Abstract
The subtilisin-like proprotein convertases PC1/3 (SPC3) and PC2 (SPC2) are believed to be the major endoproteolytic processing enzymes of the regulated secretory pathway. They are expressed together or separately in neuroendocrine cells throughout the brain and dispersed endocrine system in both vertebrates and invertebrates. Disruption of the gene-encoding mouse PC1/3 has now been accomplished and results in a syndrome of severe postnatal growth impairment and multiple defects in processing many hormone precursors, including hypothalamic growth hormone-releasing hormone (GHRH), pituitary proopiomelanocortin to adrenocorticotropic hormone, islet proinsulin to insulin and intestinal proglucagon to glucagon-like peptide-1 and -2. Mice lacking PC1/3 are normal at birth, but fail to grow normally and are about 60% of normal size at 10 weeks. They lack mature GHRH, have low pituitary growth hormone (GH) and hepatic insulin-like growth factor-1 mRNA levels and resemble phenotypically the “little” mouse (Gaylinn, B. D., Dealmeida, V. I., Lyons, C. E., Jr., Wu, K. C., Mayo, K. E. & Thorner, M. O. (1999) Endocrinology 140, 5066–5074) that has a mutant GHRH receptor. Despite a severe defect in pituitary proopiomelanocortin processing to mature adrenocorticotropic hormone, blood corticosterone levels are essentially normal. There is marked hyperproinsulinemia but without impairment of glucose tolerance. In contrast, PC2-null mice lack mature glucagon and are chronically hypoglycemic (Furuta, M., Yano, H., Zhou, A., Rouille, Y., Holst, J., Carroll, R., Ravazzola, M., Orci, L., Furuta, H. & Steiner, D. (1997) Proc. Natl. Acad. Sci. USA 94, 6646–6651). The PC1/3-null mice differ from a human subject reported with compound heterozygosity for defects in this gene, who was of normal stature but markedly obese from early life. The PC1/3-null mice are not obese. The basis for these phenotypic differences is an interesting topic for further study. These findings prove the importance of PC1/3 as a key neuroendocrine convertase.
Many bioactive peptides are generated through limited proteolytic digestion (processing) of precursors. Recent research has revealed that a majority of these processing events is mediated by a family of subtilisin-like proprotein convertases (the SPCs or PCs), which are serine endoproteases related structurally to the yeast-processing endoprotease kexin (1, 2). Seven members of this family, furin, PC1/3, PC2, PC4, PACE4, PC5/6 and PC7, have been identified. All possess well conserved pro-, catalytic, and downstream P-domains and a more variable C-terminal domain that determines their specific intracellular localization (1). Two of the convertases, PC1/3 and PC2, are expressed principally in the brain and neuroendocrine (NE) system where they play a major role in peptide hormone and neuropeptide precursor processing within the dense core vesicles of the regulated secretory pathway. The processing of many NE precursors, including proinsulin, proglucagon, and proopiomelanocortin (POMC), are mediated mainly, if not exclusively, by PC1/3 and PC2 through limited endoproteolysis, usually at paired basic amino acid sites (1). Although PC1/3 and PC2 are structurally similar and often functionally overlap, each has definite substrate site preferences. Combined with differences in their expression pattern in NE tissues, these convertases may process the same precursor protein to release different products having divergent or even opposing functions. An excellent example is proglucagon, which is processed by PC2 in the islet alpha cells to release mainly glucagon, whereas in the intestinal L cells, it is believed to be processed by PC1/3 to release glucagon-like peptide (GLP)-1 and GLP-2 (3, 4). In the pituitary, the conversion of POMC to adrenocorticotropic hormone (ACTH) in the anterior lobe corticotrophins appears to be mediated mainly by PC1/3 acting alone, whereas PC2 acts together with PC1/3 to cleave ACTH further into α-melanocyte-stimulating hormone (α-MSH) and corticotropin-like intermediate peptide (CLIP) in the intermediate lobe (5, 6). In the pancreatic islets of Langerhans, both PC1/3 and PC2 are expressed in the beta cells and are each capable of processing proinsulin to insulin (7). Normally, however, PC1/3 cleaves preferentially after the R31,32 doublet in the B-chain/C-peptide junction and thus facilitates the second maturation cleavage by PC2 at the C-peptide/A-chain junction, its preferred site (8).
Mice lacking PC2 activity develop normally and are fertile, but exhibit a variety of NE processing abnormalities in the brain and islets (38). Proinsulin levels in pancreatic extracts are elevated to 35% of total insulin-like material (normal level <5%). Pulse-chase studies of insulin biosynthesis indicate the impaired maturation of proinsulin accompanied by significant accumulations of des-31,32-proinsulin intermediates in the pancreatic islets of the PC2 nulls, as expected (7). In the islet alpha cells, proglucagon processing is completely blocked, and only glucagon precursors are detectable in the circulation in the PC2 nulls (9). The conversion of prosomatostatin to somatostatin-14 also is blocked both in the delta cells (38) and in the brain (G. Chiu and D.F.S., unpublished data). Other defects in the PC2 nulls include lack of production of α-MSH associated with accumulation of ACTH in the pituitary intermediate lobe (10). In the brain, there is impaired or altered processing of several neuropeptides, including melanin concentrating hormone and neuropeptide EI (11), neurotensin (12, 13), and many of the opioid peptides, including enkephalins, dynorphins, and endorphins (6, 14–16).
