Abstract
The neuroendocrine processing endoproteases PC2 and PC1/3 are expressed in the β cells of the islets of Langerhans and participate in the processing of proinsulin to insulin and C-peptide. We have previously shown that disruption of PC2 (SPC2) expression significantly impairs proinsulin processing. Here we report that disruption of the expression of PC1/3 (SPC3) produces a much more severe block in proinsulin conversion. In nulls, pancreatic and circulating proinsulin-like components comprise 87% and 91%, respectively, of total insulin-related immunoreactivity. Heterozygotes also show a more than 2-fold elevation in proinsulin levels to ≈12%. Immunocytochemical and ultrastructural studies of the β cells reveal the nearly complete absence of mature insulin immunoreactivity and its replacement by that of proinsulin in abundant immature-appearing secretory granules. In contrast, α cell morphology and glucagon processing are normal, and there is also no defect in somatostatin-14 generation. Pulse–chase labeling studies confirm the existence of a major block in proinsulin processing in PC1/3 nulls with prolongation of half-times of conversion by 7- and 10-fold for proinsulins I and II, respectively. Lack of PC1/3 also results in increased levels of des-64,65 proinsulin intermediates generated by PC2, in contrast to PC2 nulls, in which des- 31,32 proinsulin intermediates predominate. These results confirm that PC1/3 plays a major role in processing proinsulin, but that its coordinated action with PC2 is necessary for the most efficient and complete processing of this prohormone.
The biosynthesis of insulin via its precursors, preproinsulin and proinsulin, is one of the key processes that ensures the production of sufficient amounts of insulin in the pancreatic β cell (1–3). [The efficient conversion of proinsulin to insulin requires cleavages at both junctions of the connecting segment linking the B and A chains to release insulin and C-peptide. These products normally are stored within the mature secretory granules (>95% of total insulin-related material) awaiting secretion in response to glucose and other stimuli (1).] Initial processing cleavages occur between residues 32 and 33 (R↓E) and residues 65 and 66 (R↓G) at the B and A chain junctions, respectively. Recognition of each of these sites by the neuroendocrine convertases PC1/3 (SPC3) and PC2 (SPC2) (1, 4, 5) involves interactions with 4–6 residues upstream and at least two residues downstream, i.e., in human proinsulin resides 27–34 and 60–67, surrounding these two sites (6, 7). The initial cleavage products, consisting of insulin extended at the B chain C terminus by R31-R32 and C-peptide extended C-terminally by K64-R65, are then trimmed by removal of these basic residues by neuroendocrine carboxypeptidase E (CPE) (8) to yield the mature β cell secretory products. These convertases and CPE act mainly within maturing dense-core granules in the regulated secretory pathway (9, 10). In a previous study, we have demonstrated that mice lacking the convertase PC2 have a significant block in proinsulin processing that leads to the accumulation of proinsulin at levels of approximately 35% of total insulin-related material in pancreatic extracts, accompanied by significant amounts of des-31,32 proinsulin intermediate generated by the preferential cleavage of the B chain—C-peptide junction (11, 12). The finding that PC2 accounted for at most only one-third of normal proinsulin processing suggested that other convertases in the secretory granules account for the conversion of the remainder. In the present study, we have examined the effects of disruption of the gene encoding PC1/3 on the biosynthesis and conversion of proinsulin to insulin in islets from PC1/3 null mice and have carried out immunohistochemical analyses to assess both islet morphology and the nature of the stored material within the β cell secretory granules. The results indicate the existence of a far more severe block in proinsulin processing as one of the major phenotypes of this knockout, in comparison to that seen in PC2 null mice.
Materials and Methods
Animals.
The PC1/3 null mutant mouse line was generated as described (13). For all experiments, 8- to 12-week-old PC1/3 null mice and control (+/+) littermates of the same age were used. The care and treatment of all animals was in accordance with National Institutes of Health and institutional guidelines.
Insulin Biosynthesis.
Islets of Langerhans were isolated as described (14). Isolated islets were cultured overnight in RPMI medium containing 11 mM glucose and 10% FCS. After overnight culture, islets were washed and then pulse-labeled in groups of 300–400 in 100 μl of medium (12) containing 27.7 mM glucose and 500 μCi (1 μCi = 37 GBq) each of [35S]methionine (1,000 Ci/mmol) and [3H]leucine (300 μCi/mmol) (Amersham Pharmacia) for 45 min at 37°C. Labeled islets were then rinsed, divided into batches, and incubated for chase periods up to 3 h in medium containing 5 mM glucose and 20 μg/ml of unlabeled methionine and leucine. After the pulse and chase incubations, the islets were washed and frozen. These samples were later thawed and resuspended in immunoprecipitation buffer (0.05 M Tris⋅HCl/0.1 M NaCl/2.5 mg/ml BSA/1% Triton X-100, pH 7.6) containing a mixture of protease inhibitors and sonicated. The supernatants after centrifugation for 2 min at 12,000 × g were then treated with an immunoaffinity absorbent consisting of guinea pig anti-insulin Ig fraction coupled to Bio-Rad Affi-Gel 20 agarose beads (12). Insulin and proinsulin-related immunoreactive proteins were eluted from the beads with 30% acetonitrile/1 M acetic acid and then analyzed by HPLC as described (12). The [35S] and [3H] radioactivity in each fraction was then measured by scintillation counting.
