initially conductive and nonelectroluminescent, all silver oxide films were activated by passing ∼1 A (at ∼5 V) through the film for several seconds at room temperature, resulting in a dramatic increase in resistance from ∼5 Ω to ∼106 Ω. This electrogenerated “discolored” region surrounds an extremely narrow chemical boundary that provides essentially all of the 1-MΩ resistance and is the site of all electroluminescent species (Fig. 1A). This electrically discolored region shows optical and x-ray photoelectron spectroscopic signatures characteristic of AgO films, (20, 26, 27) but remains an excellent conductor except at the site of electroluminescence. The increased resistivity and resulting electroluminescence do not arise in AgO films that are only heated in an oven without application of high currents.
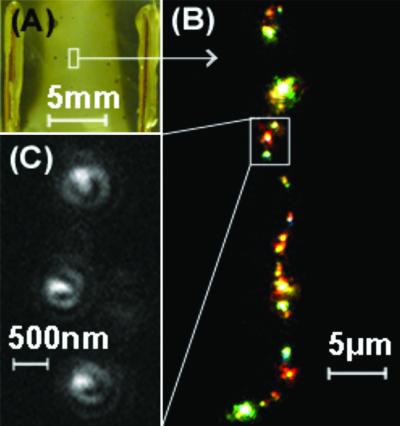
Fig 1.
(A) Discolored AgO region between copper electrodes on a glass substrate in vacuum [∼10−5 torr (1 torr = 133 Pa)]. dc potentials of 9 V were applied across the film. (B) Multicolored electroluminescence from single Agn (n = 2 to ∼8) molecules occurs within the electrically discolored region. (C) These features exhibit dipole emission patterns and blinking dynamics (not shown) characteristic of single-molecule behavior.
All such discolored silver oxide films produce dynamic, multicolored electroluminescence from many diffraction-limited sites along this single resistive line perpendicular to the applied field (Fig. 1B). For a given applied field, each electroluminescent spot can produce any of many different colors spanning the visible spectrum. At all applied dc voltages (3.5–15 V), all diffraction-limited spots show both slow, high duty cycle blinking and dipole emission patterns (12) characteristic of single molecules (Fig. 1C). The similarity to photoactivated single silver nanocluster fluorescence (20, 28) indicates that both fluorescence and electroluminescence occur from individual Agn molecules. Since each Agn molecule has discrete electronic energy levels defined by its size and geometry (21–24), radiative electron–hole pair recombination occurs only at the Ag nanoclusters within the discolored AgO regions of the films. This narrow recombination zone leads to extremely low film dielectricity, except at the individual electroluminescent molecules.
No electroluminescence or nonohmic current was observed for applied dc voltages less than ∼3.5 V. As applied voltage is increased above this value, a nonmonotonic increase of electroluminescence intensity occurs, exhibiting several clearly resolved peaks independent of electrode spacing (Fig. 2A). Corresponding sharp increases are also observed in the voltage-dependent current flow, indicating conduction through the emissive Agn nanoclusters that must span the gap between the two silver/silver oxide electrodes (Fig. 2B) (29). As dc voltage is increased to 15 V, film resistance increases to >20 MΩ, as individual emissive sites “burn out” from Joule heating, yielding overall spectral shifts to higher frequency (Fig. 2C). This process results in peaks instead of plateaus in both current flow (30) and total electroluminescence. High applied voltages force large currents to flow through this interface that lead to bright single-molecule emission. Such conditions, however, melt and restructure the interface, causing short-lived and unstable, blinking single molecules. The independence of emission and conductivity on polarity and ground level suggest that electroluminescence results from a field-dependent electron extraction (hole injection) and subsequent electron reinjection and radiative recombination. Since the electrode-spanning Agn features are likely approximately 2–8 atoms, or subnanometer in size, the low voltages applied here can readily produce the very large fields necessary for extraction of electrons (∼109 V/m) (30).
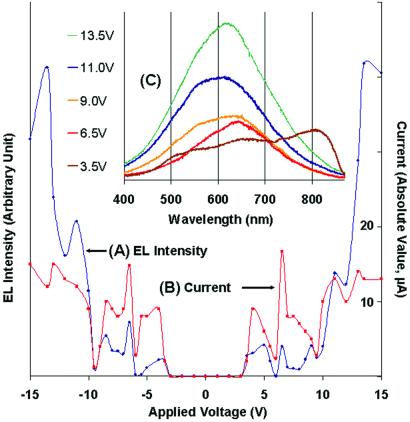
Fig 2.