Although PC1/3 also has been under extensive biochemical, cell biological, and genetic study for some time, a comprehensive assessment of its actions in vivo is lacking. A female human subject with compound heterozygosity for inactivating PC1/3 mutations has been identified (17, 18). Her symptoms include severe obesity, amenorrhea, and postprandial hypoglycemia. Further studies revealed the presence of multiple endocrine defects, including low-serum estradiol, follicle-stimulating hormone (FSH) and luteinizing hormone (lutropin LH), and very high circulating levels of proinsulin and ACTH precursors (POMC and/or intermediates; ref. 17). However, detailed studies of the involvement of PC1/3 in the biosynthesis of many other important neuropeptides under physiological conditions is not feasible without an animal model. In this study, we have successfully created a mouse line completely lacking PC1/3 by deleting a portion of the PC1/3 promoter and the first exon.
Materials and Methods
RIA of Hypothalamic Growth Hormone-Releasing Hormone (GHRH) and Insulin-Like Growth Factor-1 (IGF-1).
A rat IGF-1 RIA kit (DSL-2900, Diagnostic Systems Laboratories, Webster, TX) was used for mouse serum IGF-1 assays. The cross-reactivity of mouse IGF-1 is 75%. Sera were extracted and assayed according to manufacturer's instructions.
Hypothalamic GHRH Assay.
Hypothalamic peptides from wild-type (wt), heterozygous, and null animals of 8 weeks of age were extracted and assayed, as described (19, 20). The final dilution of antiserum (kindly provided by L. Frohman, Univ. of Illinois, Chicago) was 1:50,000, and 6,000 cpm tracer was used in each tube.
Analysis of Whole Pituitary ACTH by RIA.
Pituitaries from PC1/3 wt and null animals were collected and individually homogenized via sonication in 150 μl of ice-cold 5 N acetic acid with 2 mg/ml BSA. The samples were size-fractionated, as described (21). Aliquots (10 μl) of each fraction were subjected in duplicate to RIA by using the anti-ACTH antiserum “Kathy.” The final dilution of the antibody was 1:8,000.
Pituitary POMC-Derived Peptide Biosynthesis.
Pituitary metabolic-labeling experiments were performed according to procedures described (21). An antiserum (JH93) which recognizes the N-terminal region of ACTH, as well as larger forms, was used for the immunoprecipitation. The immunoprecipitated materials then were resolved on borate-SDS/PAGE tube gels. These gels then were sliced, and the radioactivity in each slice was determined by liquid scintillation counting (6, 21).
Glucose Tolerance Tests.
Nine wt, 11 heterozygous, and 8 PC1/3 knockout mice of 16 weeks of age were fasted for 5 h. Two milligrams of D-glucose per gram of body weight were injected i.p. Blood samples were taken from the tail at 1, 30, 60, and 120 min after injection, and the glucose content was estimated by using a Precision Q.I.D. glucose meter from MediScience (Bedford, MA).
Gel Filtration and RIA of Intestinal Tracts.
Acid ethanol extracts of small intestine were prepared as described (22) and resolved by gel filtration over a K16/100 G50 Sephadex (fine) column (Amersham Pharmacia) in a neutral buffer (50 mM sodium phosphate/0.6 mM thimerosol/10 mM EDTA/0.1 M NaCl/0.1% human serum albumin, pH 7.5). The elution positions (Kd) for GLP-17–37 and GLP-2 were established with standards. Fractions were assayed by RIA by using rabbit polyclonal antibodies as described (23–25).
Results
PC1/3 Expression Is Abolished in Homozygous-Null Mice.
The targeting strategy we used (see Text, which is published as supporting information on the PNAS web site, www.pnas.org) resulted in the deletion of exon 1 and several putative upstream transcriptional control elements (CRE, ICS, GHF-1, AP-1, and Sp1) from the PC1/3 (PCSK1) gene (26). Homozygous PC1/3-null offspring were obtained by mating heterozygous male and female siblings (Fig. 6B, which is published as supporting information on the PNAS web site). Expression of PC1/3 was eliminated in all of the homozygous nulls, as determined by Northern (Fig. 7A, which is published as supporting information on the PNAS web site) as well as Western analysis (Fig. 7B). No phenotypic differences were observed among the three PC1/3-null strains that were generated.
PC1/3-Null Mice Exhibit a Severe Growth Defect.