Proinsulin and Insulin-like Immunoreactive Products in Serum and Pancreas.
Pancreatic extracts were prepared individually (six wild type, three heterozygous, six null) and analyzed as described (12). Blood was collected from the retroorbital sinus, and the serum from 12 animals of each genotype was pooled and stored at −80°C until analysis. The serum pools were applied to Sep-pak C18 cartridges (Millipore), and the retained protein fractions, containing proinsulin and insulin-like products (15), were eluted, lyophilized, dissolved in 3 M acetic acid, and applied to a 1 × 50 cm Bio-Gel P-30 column eluted with 3 M acetic acid containing 50 μg/ml of BSA. Fractions containing proinsulin or insulin were combined, dried, and redissolved for insulin RIA (16). Proinsulin values were corrected for reduced cross-reactivity in this assay by multiplying by 2.2.
Immunocytochemistry.
Pancreatic tissue from wild-type and PC1/3−/− mice was processed for light and electron microscopic immunocytochemistry and conventional electron microscopy. For light microscopy, both paraffin (5 μm) and Epon (1 μm) sections were studied. Paraffin-embedded tissue was fixed in Bouin's fluid, Epon-embedded tissue in 2% glutaraldehyde. The embedding medium was removed and the sections were incubated by the indirect immunofluorescence method. Sections were incubated for 2 h at room temperature with the primary antibodies mouse monoclonal anti-proinsulin (17, 18) at dilution 1:400, mouse monoclonal anti-insulin (19, 20), diluted 1:20, and rabbit polyclonal anti C-terminal glucagon (code 15K from R. H. Unger, University of Texas Southwestern Center, Dallas), diluted 1:100. The sections were washed, exposed to goat anti-mouse or goat anti-rabbit IgG conjugated to FITC for 1 h before being stained with 0.03% Evans blue, and observed with a Zeiss Axiophot III fluorescence microscope.
For electron microscopy immunolabeling, islets of Langerhans were dissected from the exocrine pancreatic tissue, embedded in 12% gelatin, and cryoprotected with 2.3 M sucrose before freezing with liquid nitrogen and sectioning with a cryoultramicrotome. The thin cryosections were incubated with the anti-insulin or anti-proinsulin antibodies for 1 h at room temperature, washed, and labeled with goat anti-mouse IgG-gold (gold diameter 10 nm). For conventional electron microscopy, tissue was postfixed with 1% OsO4 before dehydration and Epon embedding. The sections were stained with uranyl acetate and lead citrate and observed with a Philips CM10 electron microscope.
Results
The first indications of a block in proinsulin maturation in the islets of Langerhans in the PC1/3 null mice came from measurements of proinsulin-related and insulin components in extracts of pancreas (13) or serum resolved by gel filtration. Typical results are summarized in Table 1. The proportion of proinsulin and intermediates was more than 85% in the homozygous null islets, but was also significantly elevated in the heterozygous nulls to ∼12%, more than twice normal values. Even greater proportions of proinsulin-related components were present in the serum (91%), reflecting their slower clearance from the circulation, caused by their reduced insulin receptor binding potency (1). In contrast, pancreatic glucagon was normally processed (data not shown).
Table 1.
Proinsulin/total insulin immunoreactivity
| Genotype | Pancreas | Serum |
|---|---|---|
| PC1/3 +/+ | 5.5% | 2.5% |
| PC1/3 +/− | 12.3% | 12.2% |
| PC1/3 −/− | 87% | 91% |
Morphology of PC1/3 Islets.