Typical voltage-dependent electroluminescence (EL) intensity from (A) and concomitant absolute value of current flow through (B) all species within the 100-μm field of view. Peaks in the emission intensity vs. applied voltage curve result from preferential electron extraction from and subsequent reinjection into subsets of specifically sized and shaped Agn molecules. The absolute voltages at which these peaks occur depend slightly on discoloration, with electroluminescence onset occurring between 3.5 and 5 V. These processes result in the correlated conduction and subsequent electron–hole recombination within the Ag nanoclusters. Independent of field polarity and ground level, this must be field-induced conduction and electroluminescence. (C) Electroluminescence spectra (30-s charge-coupled device exposures, 0.15-m monochrometer) corresponding to selected data points in A and B. As applied dc voltage is increased, lower energy features burn out because of Joule heating resulting in peaks in A and B and corresponding spectral shifts to higher frequencies.
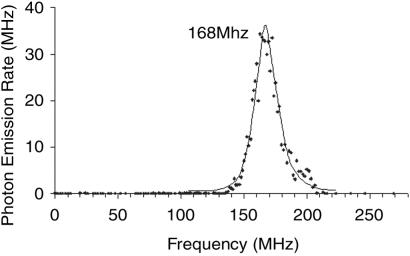
Low-frequency ac excitation also produces electroluminescence, but only when amplitudes similar to those in dc experiments are used. At very high frequencies (>150 MHz), however, >104-fold enhancements of the total single-molecule electroluminescence are observed (Fig. 3). Correspondingly, electroluminescence is observed at much lower voltages and with much greater stability than obtainable with dc excitation. Since electron extraction depends only on electric field strength, enhancement is unlikely to arise from electrons being more efficiently extracted at these specific frequencies. The field-dependent electron extraction must produce a hole that quickly decays thermally. Therefore, to enhance electroluminescence, electrons must be quickly reinjected to facilitate electron–hole recombination before the metastable hole thermally decays. While several differently sized and shaped Agn nanoclusters are present, a strong electroluminescence excitation enhancement peak is observed at 168 MHz with a linewidth of 26 MHz (full width at half maximum). Unlike its intensity, the position and shape of this peak are independent of ac amplitude. The maximum frequency suggests that an electron should be reinjected within ∼3.0 ns for maximum enhancement, whereas the linewidth suggests ∼13-ns total electroluminescence lifetime of the Agn. Effectively an average single-molecule electroluminescence excitation-enhancement spectrum, this peak results from the enhancement of many single-molecule emitters. For each Agn nanocluster, the linewidth results from a combination of electron extraction, thermal decay, reinjection, and recombination processes. Using time-correlated single-photon counting, we measured the emissive lifetime to be <1.5 ns, which was limited by the falling edge of the excitation pulse. Thus, the majority of the linewidth results from electron extraction, thermal decay, and reinjection within each of the differently sized Agn molecules along the heterogeneous interface.
Fig 3.
Typical electroluminescence intensity of ∼100 single Agn molecules within a 20-μm-length luminescent region vs. sinusoidal excitation frequency (amplitude: ±2.5 V).
Because of the low electrode dielectricity and the extremely narrow recombination zone produced by single molecules spanning the two conductive electrodes, electron extraction and subsequent reinjection can occur at very high frequencies. Therefore, a window of high ac excitation frequencies within which the excitation period is long enough to facilitate hole injection but short enough to minimize thermal decay of holes before electron reinjection and recombination results in greater than four orders of magnitude electroluminescence enhancements on the single-molecule level. Because hole decay is so fast, only spin-allowed transitions have radiative lifetimes short enough to emit. Thus, while all possible spin states should form, those states with radiative lifetimes longer than the thermal lifetime of the injected hole will rapidly decay nonradiatively. This mechanism enables spin-allowed transitions to emit and be quickly regenerated without waiting for long-lived spin-forbidden transitions to occur, thereby leading to an overall enhancement of electroluminescence over dc excitation by the ratio of spin-forbidden to spin-allowed transition lifetimes.