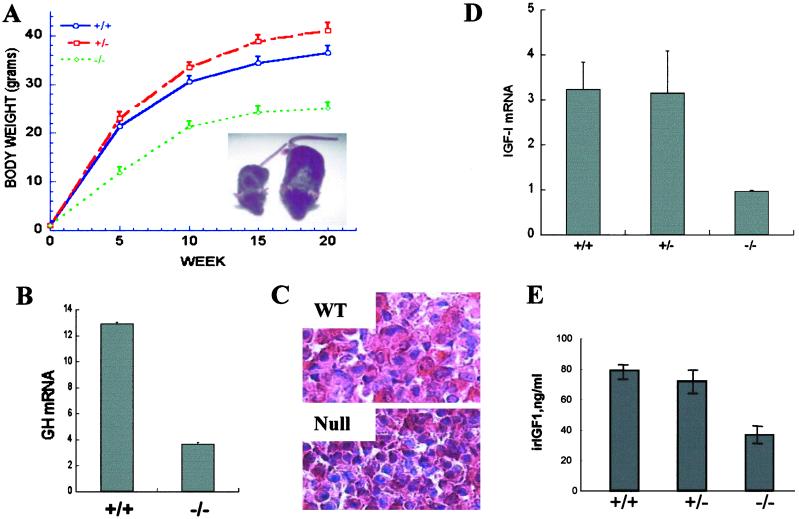
Of 17 litters examined, there were 130 live births in total. Genotyping revealed 37 (+/+), 72 (+/−), and only 21 (−/−) pups. The non-Mendelian ratio indicates some degree of prenatal lethality of the nulls. Furthermore, only 7 of these 21 PC1/3 nulls survived beyond 7 days. At birth, there was no apparent difference among pups of a litter. However, by day three nulls were readily distinguishable from wt and heterozygous littermates by their smaller overall size. Their body weights were about 60% of wt and heterozygous littermates by week 6 (Fig. 1A), and growth retardation persisted into adulthood. Because postnatal growth depends on GH, we measured pituitary GH mRNA and found it to be significantly reduced to 25–30% of normal (Fig. 1B). Immunoreactive GH was present in anterior pituitary (AP) somatotrophs, but the cells appeared shrunken and inactive, suggesting a lack of stimulation (Fig. 1C). These findings are similar to those in a mouse line lit/lit, the little mouse (27), which has a missense mutation in the GHRH receptor that prevents GHRH binding that results in reduced GH secretion and somatotroph hypoplasia in the pituitary (28, 29). GH is normally released in a pulsatile fashion, and the level oscillates by severalfold, depending on circadian rhythm and physiological conditions. However, its release can be indirectly monitored by measuring circulating IGF-1 levels, which are relatively stable (30). Because the liver is a major source of circulating IGF-1, we studied hepatic IGF-1 expression first. Northern blot analysis showed a twofold decrease in liver IGF mRNA in the null mice (Fig. 1D). The serum IGF-1 level also was decreased significantly in the null mice, as determined by RIA (Fig. 1E). Based on these observations, we suspected that defective GHRH production might be related to the growth retardation in the null mice.
Fig 1.
PC1/3-null mice exhibit growth retardation and display abnormal growth-related hormone levels. (A) The growth curve of PC1/3-null mice (18 −/−, 20 +/−, and 17 +/+ males were measured). (Inset) One example of body size difference between wt (Left) and null mice at 6 weeks of age. (B) GH mRNA level is decreased in null-mouse pituitary. Northern blot analysis of pituitary GH mRNA was quantified by PhosphorImager (Cyclone, Packard). The loading was normalized against GAPDH. n = 3. (C) GH staining in anterior pituitary lobe reveals smaller somatotrophs with shrunken nuclei in nulls (magnification: ×100). (D) Liver IGF-1 mRNA is decreased in PC1/3-null mice. A Northern blot was analyzed as described in B. n = 2. (E) Circulating IGF-1 is decreased in null mice. n = 3.
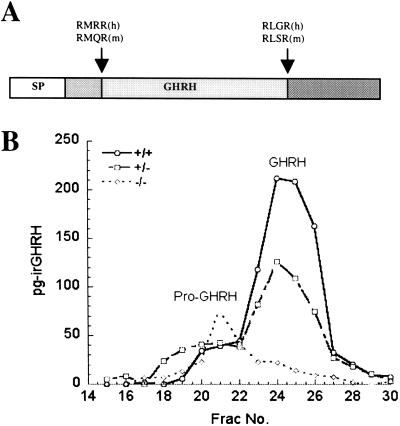
GHRH is normally synthesized in the hypothalamus as a precursor (proGHRH) that needs to be processed to exert its full biological function (ref. 31; Fig. 2A). Northern blot analysis of hypothalamic RNA revealed a significant increase (160% of control) in GHRH mRNA (data not shown), as has been noted also in the little mouse (31). To determine whether proGHRH processing was hindered in null mice, we prepared hypothalamic extracts and fractionated them by gel exclusion chromatography over a P-30 Biogel column. RIA of the fractions (Fig. 2B) showed that mature GHRH was very low or undetectable in the null animals, whereas earlier-eluting higher molecular weight material was increased, indicating that normal processing of proGHRH is blocked.
Fig 2.
GH-releasing hormone processing is impaired in PC1/3-null mice. (A) Schematic representation of proGHRH (29). Arrowheads indicate sites of proteolytic processing, which are essential for the conversion of proGHRH to mature GHRH. (B) Gel filtration profiles demonstrate shift of GHRH immunoreactivity to the position of proGHRH in PC1/3-null mice.
Impaired Processing of POMC Results in ACTH Deficiency.