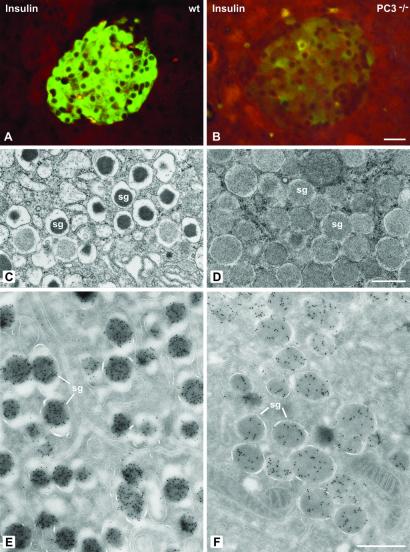
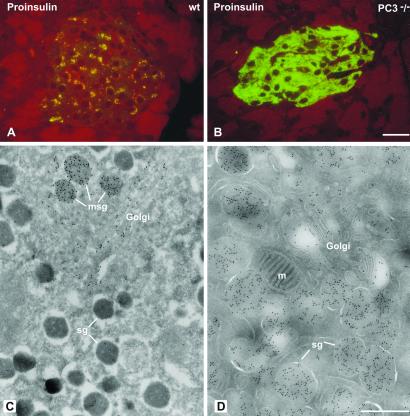
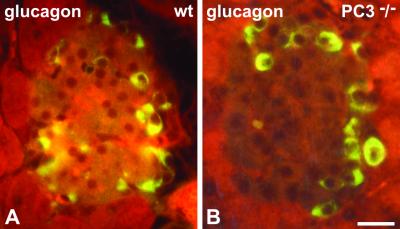
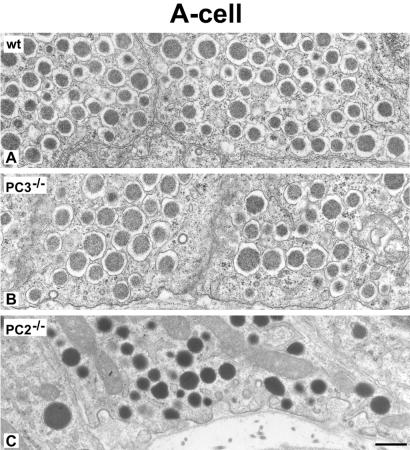
We then examined the morphology of the insulin-producing β cells in the islets in the PC1/3 nulls. The β cells were the predominant cell type in these islets as in wild-type islets (Figs. 1 and 2). However, on immunostaining with antisera specific for insulin almost no staining was observed throughout the islets in the nulls, in sharp contrast to the heavy insulin immunofluorescence seen in wild-type islets (Fig. 1 A and B). Electron microscopic examination revealed large numbers of mature-appearing secretory granules in the (+/+) islets (Fig. 1C) whereas the secretory granules in the null β cells were markedly immature in appearance, containing large amounts of homogeneous lighter density more characteristic of the proinsulin-rich newly formed secretory granules usually seen only near the Golgi apparatus in normal β cells (Fig. 1 C and D). Immunogold labeling of these granules with the insulin-specific antiserum in the PC1/3 null β cells was markedly reduced as compared with that in the (+/+) granules (Fig. 1 E and F). In contrast, immunofluorescent staining with a proinsulin-specific mAb resulted in bright labeling of the β cells in the (−/−) islets, whereas staining in the wild-type β cells was restricted to the Golgi area (Fig. 2 A and B). By immunogold staining with this antibody, it is evident that all of the immature-appearing secretory granules in the null β cells are strongly positively stained (Fig. 2D), whereas only a few newly formed secretory granules near the Golgi apparatus are stained in wild-type mice (Fig. 2C). Thus, these findings clearly demonstrate that the PC1/3 null β cells contain a very high proportion of stored proinsulin-related material and little, if any, mature insulin. We then examined the overall morphology of the islets in the PC1/3 nulls. In contrast to the PC2 nulls, there was no evident hyperplasia of the glucagon-producing α cells in the PC1/3 nulls (Fig. 3), and β cells were the predominant cell type, as in the normal wild-type pancreas (see Figs. 1–3). Electron microscopic examination of the α cells also revealed the presence of normal numbers of mature secretory granules, whereas those of the PC2 nulls contained immature-appearing granules (Fig. 4). Previous studies have demonstrated that the latter contain large amounts of unprocessed proglucagon (21).
Fig 1.
Insulin immunoreactivity is decreased in PC3−/− mice. (A and B) Paraffin sections of pancreatic islets of wild-type (wt) (A) and knockout (B) mice incubated with the anti-insulin antibody by the immunofluorescence method. In control islet (A) the mass of insulin B cells is brightly immunostained, whereas in PC3−/− islet (B), the insulin cells show a very strong reduction in labeling. The reduced level of insulin immunoreactivity was also detectable on thin sections labeled with insulin antibody revealed by anti-mouse IgG-gold. The quantitation indicated 502 ± 24 gold particles/μm2 of 20 secretory granules (sg, E) versus 169 ± 9 gold particles/μm2 (PC3−/− mice, F). The secretory granules in PC3−/− mice also had a different morphology characterized by a pale content and a thin halo (D), as compared with the characteristic dense core and wide halo in the control wt mice (C). (Bars: A and B, 20 μm; C–F, 0.5 μm.)
Fig 2.