To compensate for the fast hole decay with dc and low-frequency ac excitation, applied voltages must be increased to provide sufficient current flow to increase the possibility of injecting electrons within the lifetime of the injected hole. This constraint leads to the observed increased thermal decay and film destruction under high-voltage dc excitation (31). Under ac excitation, however, single molecules can be cycled and strong emission observed with greatly reduced blinking for well over 5 hr under continuous operation in vacuum, corresponding to >1010 photons from individual molecules. This electroluminescence excitation method should be generally applicable to systems with highly conductive electrodes and recombination zones of molecular dimensions. In such geometries, charge injection is not limited by material dielectricity. Therefore, on the single-molecule level, electroluminescence can be greatly enhanced relative to bulk materials, as long as injected hole lifetimes are comparable to or longer than recombination lifetimes. This is likely a general scheme for electroluminescence enhancement with great applications in molecular electronics and nanotechnology.
To illustrate the generality of single-molecule electroluminescence enhancement, we examined the optical and electroluminescent behavior of similarly discolored, slightly oxidized thin (∼15-nm) copper films. While we have been unable to observe fluorescence from single Cun nanoclusters, we have observed their electroluminescence, but only when excited with high dc voltages or at extremely high ac frequencies. Thus, individual copper nanoclusters can be made to be strongly electroluminescent with enhancements over dc excitation similar to that in silver (>104). Whereas the copper and silver nanocluster electroluminescence mechanisms are likely identical, the behavior is not. The maximum enhancement frequency for Cu (180 MHz) is higher than for silver, and the linewidth is similar (25 MHz full width at half maximum), indicating that charge needs to be injected more quickly to enhance electroluminescence. While copper and silver have similar chemical properties and are stable with respect to ionization and charge injection, the generality of this mechanism should also enable other molecules that are nonelectroluminescent in bulk to electroluminesce on the single-molecule level. The key conditions are that individual molecules must span highly conducting electrodes (e.g., Au, Ag, Cu), thereby having an extremely narrow recombination zone and low overall dielectricity until the sample is reached, have ionized lifetimes comparable to radiative lifetimes, and be somewhat stable relative to ionization (requiring organic molecules to operate in ultrahigh vacuum conditions). While this mechanism does not avoid excited state population with disadvantageous spin statistics (5), it does prevent molecules from residing in long-lived excited states. This enables higher rates of radiative spin-allowed transitions, thereby greatly enhancing electroluminescence intensities at very low applied voltages.
This room-temperature observation of single-molecule electroluminescence has resulted in in situ generation of nanoscale emissive species, the total emission from which can be increased significantly through ac excitation on the single-molecule level. The field-dependent electron extraction likely creates an cationic nanocluster that, when subsequent reinjection and electron–hole recombination occur quickly enough, produces an electroluminescence enhancement mechanism that is likely applicable to a wide array of single-molecule-based optoelectronics systems. Because the injected hole lifetime is comparable to the fluorescence lifetime but short relative to the radiative lifetimes of spin-forbidden transitions, great enhancements of electroluminescence (>104) are observed relative to both dc single-molecule excitation and dc- or ac-generated bulk electroluminescence on a per molecule basis.
Acknowledgments
We gratefully acknowledge experimental assistance from L. A. Peyser and A. P. Bartko, loan of equipment from L. A. Lyon, and funding from the A. P. Sloan Foundation.