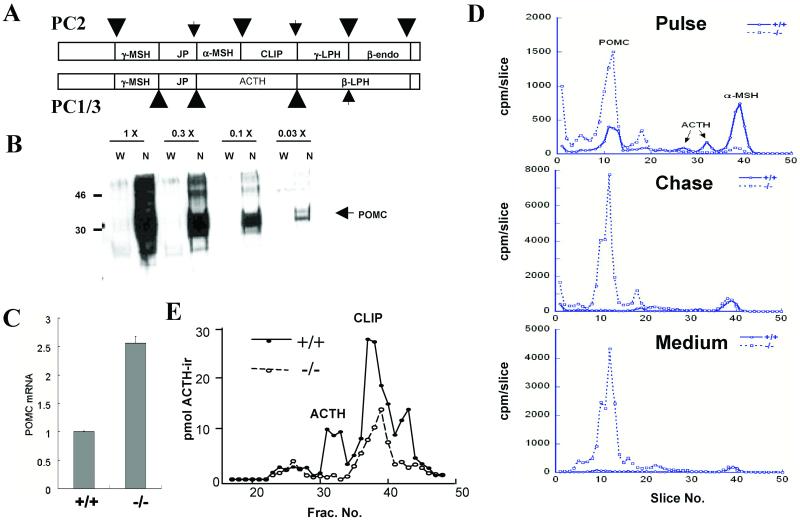
ACTH is produced in anterior pituitary corticotrophs by processing of its precursor, POMC. Previous studies have suggested that PC1/3 is the major convertase in these cells, and that it is responsible for the generation of ACTH, while in the pituitary intermediate lobe (IL), both PC1/3 and PC2 are expressed with POMC, and this results in further cleavages to generate large amounts of β-endorphin and to break ACTH into two fragments, α-MSH and CLIP (refs. 5 and 6; Fig. 3A). To determine whether POMC processing is compromised in PC1/3 nulls, we first examined levels of POMC and its mRNA in whole pituitaries. These studies revealed a dramatic increase in the level of unprocessed POMC (Fig. 3B) and up-regulation of POMC mRNA in the pituitary (Fig. 3C). To assess the processing of POMC in greater detail, we examined ACTH biosynthesis by metabolic labeling. Anterior pituitaries were first incubated with [35S]methionine for 2 h and then for an additional 2 h in medium containing unlabeled methionine. Cells and media were extracted, immunoprecipitated, and resolved on borate-SDS tube gels, as described in Materials and Methods. These results revealed a severe block in POMC processing and the absence of mature ACTH in cells or media from the null pituitaries (Fig. 3D). The absence of ACTH was confirmed by RIA of extracts of whole pituitaries resolved by gel permeation chromatography (Fig. 3E). Despite the lack of normal-sized ACTH, measurements of plasma corticosterone revealed no significant differences in the null animals (data not shown).
Fig 3.
POMC processing is impaired in PC1/3-null mice. (A) Schematic representation of POMC processing by PC1/3 and PC2 (5 and 6). Partially processed sites are indicated by small arrows. JP, joining peptide; LPH, lipotropin; endo, endorphin; MSH, melanocyte-stimulating hormone. (B) Western blot shows elevated whole pituitary POMC level in PC1/3-null mice. Samples are arranged in successive three-fold serial dilution (L→R), starting with 0.4 pituitary equivalents (1×). W, wild type; N, null. (C) POMC mRNA is up-regulated in pituitaries of PC1/3-null mice. Samples were prepared and analyzed as in Fig. 1B, n = 3. (D) ACTH is absent in PC1/3-null mice. Anterior pituitaries were labeled as described in Materials and Methods. Samples were immunoprecipitated with antiserum JH93 and size-separated by using a borate-SDS/PAGE tube-gel system. The gels were sliced, and the radioactivity in each slice was determined by scintillation counting. (E) ACTH RIA of whole pituitary extracts resolved by gel permeation chromatography. Antiserum specific to the C-terminal portion of ACTH was used to estimate levels of ACTH-ir peptides in PC1/3-null and wt pituitaries. No intact ACTH was observed in the PC1/3 null; this product is normally generated by PC1/3 in the anterior lobe. However, PC1/3 nulls contain a smaller ACTH-derived peptide (CLIP), arising mainly from PC2 action in the pituitary intermediate lobe.
Processing of Proinsulin Is Severely Impaired in PC1/3 Nulls.
Both PC1/3 and PC2 are expressed in the islet beta cells, whereas only PC2 is expressed in the islet non-beta endocrine cells. The PC2-null mice exhibited significant elevations of proinsulin to about 35% of total insulin-related immunoreactivity in pancreatic extracts (7). In contrast, the PC3-null pancreas extracts contained a much greater proportion of proinsulin-like material (Fig. 8, which is published as supporting information on the PNAS web site). Proinsulin and intermediate forms made up over 85% of total immunoreactivity, whereas mature insulin was less than 15% of total. Interestingly, PC1/3 heterozygotes also exhibited a significant elevation of the pancreatic proinsulin level by more than twofold compared with wt extracts (12.8 vs. 5.3%).
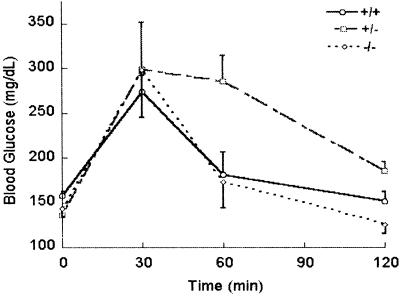
To assess the physiological effects of the severe block in proinsulin processing, we examined the glucose tolerance of the PC1/3 nulls in mice 16 weeks of age. The results showed that the fasting blood sugar level, as well as the rise in response to glucose injection in the homozygous nulls, was very similar to that of wt mice (Fig. 4). Interestingly, the glycemic response of the heterozygous animals was significantly prolonged compared with the nulls and wts. Because the heterozygous nulls tended to be mildly obese, this result may reflect a mild degree of insulin resistance. However, none of these animals has developed diabetes.
Fig 4.
Intraperitoneal glucose tolerance test. The tests were performed on fasted animals 16 weeks of age as described in Materials and Methods.
Impaired Proglucagon Processing in the Intestinal Tract.