Proinsulin immunoreactivity is increased in PC3−/− mice. (A and B) Staining by immunofluorescence for proinsulin on semithin Epon sections from wild-type (wt) and mutant mice. In wt islets proinsulin staining has the characteristic Golgi-like perinuclear distribution (A); in mutant PC3−/− mice, proinsulin staining is abundant throughout the entire cytoplasm (B). Proinsulin labeling at the ultrastructural level is restricted to the Golgi complex and the maturing secretory granules (msg) in wt mice (C). In PC3−/− mice, gold labeling is present over the Golgi complex and the entire population of secretory granules (sg) (D). m = Mitochondrion. (Bars: A and B, 20 μm; C and D, 0.5 μm.)
Fig 3.
Glucagon immunoreactivity shows a similar pattern of distribution in wild-type (wt) and PC3 null mice. Shown are paraffin sections of pancreatic islets from wild-type (A) and PC3 null (B) mice incubated with C-terminal anti-glucagon antibody by the immunofluorescence method. The amount and distribution of immunoreactive cells are similar in wt and null islets. (Bar: 20 μm.)
Fig 4.
The morphology of glucagon cell secretory granules is unaltered in PC3 null mice. Shown are thin section electron micrographs comparing the morphologic appearance of α cell secretory granules in wild-type (wt) (A), PC3(−/−) (B), and PC2(−/−) (C) mice. PC3 null and wt secretory granules show a similar aspect, characterized by a dense granule core separated by a distinct clear halo from the limiting membrane. By contrast, in PC2 null glucagon cell granules, the halo is absent and the denser content reaches the granule limiting membrane. (Bar: 0.5 μm.)
Analysis of Insulin Biosynthesis.
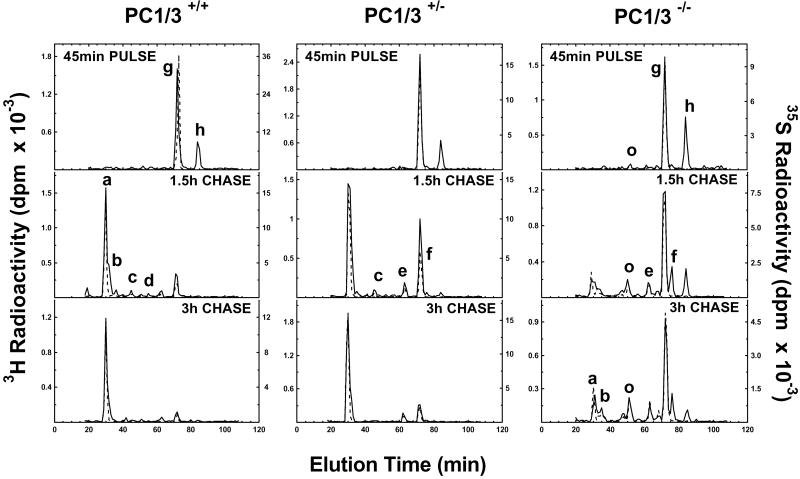
We next carried out a series of metabolic labeling studies to examine the biosynthesis and kinetics of conversion of the two mouse proinsulins into their respective insulins. The two mouse proinsulins arise from two nonallelic preproinsulin genes and differ at five positions, two in the insulins and three in the C-peptides (22). Two of these differences alter the P4 residues (i.e., 4 aa upstream) in both of the cleavage sites for conversion of proinsulin to insulin, which in turn influences their rates of processing such that proinsulin I, which has basic P4 residues in both sites, is converted more rapidly than proinsulin II (12). A single methionine residue in mouse proinsulin II replaces lysine at position B29 (P4) and allows it to be readily distinguished from mouse proinsulin I in dual-labeling experiments with [35S]- methionine and [3H]leucine. Pulse–chase labeling experiments, shown in Fig. 5, were carried out, consisting of a 45-min pulse at high glucose to enhance incorporation of amino acids into proinsulin, followed by a chase incubation at low glucose for periods of 1.5 and 3 h. The results indicate a severe block in conversion of both proinsulins to insulin in the homozygous nulls, extending throughout the entire chase period and accompanied by the appearance of significant amounts of des-64,65 proinsulin intermediate (Fig. 5 Right). It should be noted that because of the presence of CPE, along with the converting endoproteases in the insulin secretory granules (10), the lysine-arginine pair at positions 64 and 65 in the C-domain is rapidly removed as soon as endoproteolytic cleavage by PC2 occurs between residues 65 and 66 to initiate the generation of this intermediate. Thus, no C-terminally extended forms are normally seen in these experiments unless CPE is lacking or defective (23). Interestingly, the PC1/3 heterozygotes also show a significant block in proinsulin conversion, which is especially evident at the 1.5-h chase interval (Fig. 5 Center).
Fig 5.