References
- 1.Rogers J. A., Bao, Z. N. & Dhar, L. (1998) Appl. Phys. Lett. 73, 294-296. [Google Scholar]
- 2.Bao Z., Rogers, J. A., Dodabalapur, A., Lovinger, A. J., Katz, H. E., Raju, V. R., Peng, Z. & Galvin, M. E. (1999) Opt. Mater. 12, 177-182. [Google Scholar]
- 3.Pinner D. J., Friend, R. H. & Tessler, N. (1999) J. Appl. Phys. 86, 5116-5130. [Google Scholar]
- 4.Braun D., Moses, D., Zhang, C. & Heeger, A. J. (1992) Appl. Phys. Lett. 61, 3092-3094. [Google Scholar]
- 5.Burin A. L. & Ratner, M. A. (1998) J. Chem. Phys. 109, 6092-6102. [Google Scholar]
- 6.Nirmal M. & Brus, L. (1999) Acc. Chem. Res. 32, 407-414. [Google Scholar]
- 7.Moerner W. E. & Orrit, M. (1999) Science 283, 1670-1676. [DOI] [PubMed] [Google Scholar]
- 8.Lounis B. & Moerner, W. E. (2000) Nature (London) 407, 491-493. [DOI] [PubMed] [Google Scholar]
- 9.Fourkas J. T. (2001) Opt. Lett. 26, 211-213. [DOI] [PubMed] [Google Scholar]
- 10.VandenBout D. A., Yip, W. T., Hu, D. H., Fu, D. K., Swager, T. M. & Barbara, P. F. (1997) Science 277, 1074-1077. [Google Scholar]
- 11.Macklin J. J., Trautman, J. K., Harris, T. D. & Brus, L. E. (1996) Science 272, 255-258. [Google Scholar]
- 12.Bartko A. P. & Dickson, R. M. (1999) J. Phys. Chem. B 103, 11237-11241. [Google Scholar]
- 13.Nie S. M. & Emory, S. R. (1997) Science 275, 1102-1106. [DOI] [PubMed] [Google Scholar]
- 14.Kneipp K., Wang, Y., Kneipp, H., Perelman, L. T., Itzkan, I., Dasari, R. & Feld, M. S. (1997) Phys. Rev. Lett. 78, 1667-1670. [DOI] [PubMed] [Google Scholar]
- 15.Ha T., Glass, J., Enderle, T., Chemla, D. S. & Weiss, S. (1998) Phys. Rev. Lett. 80, 2093-2096. [Google Scholar]
- 16.Michaels A. M., Nirmal, M. & Brus, L. E. (1999) J. Am. Chem. Soc. 121, 9932-9939. [Google Scholar]
- 17.Schon J. H., Meng, H. & Bao, Z. N. (2001) Science 294, 2138-2140. [DOI] [PubMed] [Google Scholar]
- 18.Klein D. L., Roth, R., Lim, A. K. L., Alivisatos, A. P. & McEuen, P. L. (1997) Nature (London) 389, 699-701. [Google Scholar]
- 19.Yuan Z., Kardynal, B. E., Stevenson, R. M., Shields, A. J., Lobo, C. J., Cooper, K., Beattie, N. S., Ritchie, D. A. & Pepper, M. (2002) Science 295, 102-105. [DOI] [PubMed] [Google Scholar]
- 20.Peyser L. A., Vinson, A. E., Bartko, A. P. & Dickson, R. M. (2001) Science 291, 103-106. [DOI] [PubMed] [Google Scholar]
- 21.Bonacic-Koutecky V., Pittner, J., Boiron, M. & Fantucci, P. (1999) J. Chem. Phys. 110, 3876-3886. [Google Scholar]
- 22.Yoon J., Kim, K. S. & Baeck, K. K. (2000) J. Chem. Phys. 112, 9335-9342. [Google Scholar]
- 23.Harbich W., Fedrigo, S., Meyer, F., Lindsay, D. M., Lignieres, J., Rivoal, J. C. & Kreisle, D. (1990) J. Chem. Phys. 93, 8535-8543. [Google Scholar]
- 24.Konig L., Rabin, I., Schulze, W. & Ertl, G. (1996) Science 274, 1353-1355. [DOI] [PubMed] [Google Scholar]
- 25.Duan X. F., Huang, Y., Cui, Y., Wang, J. F. & Lieber, C. M. (2001) Nature (London) 409, 66-69. [DOI] [PubMed] [Google Scholar]
- 26.Varkey A. J. & Fort, A. F. (1993) Sol. Energy Mater. Sol. Cells 29, 253-259. [Google Scholar]
- 27.Hoflund G. B., Hazos, Z. F. & Salaita, G. N. (2000) Phys. Rev. B 62, 11126-11133. [Google Scholar]
- 28.Mihalcea C., Buchel, D., Atoda, N. & Tominaga, J. (2001) J. Am. Chem. Soc. 123, 7172-7173. [DOI] [PubMed] [Google Scholar]
- 29.Reed M. A., Zhou, C., Muller, C. J., Brugin, T. P. & Tour, J. M. (1997) Science 278, 252-254. [Google Scholar]
- 30.Driskill-Smith A. A. G., Hasko, D. G. & Ahmed, H. (1997) Appl. Phys. Lett. 71, 3159-3161. [Google Scholar]
- 31.Tessler N., Harrison, N. T., Thomas, D. S. & Friend, R. H. (1998) Appl. Phys. Lett. 73, 732-734. [Google Scholar]