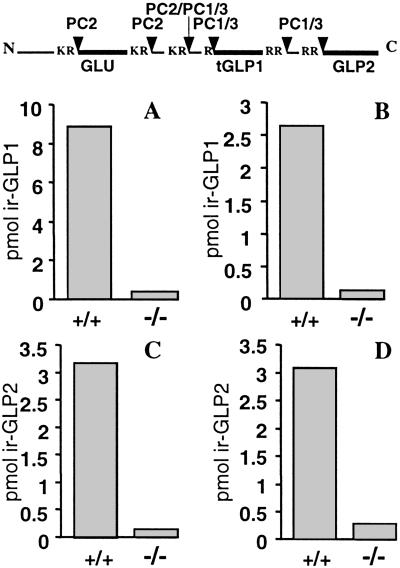
Recent research has indicated that proglucagon is processed differently in the intestinal neuroendocrine L cells to give rise to GLP-1 and GLP-2 (3, 4). It has been proposed that PC1/3 may play an essential role in this aspect of the differential processing of proglucagon, in contrast to the role of PC2 in generating glucagon from this precursor in the islet alpha cells (Fig. 5 Top). Accordingly, we have prepared extracts of small intestine in wt and PC1/3-null animals and have measured the levels of GLP-1 and GLP-2 after gel chromatography by using a variety of antisera (Fig. 5 A–D). These results indicate the existence of a severe block in the production of both peptides and are thus consistent with the proposed role of PC1/3 as the essential convertase in the L cells.
Fig 5.
GLP-1 and GLP-2 are not detectable in the PC1/3-null mice. (Top) Schematic of proglucagon indicating sites of processing by PC2 and PC1/3 (1, 3, 4). Samples from wt and null mice small intestines were extracted and fractionated. GLP-1 and GLP-2 in each fraction were determined by RIA. The readings from GLP-containing fractions were pooled as depicted in the bar graphs. GLP-1 immunoreactivity was measured by using (A) antiserum 2135, an antiserum that measures all GLP-1-containing forms, or (B) antiserum 93242, which is specific for the NH2 terminus of GLP7–37 (tGLP) and reacts minimally with N-terminally extended forms of GLP-1. GLP-2 immunoreactivity was measured by using (C) antiserum 312–01, which measures all GLP-2-containing forms, or (D) antiserum 92160, which is specific for N-terminal GLP-2 and reacts minimally with N-terminally extended or truncated forms of GLP-2.
GLP-2 has been suggested to play a role in normal intestinal growth and functional maintenance (32). Of interest here may be our observation that the PC1/3-null animals suffer from chronic mild diarrhea associated with bulky, moist stools. However, histologic examination of the gastrointestinal tract has not revealed any gross abnormalities, nor are there any signs of underlying chronic infection or an inflammatory process in the mucosa. These findings may be consistent with proposed inhibitory effects of GLP-1 and GLP-2 on intestinal motility (33).
Discussion
A number of convertase genes have now been disrupted with varying consequences. Disruption of the genes encoding the constitutive pathway convertases furin and PACE4 are both embryonic lethals because of the lack of maturation of developmentally important growth factors (34, 35), whereas disruption of PC7 has no apparent phenotype (13). PC4 disruption leads to defective processing of proPACAP in testis and may result in reduced fertility (36, 37).
Because of the widespread expression of PC2 and PC1/3 throughout the brain and neuroendocrine system, it was anticipated initially that disruption of either of these genes also might present problems because of embryonic lethality. However, lethality was not seen in the PC2-nulls (38). Moreover, the identification of a 43-year-old human subject lacking PC1/3 caused by compound heterozygosity for two inactivating mutations in the PCSK1 gene argued against embryonic lethality (17, 18). It has been suggested that her survival through development might have been the consequence of the presence of a small but significant amount of enzymatic activity in one of the two mutant forms of PC1/3 in this individual. Although this possibility remains to be completely excluded, it now seems less likely to be of any significance in the light of the findings reported here that the complete absence of the PC1/3 protein is compatible with normal development and birth, although with a substantial degree of prenatal and perinatal mortality.
PC1/3-null mice appear normal at birth and exhibit no discernable developmental abnormalities. But, unlike the human subject described above, they almost immediately begin to lag in growth and are significantly, but proportionately, reduced in size by the age of 6 weeks. Such growth failure does not appear to be caused by malnourishment. Reducing the litter size does not promote growth or survival. The growth defect they exhibit is most typical of that caused by the lack of GH, as has been shown both clinically in humans and also in several mouse strains lacking GH (39, 40). One of the most interesting and relevant of these mutant mice is the “little” mouse, which was described originally at The Jackson Laboratories by Beamer and Eicher (27). Like the “little” mouse, PC1/3-null mice were able to grow significantly during pregnancy (data not shown), indicating both their retention of responsiveness to pregnancy growth hormones as well as their relatively normal fertility. The (lit/lit) strain exhibits a pattern of disturbed postnatal growth that has been traced to a point mutation in the GHRH receptor that prevents ligand binding (28, 29). This G protein-coupled receptor is expressed in the anterior pituitary somatotrophs where it normally transmits growth signals from the hypothalamus via GHRH (29, 31). Several pieces of evidence pointed to a similar defect in GH secretion in the PC1/3 nulls, including elevated levels of GHRH mRNA in hypothalamus and significantly reduced levels of circulating IGF-1 and of hepatic IGF-1 mRNA. And, whereas the anterior pituitary in the PC1/3 nulls did not show the severe depletion of somatotrophs as seen in the “little” mouse (29), the somatotrophs were smaller in size, with shrunken nuclei suggestive of inactivity. In accord with this finding, GH mRNA was significantly reduced. Finally, direct examination of hypothalamic extracts resolved by gel chromatography confirmed the existence of a severe block in GHRH processing. Although GHRH processing was not examined in the human subject with PC1/3 defects, circulating GH levels were somewhat subnormal (17). Of interest is a sequence difference between human and mouse proGHRH in the N-terminal cleavage site, in which the P2 residue in the human is Arg, whereas in the mouse it is Gln (see Fig. 2A). This difference likely impairs cleavage at this site, which is critical for activation of GHRH (41). Because PC1/3 has been shown to process RXXR-type sequences in the regulated secretory pathway (42, 43), it is likely that GHRH production is more dependent on PC1/3 in the mouse as opposed to the human subject. However, there could also be species differences in convertase expression in GHRH-producing cells. Further studies should help to evaluate the importance of PC1/3 for this cleavage and its effect on the activity of GHRH.