Conversion of proinsulin to insulin in pulse–chase metabolic labeling. Islets from wild-type (Left), heterozygous (+/−) (Center), or null (−/−) (Right) mice were labeled with 35S-Met (dashed lines) or 3H-Leu (solid lines) in high glucose for 45 min and then chased in low glucose for a total of 3 h. Proteins were extracted from islets at indicated times, immunopurified, and resolved on HPLC, as described in Materials and Methods. Peaks are identified (see ref. 12) as follows: a = mouse insulin II; b = mouse insulin I; c and d = des-31,32 mProinsulins II and I, respectively; e and f = des-64,65 mProinsulins II and I, respectively; g and h = intact mProinsulins II and I, respectively; and o = oxidized mProinsulin II. Note the rapid conversion of both proinsulins I and II in wild-type islets, whereas conversion is clearly slowed in heterozygous islets at 1.5 h and markedly reduced throughout the chase period in null islets, accompanied by the appearance of des-64,65 intermediate peaks (e and f).
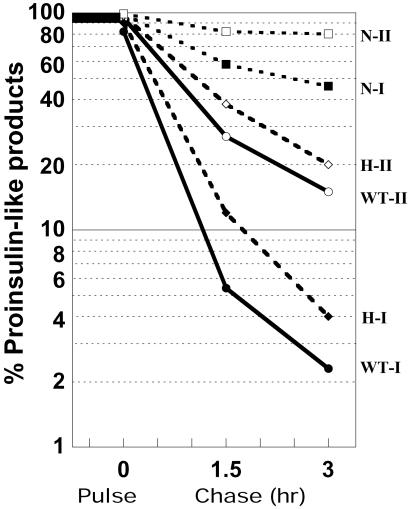
There were significant differences in the generation of proinsulin intermediate forms in these experiments as compared with similar experiments performed previously with the PC2 nulls. In those experiments, des-31,32 intermediates predominated, with levels of up to approximately 20% of both of the two proinsulin isoforms (12). In the present experiments, this pattern was reversed, reflecting the preference of PC2 for cleavage at the C peptide–A chain junction (Fig. 6). The HPLC data indicate a greater buildup of des-64,65 proinsulin intermediates in both the heterozygotes and homozygous nulls, but only in the nulls were levels above 10% seen and then only in the case of des-64,65, proinsulin I, which reached levels of 25–30% during the chase period (Fig. 6). This finding is consistent with the presence of a P4 arginine residue at this site in proinsulin I (12), which clearly enhances its susceptibility for cleavage by PC2, the only convertase now present in the β cell secretory granules in the PC1/3 nulls. The data in Fig. 6 also demonstrate the excellent balance of the proteolytic processing in the wild-type islets where conversion by the conjoint action of both PC2 and PC1/3 is very rapid and without any attendant build-up of intermediates (Fig. 5 Left).
Fig 6.
Time course of accumulation of des-64,65 proinsulin intermediates in wild-type (+/−) and null islets during a 3-h chase after a 45-min pulse. Note much greater accumulation in nulls of both proinsulin I and II intermediates. The much greater production of the des-64,65 intermediate I is likely caused by the presence of a P4 arginine residue at position 62 in proinsulin I rather than glutamine, as in proinsulin II. ◊, Wild type; ○, heterozygotes; •, nulls. Dashed line denotes des- 64,65 proinsulin I and solid line indicates des-64,65 proinsulin II.
Analysis of the data on rates of conversion of both proinsulins to insulin, modeled as pseudofirst-order reactions is shown on semilog plots in Fig. 7. These data indicate a tendency of the conversion rates to decrease somewhat during the second chase period in all instances. We therefore have used the data from the more rapid first chase period to determine half-times for conversion, as shown in Table 2. These data indicate that proinsulin I was converted approximately 2-fold more rapidly than proinsulin II in all cases. Comparing nulls to wild type, the half-time rate of conversion of proinsulin I was increased 7-fold and that of proinsulin II 10-fold, whereas the half-times for both proinsulins in the heterozygous nulls were increased to 150% of wild-type values. These findings are consistent with the observed increases of unconverted proinsulin seen in pancreatic extracts and serum from both null and heterozygous animals (Table 1). The retention of large amounts of unprocessed proinsulin-related material in the secretory granules in the PC1/3 null pancreas (Fig. 2) suggests that active intragranular processing of proinsulin may be terminated by some kind of active process after the first few hours of granule maturation, despite the maintenance of suitable conditions of pH and calcium ion concentration for continued convertase action in mature granules.
Fig 7.
Semilog plot of the disappearance rates of proinsulin-like components during a 3-h chase in islets of PC1/3 (+/+), (+/−), and (−/−) mice. Black symbols denote proinsulin I, and white symbols denote proinsulin II components. N, null; H, heterozygous; WT, wild type.
Table 2.
Proinsulin disappearance rates during chase period
| Genotype
|
Half-time in h | |
|---|---|---|
| PI-I | PI-II | |
| PC1/3 (+/+) | 0.38 | 0.75 |
| PC1/3 (+/−) | 0.53 | 1.2 |
| PC1/3 (−/−) | 2.6 | 7.3 |
Discussion
The prohormone convertase family of subtilisin-related proteases consists of two main divisions: those including furin, PACE4, PC6B, and PC7 that are localized in the distal Golgi/trans-Golgi network and act predominantly on the precursors of growth factors, growth factor receptors, and viral or other secreted cell surface glycoproteins (24), and the others consisting of PC2, PC1/3, PC4, and PC5/6A that localize to dense core vesicles in the regulated secretory pathway and process the rich variety of neuropeptides and peptide hormone precursors before their storage and release (4, 5). Of these, PC1/3 and PC2 are the most widely distributed within the brain and neuroendocrine system, being either coexpressed or expressed separately in various cell populations (5, 25). PC4 is expressed mainly, if not exclusively, in gonadal cells (26) whereas PC5/6A is expressed in gut and to a lesser extent in brain (27). In the islets of Langerhans both PC2 and PC1/3 are expressed in the insulin-producing β cells whereas PC2 appears to be the sole secretory granule convertase expressed in the non-β cell populations—the α, γ, and δ cells that express glucagon, pancreatic polypeptide, and somatostatin, respectively (11, 28). These latter cell types are therefore more severely affected in the PC2 null islets (11, 21) and exhibit marked hyperplasia and hypertrophy, associated with the inability to process their respective precursors to normal bioactive products. Because of the lack of glucagon, the PC2 nulls are chronically mildly hypoglycemic (11). Administration of exogenous glucagon i.p. via implanted osmotic minipumps (29) reverses the excessive secretory activity and hyperplasia of the PC2 null α cells and restores normal blood glucose levels. None of these changes occurs in the PC1/3 null mice. Glucagon is processed normally, the blood glucose level is normal (13), and the islet morphology remains normal, as shown here with the central β cell mass being predominant. Surprisingly, pancreatic glucagon content is elevated in PC1/3 nulls (1.6-fold) and the serum level is also elevated by about 2.5-fold (data not shown). In contrast to the situation in islets, proglucagon processing to GLP1 and GLP2 in intestinal L cells is blocked in PC1/3 nulls, a defect likely to influence insulin responses to ingested food through loss of GLP1's incretin effect (13). Somatostatin-14 is also present in normal amounts in pancreatic extracts from PC1/3 null mice (data not shown) in contrast to the production of only somatostatin-28, a larger intermediate form of somatostatin in the pancreatic islets in PC2 nulls (11).
Our findings in both biosynthetic and pancreatic extraction studies confirm the major importance of PC1/3 for normal proinsulin processing and strongly support the proposal that PC1/3 acts first on proinsulin to cleave at the B chain—C-peptide junction, thereby generating an intermediate, split 32,33 proinsulin, that is rapidly converted to the des-31,32 intermediate by CPE, which removes the two C-terminal arginine residues at B31 and B32. This intermediate has been shown to be a preferred substrate for PC2 cleavage at the remaining C-peptide—A chain junction (30). Thus although either enzyme can cleave at both sites in the absence of the other, the efficiency and completeness of conversion is greatly enhanced by their coordinated action. PC1/3 is activated earlier than PC2 and also functions well at a somewhat less acidic pH (5.5–6.0), whereas PC2 activation has special requirements and occurs later (9). PC2 also has a more acidic pH optimum (≈5.0). All of these properties are in keeping with the proposed two-step concerted mechanism of proinsulin activation in which PC1/3 begins intragranular processing and PC2 finishes it (2).
It should be noted that whereas PC1/3 clearly is more important than PC2 for the processing of proinsulin to insulin in the β cells as shown here, proinsulin is only one of many precursors that are processed in the β cell, although it is surely the most abundant and most important one. Some other β-granule constituents that have been identified are islet amyloid polypeptide (IAPP/amylin), chromogranin A and products, transthyretin, thyrotropin-releasing hormone, and proopiomelanocortin (31, 32). All of these require proteolytic processing and may therefore undergo the action of either PC2, PC1/3, or perhaps both. IAPP is of special interest because it not only has been shown to exert a mild insulin antagonistic action, but also tends to form fibrillar amyloid deposits in the islets of type 2 diabetes in humans in late stages of this disorder. It is therefore of interest whether pro-IAPP or its partially processed intermediates might contribute to the tendency of the human peptide to aggregate. Studies by Wang et al. (33) have shown that PC2 null mice exhibit a loss of cleavage of the N-terminal propeptide of IAPP. Further studies should assess the effects of PC1/3 deficiency. Preliminary findings, however, indicate that PC2 may be the dominant activity in processing this β cell product (34) in contrast to the situation with insulin.