The PC1/3-null mutation also disrupts the normal hypothalamic-pituitary-adrenal axis by preventing the maturation of POMC to ACTH in the anterior pituitary corticotrophins that control adrenal cortical secretion of corticosterone. The absence of PC1/3 disturbs POMC processing in both anterior and intermediate pituitary lobes, leading to striking accumulations of unprocessed POMC in anterior lobe and to a lesser extent in IL (not shown), and to significant up-regulation of POMC mRNA. In the anterior lobe, some α-MSH is evident in the pulse-chase studies (Fig. 3D). However, much of this may be caused by contamination with small amounts of IL tissue containing high levels of PC2, which cleaves ACTH into α-MSH/CLIP (see Fig. 3A), as confirmed in separate IL-labeling studies (data not shown). However, although no mature ACTH is seen in anterior or intermediate pituitary lobes in the PC1/3 nulls, blood corticosterone levels are nonetheless maintained at normal levels. This is also paralleled in the human subject, where circulating cortisone levels were normal but accompanied by large compensatory increases in circulating ACTH precursors (POMC or intermediates). It is likely that some larger ACTH-containing intermediates, especially those with cleaved ACTH N-termini, retain some corticotropic activity. In contrast, POMC-null mice lack adrenal tissue and have no detectable adrenal steroids (44).
The severe block in proinsulin processing caused by PC1/3 deficiency is described in greater detail in the accompanying paper (45). Its existence in both mouse and man demonstrates that PC1/3 is of greater importance in proinsulin processing than PC2. This finding arises in part from the fact that PC1/3 cleaves preferentially at the B-chain/C-peptide junction, creating an intermediate product, des-31,32 proinsulin, that is a better substrate for PC2 cleavage at the A chain junction than is intact proinsulin (8). Thus, the two convertases work sequentially to process proinsulin to insulin efficiently, much as PC1/3 and PC2 cooperate in producing α-MSH from POMC in the intermediate lobe of the pituitary.
In contrast, the differential processing of proglucagon to either glucagon in islet alpha cells or GLP-1 and GLP-2 in the intestinal L cells relies on the independent expression and action of each convertase. Thus, PC2 cleaves proglucagon to release only glucagon (9), whereas the results reported here demonstrate that production of GLP-1 and GLP-2 in the intestinal tract depends completely on PC1/3. The lack of GLP-1 would be expected to result in the loss of its “incretin” action to promote the secretion of insulin in response to orally ingested food (46). Disruption of the gene encoding the GLP-1 receptor impairs this response significantly, but does not result in diabetes (47). The normal glucose tolerance of the PC1/3 mice in response to i.p. injected glucose indicates that the extreme hyperproinsulinemia (45) does not impair glucose homeostasis. Interestingly, the heterozygotes exhibit moderate obesity and a more prolonged rise in glucose during the glucose tolerance test, suggesting the possible existence of mild insulin resistance. The basis of these metabolic differences in the (+/-) mice will require further studies to differentiate factors such as increased food intake vs. decreased activity/energy expenditure. Severe early-onset obesity was seen in the human PC1/3-deficient subject but was not evident in our null mice, emphasizing the importance of genetic background on such complex multifactorial phenotypes.
In conclusion, the results of these studies strongly support the proposed role of PC1/3 as a key NE convertase. PC1/3 and/or PC2 now account for the normal endoproteolytic processing in vivo of proinsulin, proglucagon, prosomatostatin, propancreatic peptide, POMC, proGHRH, and the neuropeptide melanin-concentrating hormone, NEI, neurotensin, and several opioid peptides (7, 9–16, 38). Assessment of the putative role in vivo of other convertases, such as PC5/6A (48), will require animal models lacking their expression. Further studies of the PC1/3 and PC2-null mice to examine the processing of other hypothalamic-releasing hormones and additional neuropeptides and gut hormones may reveal still other functions of these convertases.
Supplementary Material
Acknowledgments
We thank Gary Thomas for helpful review and advice, Dick Mains for POMC antibodies, Lawrence Frohman and William Wehrenberg for mouse GHRH antibodies, Kelly Mayo for mouse GHRH cDNA, Linda Degenstein for embryo injections, Jingquan Lan for assistance with immunohistochemistry, Barbara Danielle and Meghan Moriarty for assistance with mouse breeding and care, and Rosie Ricks for expert assistance in preparing this manuscript. This work was supported by National Institutes of Health Grants DK20595 (Diabetes Research and Training Center) and DK13914, Howard Hughes Medical Institute Grants DK49703 and DA05084 (to I.L.), the Danish Medical Research Council, and the Novo Nordisk Foundation (to J.H.).