In human, PC1/3 deficiency has been reported to be associated with severe hyperproinsulinemia with plasma levels of proinsulin in excess of 90% and des-64,65 proinsulin as the main intermediate form, as found in our PC1/3 null mice (35, 36). This interesting patient also exhibited gestational diabetes and severe early onset obesity without any clinically evident growth defect. The lack of obesity in our mice could reflect the lack of growth hormone and its normal insulinotropic effect (13). On the other hand, the genetic background of the human subject could also well have been an important contributing factor to the development of obesity. The effects of PC1/3 deficiency on the processing of various neuropeptides involved in regulation of food intake and energy metabolism is thus an important issue. We have examined the processing of pro-CART (cocaine-amphetamine-related transcript), a neuroendocrine precursor that gives rise to a potent anorexogenic peptide, CART, in the hypothalamus (37, 38). CART appears to be an important downstream target of leptin that may mediate its suppression of appetite. However, PC1/3 deficiency does not appear to significantly impair CART processing in vivo (A. Dey and D.F.S., unpublished results). However, it is likely that PC1/3 deficiency impairs hypothalamic processing of proglucagon to generate GLP1 (13), another potent anorexogenic peptide (39). Further analysis of the processing of these and other hypothalamic regulatory peptides in PC2 and PC1/3 mice may provide useful new insights into the central mechanisms that contribute to the development of obesity.
Acknowledgments
We thank John Hutton for helpful review and advice and Rosie Ricks for expert assistance in preparing this report. This work was supported by National Institutes of Health Grants DK20595 (Diabetes Research and Training Center) and DK13914, the Howard Hughes Medical Institute (to D.F.S.), and Swiss National Science Foundation grants (to L.O.).
Abbreviations
CPE, carboxypeptidase E
References
- 1.Steiner D., Chan, S. & Rubenstein, A. (2000) in Handbook of Physiology, The Endocrine System, eds. Jefferson, L. & Cherrington, A. (Oxford Univ. Press, New York), Vol. II, pp. 49–77. [Google Scholar]
- 2.Steiner D. (2001) in The Enzymes, eds. Dalbey, R. E. & Sigman, D. S. (Academic, New York), Vol. XXII, pp. 163–198. [Google Scholar]
- 3.Goodge K. A. & Hutton, J. C. (2000) Semin. Cell. Dev. Biol. 11, 235-242. [DOI] [PubMed] [Google Scholar]
- 4.Zhou A., Webb, G., Zhu, X. & Steiner, D. F. (1999) J. Biol. Chem. 274, 20745-20748. [DOI] [PubMed] [Google Scholar]
- 5.Seidah N. G. & Chrétien, M. (1999) Brain Res. 848, 45-62. [DOI] [PubMed] [Google Scholar]
- 6.Siezen R. J., Creemers, J. W. & Van de Ven, W. J. (1994) Eur. J. Biochem. 222, 255-266. [DOI] [PubMed] [Google Scholar]
- 7.Lipkind G., Gong, Q. & Steiner, D. F. (1995) J. Biol. Chem. 270, 13277-13284. [DOI] [PubMed] [Google Scholar]
- 8.Fricker L. (1988) Annu. Rev. Physiol. 50, 309-321. [DOI] [PubMed] [Google Scholar]
- 9.Cameron A. & Apletalina, E. (2001) in The Enzymes, eds. Dalbey, R. E. & Sigman, D. S. (Academic, New York), Vol. XXII, pp. 291–332. [Google Scholar]
- 10.Guest P. C., Arden, S. D., Rutherford, N. G. & Hutton, J. C. (1995) Mol. Cell. Endocrinol. 113, 99-108. [DOI] [PubMed] [Google Scholar]
- 11.Furuta M., Yano, H., Zhou, A., Rouille, Y., Holst, J., Carroll, R., Ravazzola, M., Orci, L., Furuta, H. & Steiner, D. (1997) Proc. Natl. Acad. Sci. USA 94, 6646-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuta M., Carroll, R., Martin, S., Swift, H., Ravazzola, M., Orci, L. & Steiner, D. (1998) J. Biol. Chem. 273, 3431-3437. [DOI] [PubMed] [Google Scholar]
- 13.Zhu X., Zhou, A., Dey, A., Norrbom, C., Carroll, R., Zhang, C., Laurent, V., Lindberg, I., Ugleholdt, R., Holst, J. J. & Steiner, D. F. (2002) Proc. Natl. Acad. Sci. USA 99, 10293-10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lernmark A., Nathans, A. & Steiner, D. (1976) J. Cell Biol. 71, 606-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wroblewski V. J., Masnyk, M. & Kaiser, R. E. (1993) Diabetes 42, 1407-1414. [DOI] [PubMed] [Google Scholar]
- 16.Tager H., Rubenstein, A. & Steiner, D. (1975) Methods Enzymol. 37, 326-345. [DOI] [PubMed] [Google Scholar]
- 17.Madsen O. D., Cohen, R. M., Fitch, F. W., Rubenstein, A. H. & Steiner, D. F. (1983) Endocrinology 113, 2135-2144. [DOI] [PubMed] [Google Scholar]
- 18.Orci L., Ravazzola, M., Amherdt, M., Madsen, O., Vassalli, J. & Perrelet, A. (1985) Cell 42, 671-681. [DOI] [PubMed] [Google Scholar]
- 19.Storch M. J., Petersen, K. G., Licht, T. & Kerp, L. (1985) Diabetes 34, 808-811. [DOI] [PubMed] [Google Scholar]
- 20.Orci L., Ravazzola, M., Storch, M. J., Anderson, R. G., Vassalli, J. D. & Perrelet, A. (1987) Cell 49, 865-868. [DOI] [PubMed] [Google Scholar]
- 21.Furuta M., Zhou, A., Webb, G., Carroll, R., Ravazzola, M., Orci, L. & Steiner, D. F. (2001) J. Biol. Chem. 276, 27197-27202. [DOI] [PubMed] [Google Scholar]
- 22.Wentworth B. M., Schaefer, I. M., Villa-Komaroff, L. & Chirgwin, J. M. (1986) J. Mol. Evol. 23, 305-312. [DOI] [PubMed] [Google Scholar]
- 23.Naggert J., Fricker, L., Varlamov, O., Nishina, P., Rouille, Y., Steiner, D., Carroll, R., Paigen, B. & Leiter, E. (1995) Nat. Genet. 10, 135-142. [DOI] [PubMed] [Google Scholar]
- 24.Molloy S. S. & Thomas, G. (2001) in The Enzymes, eds. Dalbey, R. E. & Sigman, D. S. (Academic, New York), Vol. XXII, pp. 199–235. [Google Scholar]
- 25.Schäfer M. K.-H., Day, R., Cullinan, W. E., Chretien, M., Seidah, N. G. & Watson, S. J. (1993) J. Neurosci. 13, 1258-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tadros H., Chretien, M. & Mbikay, M. (2001) J. Reprod. Immunol. 49, 133-152. [DOI] [PubMed] [Google Scholar]
- 27.De Bie I., Marcinkiewicz, M., Malide, D., Lazure, C., Nakayama, K., Bendayan, M. & Seidah, N. G. (1996) J. Cell Biol. 135, 1261-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcinkiewicz M., Ramla, D., Seidah, N. G. & Chretien, M. (1994) Endocrinology 135, 1651-1660. [DOI] [PubMed] [Google Scholar]
- 29.Webb G. C., Akbar, M. S., Zhao, C., Swift, H. H. & Steiner, D. F. (2002) Diabetes 51, 398-405. [DOI] [PubMed] [Google Scholar]
- 30.Rhodes C., Lincoln, B. & Shoelson, S. (1992) J. Biol. Chem. 267, 22719-22727. [PubMed] [Google Scholar]
- 31.Nishi M., Sanke, T., Nagamatsu, S., Bell, G. & Steiner, D. (1990) J. Biol. Chem. 265, 4173-4176. [PubMed] [Google Scholar]
- 32.Hummel A. & Zuhlke, H. (1994) Biol. Chem. Hoppe-Seyler 375, 811-815. [DOI] [PubMed] [Google Scholar]
- 33.Wang J., Xu, J., Finnerty, J., Furuta, M., Steiner, D. F. & Verchere, C. B. (2001) Diabetes 50, 534-539. [DOI] [PubMed] [Google Scholar]
- 34.Marzban, L., Wang, J., Trigo, G., Steiner, D. F. & Verchere, C. B. (2002) Diabetes, in press.
- 35.O'Rahilly S., Gray, H., Humphreys, P., Krook, A., Polonsky, K., White, A., Gibson, S., Taylor, K. & Carr, C. (1995) N. Engl. J. Med. 333, 1386-1390. [DOI] [PubMed] [Google Scholar]
- 36.Jackson R., Creemers, J., Ohagi, S., Raffin-Sanson, M.-L., Sanders, L., Montague, C., Hutton, J. & O'Rahilly, S. (1997) Nat. Genet. 16, 303-306. [DOI] [PubMed] [Google Scholar]
- 37.Douglass J., McKinzie, A. A. & Couceyro, P. (1995) J. Neurosci. 15, 2471-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thim L., Kristensen, P., Nielsen, P. F., Wulff, B. S. & Clausen, J. T. (1999) Proc. Natl. Acad. Sci. USA 96, 2722-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drucker D. J. (2001) Endocrinology 142, 521-527. [DOI] [PubMed] [Google Scholar]