Abbreviations
PC, proprotein convertase
NE, neuroendocrine
POMC, proopiomelanocortin
ACTH, adrenocorticotropic hormone
α-MSH, melanocyte-stimulating hormone
GLP, glucagon-like peptide
GHRH, growth hormone-releasing hormone
IGF, insulin-like growth factor
GH, growth hormone
wt, wild type
IL, pituitary intermediate lobe
References
- 1.Zhou A., Webb, G., Zhu, X. & Steiner, D. F. (1999) J. Biol. Chem. 274, 20745-20748. [DOI] [PubMed] [Google Scholar]
- 2.Muller L. & Lindberg, I., (2000) Progress in Nucleic Acid Research and Molecular Biology (Academic, New York), Vol. 63, pp. 69–108. [DOI] [PubMed] [Google Scholar]
- 3.Rouillé Y., Martin, S. & Steiner, D. (1995) J. Biol. Chem. 270, 26488-26496. [DOI] [PubMed] [Google Scholar]
- 4.Rouillé Y., Kantengwa, S., Irminger, J.-C. & Halban, P. A. (1997) J. Biol. Chem. 272, 32810-32816. [DOI] [PubMed] [Google Scholar]
- 5.Mains R. E. & Eipper, B. A. (2000) in Handbook of Physiology, ed. McEwen, B. S. (Oxford Univ. Press, London), Vol. IV, pp. 85–101. [Google Scholar]
- 6.Zhou A., Bloomquist, B. T. & Mains, R. E. (1993) J. Biol. Chem. 268, 1763-1769. [PubMed] [Google Scholar]
- 7.Furuta M., Carroll, R., Martin, S., Swift, H., Ravazzola, M., Orci, L. & Steiner, D. (1998) J. Biol. Chem. 273, 3431-3437. [DOI] [PubMed] [Google Scholar]
- 8.Rhodes C., Lincoln, B. & Shoelson, S. (1992) J. Biol. Chem. 267, 22719-22727. [PubMed] [Google Scholar]
- 9.Furuta M., Zhou, A., Webb, G., Carroll, R., Ravazzola, M., Orci, L. & Steiner, D. F. (2001) J. Biol. Chem. 276, 27197-27202. [DOI] [PubMed] [Google Scholar]
- 10.Laurent V., Kimble, A., Peng, B., Zhu, P., Pintar, J., Steiner, D. & Lindberg, I. (2002) Proc. Natl. Acad. Sci. USA 99, 3087-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viale A., Ortola, C., Hervieu, G., Furuta, M., Barbero, P., Steiner, D. F., Seidah, N. G. & Nahon, J.-L. (1999) J. Biol. Chem. 274, 6536-6545. [DOI] [PubMed] [Google Scholar]
- 12.Rovère C., Barbero, P. & Kitabgi, P. (1996) J. Biol. Chem. 271, 11368-11375. [DOI] [PubMed] [Google Scholar]
- 13.Villeneuve, P., Feliciangeli, S., Croissandeau, G., Seidah, N. G., Mbikay, M., Kitabgi, P. & Beaudet, A. (2002) J. Neurochem., in press. [DOI] [PubMed]
- 14.Johanning K., Juliano, M. A., Juliano, L., Lazure, C., Lamango, N. S., Steiner, D. F. & Lindberg, I. (1998) J. Biol. Chem. 273, 22672-22680. [DOI] [PubMed] [Google Scholar]
- 15.Berman Y., Mzhavia, M., Polonskaia, A., Furuta, M., Steiner, D., Pintar, J. & Devi, L. A. (2000) J. Neurochem. 75, 1763-1770. [DOI] [PubMed] [Google Scholar]
- 16.Day R., Lazure, C., Basak, A., Boudreault, A., Limperis, P., Dong, W. & Lindberg, I. (1998) J. Biol. Chem. 273, 829-836. [DOI] [PubMed] [Google Scholar]
- 17.O'Rahilly S., Gray, H., Humphreys, P., Krook, A., Polonsky, K., White, A., Gibson, S., Taylor, K. & Carr, C. (1995) N. Engl. J. Med. 333, 1386-1390. [DOI] [PubMed] [Google Scholar]
- 18.Jackson R., Creemers, J., Ohagi, S., Raffin-Sanson, M.-L., Sanders, L., Montague, C., Hutton, J. & O'Rahilly, S. (1997) Nat. Genet. 16, 303-306. [DOI] [PubMed] [Google Scholar]
- 19.Miki N., Ono, M., Asakawa-Yasumoto, K., Aoki, T., Murata, Y., Ishituka, Y., Demura, H. & Sasaki, F. (1994) J. Neuroendocrinol. 6, 71-78. [DOI] [PubMed] [Google Scholar]
- 20.Miki N., Ono, M., Miyoshi, H., Tsushima, T. & Shizume, K. (1989) Life Sci. 44, 469-476. [DOI] [PubMed] [Google Scholar]
- 21.Westphal C., Muller, L., Zhou, A., Zhu, X., Bonner-Weir, S., Steiner, D., Lindberg, I. & Leder, P. (1999) Cell 96, 689-700. [DOI] [PubMed] [Google Scholar]
- 22.Holst J. J. & Bersani, M. (1991) Methods Neurosci. 5, 3-22. [Google Scholar]
- 23.Gutniak M. K., Larsson, H., Heiber, S. J., Juneskans, O. T., Holst, J. J. & Ahren, B. (1996) Diabetes Care 19, 843-848. [DOI] [PubMed] [Google Scholar]
- 24.Ørskov C., Rabenhoj, L., Wettergren, A., Kofod, H. & Holst, J. J. (1994) Diabetes 43, 535-539. [DOI] [PubMed] [Google Scholar]
- 25.Hartmann B., Johnsen, A. H., Orskov, C., Adelhorst, K., Thim, L. & Holst, J. J. (2000) Peptides 21, 73-80. [DOI] [PubMed] [Google Scholar]
- 26.Ftouhi N., Day, R., Mbikay, M., Chretien, M. & Seidah, N. (1994) DNA Cell Biol. 13, 395-407. [DOI] [PubMed] [Google Scholar]
- 27.Beamer W. & Eicher, E. M. (1976) J. Endocrinol. 71, 37-45. [DOI] [PubMed] [Google Scholar]
- 28.Gaylinn B. D., Dealmeida, V. I., Lyons, C. E., Jr., Wu, K. C., Mayo, K. E. & Thorner, M. O. (1999) Endocrinology 140, 5066-5074. [DOI] [PubMed] [Google Scholar]
- 29.Lin S.-C., Lin, C. R., Gukovsky, I., Lusis, A. J., Sawchenko, P. E. & Rosenfeld, M. G. (1993) Nature (London) 364, 208-213. [DOI] [PubMed] [Google Scholar]
- 30.Clemmons D. R. (2001) in Endocrinology, eds. DeGroot, L. & Jameson, J. (Saunders, Philadelphia), Vol. 1, pp. 439–460. [Google Scholar]
- 31.Mayo K. E., Godfrey, P. A., Suhr, S. T., Kulik, D. J. & Rahal, J. O. (1995) Recent Prog. Horm. Res. 50, 35-73. [DOI] [PubMed] [Google Scholar]
- 32.Drucker D. J. (2001) J. Clin. Endocrinol. Metab. 86, 1759-1764. [DOI] [PubMed] [Google Scholar]
- 33.Drucker D. J. (2002) Gut 50, 428-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roebroek A. J., Umans, L., Pauli, I. G., Robertson, E. J., van Leuven, F., Van de Ven, W. J. & Constam, D. B. (1998) Development (Cambridge, U.K.) 125, 4863-4876. [DOI] [PubMed] [Google Scholar]
- 35.Constam D. B. & Robertson, E. J. (2000) Genes Dev. 14, 1146-1155. [PMC free article] [PubMed] [Google Scholar]
- 36.Mbikay M., Tadros, H., Ishida, N., Lerner, C., De Lamirande, E., Chen, E., El-Alfy, M., Clermont, Y., Seidah, N., Chretien, M., et al. (1997) Proc. Natl. Acad. Sci. USA 94, 6842-6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M., Mbikay, M., Nakayama, K., Miyata, A. & Arimura, A. (2000) Ann. N.Y. Acad. Sci. 921, 333-339. [DOI] [PubMed] [Google Scholar]
- 38.Furuta M., Yano, H., Zhou, A., Rouille, Y., Holst, J., Carroll, R., Ravazzola, M., Orci, L., Furuta, H. & Steiner, D. (1997) Proc. Natl. Acad. Sci. USA 94, 6646-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Behringer R. R., Mathews, L. S., Palmiter, R. D. & Brinster, R. L. (1988) Genes Dev. 2, 453-461. [DOI] [PubMed] [Google Scholar]
- 40.Salvatori R., Fan, X., Mullis, P. E., Haile, A. & Levine, M. A. (2002) Mol. Endocrinol. 16, 450-458. [DOI] [PubMed] [Google Scholar]
- 41.Nillni E. A., Steinmetz, R. & Pescovitz, O. H. (1999) Endocrinology 140, 5817-5827. [DOI] [PubMed] [Google Scholar]
- 42.Nakayama K., Watanabe, T., Nakagawa, T., Kim, W., Nagahama, M., Hosaka, M., Hatsuzawa, K., Kondoh-Hashiba, K. & Murakami, K. (1992) J. Biol. Chem. 267, 16335-16340. [PubMed] [Google Scholar]
- 43.Rufaut N. W., Brennan, S. O., Hakes, D. J., Dixon, J. E. & Birch, N. P. (1993) J. Biol. Chem. 268, 20291-20298. [PubMed] [Google Scholar]
- 44.Krude H., Biebermann, H., Luck, W., Horn, R., Brabant, G. & Gruters, A. (1998) Nat. Genet. 19, 155-157. [DOI] [PubMed] [Google Scholar]
- 45.Zhu X., Orci, L., Carroll, R., Norrbom, C., Ravazzola, M. & Steiner, D. F. (2002) Proc. Natl. Acad. Sci. USA 99, 10299-10304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orskov C. (1992) Diabetologia 35, 701-711. [PubMed] [Google Scholar]
- 47.Scrocchi L. A., Brown, T. J., MaClusky, N., Brubaker, P. L., Auerbach, A. B., Joyner, A. L. & Drucker, D. J. (1996) Nat. Med. 2, 1254-1258. [DOI] [PubMed] [Google Scholar]
- 48.De Bie I., Marcinkiewicz, M., Malide, D., Lazure, C., Nakayama, K., Bendayan, M. & Seidah, N. G. (1996) J. Cell Biol. 135, 1261-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.